Comparison of the New-Generation Self-Expanding NAVITOR Transcatheter Heart Valve with Its Predecessor, the PORTICO, in Severe Native Aortic Valve Stenosis
Abstract
:1. Introduction
2. Methods
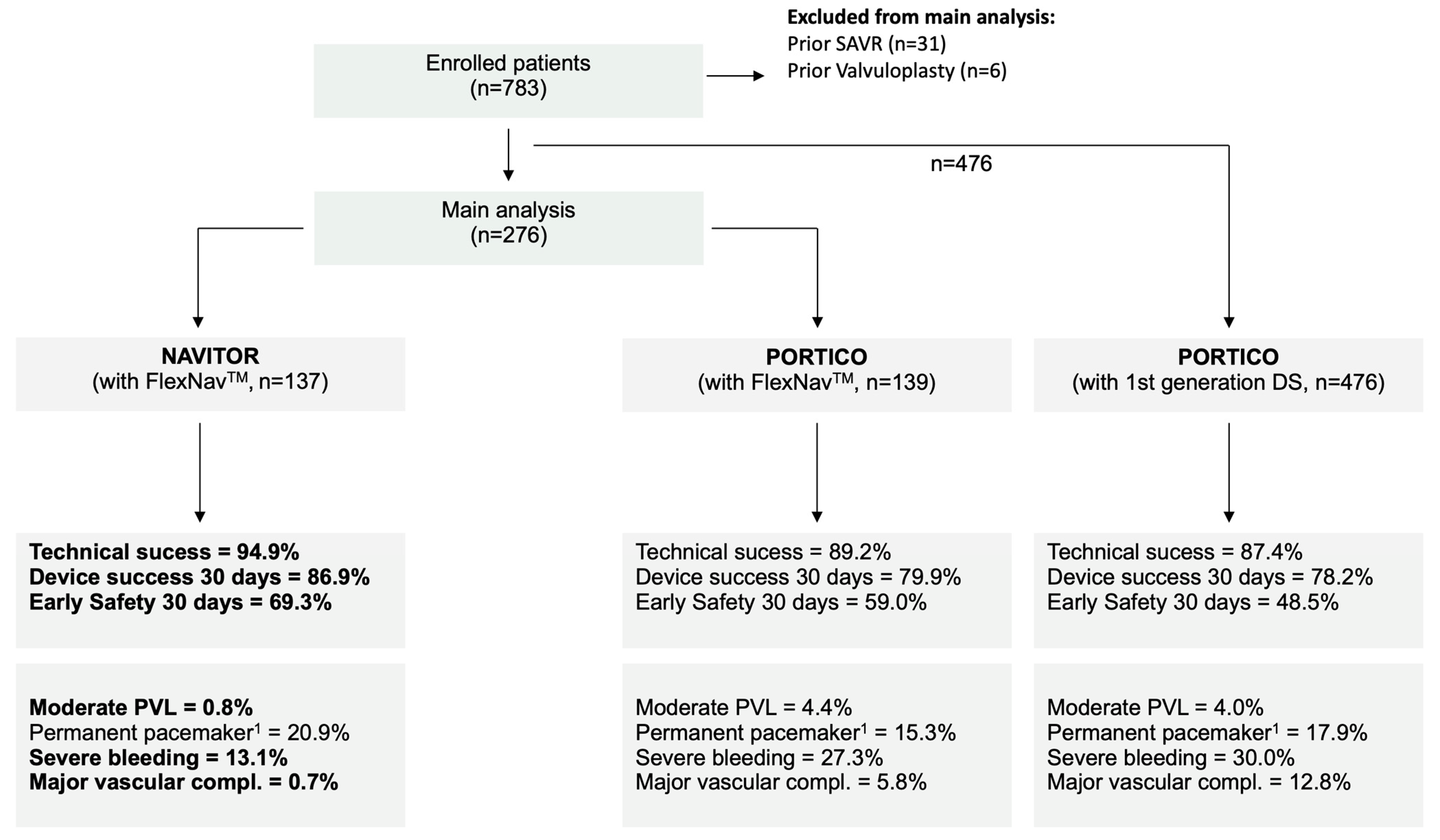
2.1. Patient Cohort
2.2. Multidetector Computed Tomography
2.3. Device Description
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Data
3.2. Procedural Data and Outcomes
3.3. Outcome Analysis up to 30 Days
4. Discussion
4.1. Procedural Outcome
4.2. Hemodynamic Outcome
4.3. Conduction Disturbances and Permanent Pacemaker Implantation
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AV | aortic valve |
| CI | cover index |
| LVOT | left ventricular outflow tract |
| MDCT | multidetector computed tomography |
| PPI | permanent pacemaker implantation |
| PVL | paravalvular leakage |
| MDCT | multidetector computed tomography |
| STJ | sinotubular junction |
| TAVR | transcatheter aortic valve replacement |
| THV | transcatheter heart valve |
| VARC | Valve Academic Research Consortium |
References
- Makkar, R.R.; Cheng, W.; Waksman, R.; Satler, L.F.; Chakravarty, T.; Groh, M.; Abernethy, W.; Russo, M.J.; Heimansohn, D.; Hermiller, J.; et al. Self-expanding intra-annular versus commercially available transcatheter heart valves in high and extreme risk patients with severe aortic stenosis (PORTICO IDE): A randomised, controlled, non-inferiority trial. Lancet 2020, 396, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, S.; Delgado, V.; Hausleiter, J.; Schoenhagen, P.; Min, J.K.; Leipsic, J.A. SCCT expert consensus document on computed tomography imaging before transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). J Cardiovasc. Comput. Tomogr. 2012, 6, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.K.; Blumenstein, J.; Liebetrau, C.; Rolf, A.; Gaede, L.; Van Linden, A.; Arsalan, M.; Doss, M.; Tijssen, J.G.P.; Hamm, C.W.; et al. Comparison of outcomes using balloon-expandable versus self-expanding transcatheter prostheses according to the extent of aortic valve calcification. Clin. Res. Cardiol. 2017, 106, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Bhumimuang, K.; Renker, M.; Fischer-Rasokat, U.; Mollmann, H.; Walther, T.; Choi, Y.H.; Nef, H.; Hamm, C.W. Determinants of paravalvular leakage following transcatheter aortic valve replacement in patients with bicuspid and tricuspid aortic stenosis. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1387–1396. [Google Scholar] [CrossRef]
- Willson, A.B.; Rodes-Cabau, J.; Wood, D.A.; Leipsic, J.; Cheung, A.; Toggweiler, S.; Binder, R.K.; Freeman, M.; DeLarochelliere, R.; Moss, R.; et al. Transcatheter aortic valve replacement with the St. Jude Medical Portico valve: First-in-human experience. J. Am. Coll. Cardiol. 2012, 60, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Smith, D. One-Year Clinical Trial Results with A Next-Generation Aortic Transcatheter Heart Valve. Pcronline. Available online: https://media.pcronline.com/diapos/EuroPCR2022/2618-20220517_1600_Room_Maillot_Smith_Dave_1111111_(5728)/Smith_Dave_20220517_1530_Room_Maillot.pdf (accessed on 4 March 2023).
- Varc-3 Writing, C.; Genereux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3, Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746. [Google Scholar] [CrossRef]
- Gaede, L.; Blumenstein, J.; Eckel, C.; Grothusen, C.; Tiyerili, V.; Sotemann, D.; Nef, H.; Elsasser, A.; Achenbach, S.; Mollmann, H. Transcatheter-based aortic valve replacement vs. isolated surgical aortic valve replacement in 2020. Clin. Res. Cardiol. 2022, 111, 924–933. [Google Scholar] [CrossRef]
- Werner, N.; Renker, M.; Dorr, O.; Bauer, T.; Nef, H.; Choi, Y.H.; Hamm, C.W.; Zahn, R.; Kim, W.K. Anatomical suitability and off-label use of contemporary transcatheter heart valves. Int. J. Cardiol. 2022, 350, 96–103. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J. Surgical or Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2017, 377, 197–198. [Google Scholar]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Dencker, D.; Taudorf, M.; Luk, N.H.; Nielsen, M.B.; Kofoed, K.F.; Schroeder, T.V.; Sondergaard, L.; Lonn, L.; De Backer, O. Frequency and Effect of Access-Related Vascular Injury and Subsequent Vascular Intervention After Transcatheter Aortic Valve Replacement. Am. J. Cardiol. 2016, 118, 1244–1250. [Google Scholar] [CrossRef]
- Chau, K.H.; Chen, S.; Crowley, A.; Redfors, B.; Li, D.; Hahn, R.T.; Douglas, P.S.; Alu, M.C.; Finn, M.T.; Kodali, S.; et al. Paravalvular regurgitation after transcatheter aortic valve replacement in intermediate-risk patients: A pooled PARTNER 2 study. EuroIntervention 2022, 17, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Mollmann, H.; Linke, A.; Nombela-Franco, L.; Sluka, M.; Dominguez, J.F.O.; Montorfano, M.; Kim, W.K.; Arnold, M.; Vasa-Nicotera, M.; Conradi, L.; et al. Procedural Safety and Device Performance of the Portico Valve from Experienced TAVI Centers: 30-Day Outcomes in the Multicenter CONFIDENCE Registry. J. Clin. Med. 2022, 11, 4839. [Google Scholar] [CrossRef]
- Fontana, G.P.; Bedogni, F.; Groh, M.; Smith, D.; Chehab, B.M.; Garrett, H.E., Jr.; Yong, G.; Worthley, S.; Manoharan, G.; Walton, A.; et al. Safety Profile of an Intra-Annular Self-Expanding Transcatheter Aortic Valve and Next-Generation Low-Profile Delivery System. JACC Cardiovasc. Interv. 2020, 13, 2467–2478. [Google Scholar] [CrossRef]
- Blumenstein, J.; Eckel, C.; Husser, O.; Kim, W.K.; Renker, M.; Choi, Y.H.; Hamm, C.W.; Al-Terki, H.; Sotemann, D.; Korbi, L.; et al. Multi-Center Comparison of Two Self-Expanding Transcatheter Heart Valves: A Propensity Matched Analysis. J. Clin. Med. 2022, 11, 4228. [Google Scholar] [CrossRef] [PubMed]
- Mollmann, H.; Holzhey, D.M.; Hilker, M.; Toggweiler, S.; Schafer, U.; Treede, H.; Joner, M.; Sondergaard, L.; Christen, T.; Allocco, D.J.; et al. The ACURATE neo2 valve system for transcatheter aortic valve implantation: 30-day and 1-year outcomes. Clin. Res. Cardiol. 2021, 110, 1912–1920. [Google Scholar] [CrossRef]
- Biersmith, M.; Alston, M.; Makki, N.; Hatoum, H.; Yeats, B.; Egbuche, O.; Biswas, M.; Orsinelli, D.; Boudoulas, K.D.; Dasi, L.; et al. Comparison of Catheterization Versus Echocardiographic-Based Gradients in Balloon-Expandable Versus Self-Expanding Transcatheter Aortic Valve Implantation. J. Invasive Cardiol. 2022, 34, E442–E447. [Google Scholar] [PubMed]
- Forrest, J.K.; Mangi, A.A.; Popma, J.J.; Khabbaz, K.; Reardon, M.J.; Kleiman, N.S.; Yakubov, S.J.; Watson, D.; Kodali, S.; George, I.; et al. Early Outcomes with the Evolut PRO Repositionable Self-Expanding Transcatheter Aortic Valve with Pericardial Wrap. JACC Cardiovasc. Interv. 2018, 11, 160–168. [Google Scholar] [CrossRef]
- Eckel, C.; Sotemann, D.; Kim, W.K.; Grothusen, C.; Tiyerili, V.; Dohmen, G.; Renker, M.; Charitos, E.; Hamm, C.W.; Choi, Y.H.; et al. Procedural Outcomes of a Self-Expanding Transcatheter Heart Valve in Small Annuli. J. Clin. Med. 2022, 11, 5313. [Google Scholar] [CrossRef]
- Kodali, S.; Pibarot, P.; Douglas, P.S.; Williams, M.; Xu, K.; Thourani, V.; Rihal, C.S.; Zajarias, A.; Doshi, D.; Davidson, M.; et al. Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards sapien valve in the PARTNER trial: Characterizing patients and impact on outcomes. Eur. Heart J. 2015, 36, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Zito, A.; Princi, G.; Lombardi, M.; D’Amario, D.; Vergallo, R.; Aurigemma, C.; Romagnoli, E.; Pelargonio, G.; Bruno, P.; Trani, C.; et al. Long-term clinical impact of permanent pacemaker implantation in patients undergoing transcatheter aortic valve implantation: A systematic review and meta-analysis. Europace 2022, 24, 1127–1136. [Google Scholar] [CrossRef]
- Husser, O.; Pellegrini, C.; Kessler, T.; Burgdorf, C.; Thaller, H.; Mayr, N.P.; Kasel, A.M.; Kastrati, A.; Schunkert, H.; Hengstenberg, C. Predictors of Permanent Pacemaker Implantations and New-Onset Conduction Abnormalities with the SAPIEN 3 Balloon-Expandable Transcatheter Heart Valve. JACC Cardiovasc. Interv. 2016, 9, 244–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirado-Conte, G.; Gomez-Alvarez, Z.; Gheorghe, L.; Asmarats, L.; Jimenez-Quevedo, P.; Regueiro, A.; Garcia Gamez, A.F.; McInerney, A.; Pedro Li, C.H.; Pozo, E.; et al. Neo-Commissural Alignment and Coronary Artery Overlap Following Portico Aortic Valve Implantation. JACC Cardiovasc. Interv. 2022, 15, 1590–1592. [Google Scholar] [CrossRef] [PubMed]
- Wong, I.; Ho, C.B.; Chui, A.S.F.; Chan, A.K.C.; Chan, K.T.; Sondergaard, L.; Lee, M.K. Neo-Commissural Alignment During Transcatheter Aortic Valve Replacement: The LACRCO Algorithm. JACC Cardiovasc. Interv. 2022, 15, 1582–1584. [Google Scholar] [CrossRef] [PubMed]
- Spilias, N.; Sabbak, N.; Harb, S.C.; Yun, J.J.; Vargo, P.R.; Unai, S.; Puri, R.; Reed, G.W.; Krishnaswamy, A.; Kapadia, S.R. A Novel Method of Assessing Commissural Alignment for the SAPIEN 3 Transcatheter Aortic Valve. JACC Cardiovasc. Interv. 2021, 14, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, L.; De Backer, O. Transcatheter aortic valve implantation: Don’t forget the coronary arteries! EuroIntervention 2018, 14, 147–149. [Google Scholar] [CrossRef]
- Tarantini, G.; Nai Fovino, L.; Scotti, A.; Massussi, M.; Cardaioli, F.; Rodino, G.; Benedetti, A.; Boiago, M.; Matsuda, Y.; Continisio, S.; et al. Coronary Access After Transcatheter Aortic Valve Replacement With Commissural Alignment: The ALIGN-ACCESS Study. Circ Cardiovasc. Interv. 2022, 15, e011045. [Google Scholar] [CrossRef] [PubMed]


| Variable | PORTICO | NAVITOR | p Value |
|---|---|---|---|
| n = 139 | n = 137 | ||
| Demographic Data | |||
| Age, years | 82.7 [80.0; 86.0] | 83.0 [80.0; 86.0] | 0.382 |
| Female sex | 85 (61.2%) | 84 (61.3%) | 1.000 |
| BMI, kg/m2 | 26.0 [23.1; 29.7] | 26.9 [24.0; 30.1] | 0.070 |
| EuroSCORE I, % | 13.9 [9.5; 22.4] | 12.1 [8.4; 19.7] | 0.141 |
| EuroSCORE II, % | 3.7 [2.2; 6.2] | 3.6 [2.1; 5.1] | 0.234 |
| eGFR, mL/min/1.73 m2 | 56.0 [40.0; 71.5] | 52.0 [38.0; 71.0] | 0.412 |
| Peripheral artery disease | 32 (23.0%) | 23 (16.8%) | 0.252 |
| Prior stroke | 16 (11.5%) | 12 (8.8%) | 0.591 |
| Atrial fibrillation | 48 (34.5%) | 56 (40.9%) | 0.335 |
| Coronary artery disease | 90 (64.7%) | 94 (68.6%) | 0.580 |
| Prior coronary intervention | 54 (38.8%) | 51 (37.2%) | 0.878 |
| Echocardiographic data | |||
| LV ejection fraction, % | 60.0 [51.0; 65.0] | 60.0 [53.0; 65.0] | 0.508 |
| Mean gradient, mmHg | 41.0 [29.5; 49.5] | 41.0 [32.0; 49.0] | 0.730 |
| AVA, cm2 | 0.7 [0.6; 0.9] | 0.8 [0.6; 0.9] | 0.527 |
| Electrocardiographic data | |||
| Right bundle branch block | 12 (9.0%) | 9 (6.7%) | 0.637 |
| Left bundle branch block | 5 (3.7%) | 9 (6.7%) | 0.418 |
| Atrioventricular block | 20 (15.0%) | 24 (17.8%) | 0.660 |
| MDCT data | |||
| Annular area, cm2 | 4.5 [4.0; 4.9] | 4.4 [3.9; 4.8] | 0.098 |
| Annulus diameter, mm | 24.3 [22.9; 25.8] | 24.0 [22.6; 25.0] | 0.061 |
| LVOT, mm | 24.0 [22.4; 25.6] | 23.4 [22.1; 25.6] | 0.252 |
| STJ, mm | 28.4 [26.6; 30.3] | 28.1 [26.0; 29.9] | 0.177 |
| Aortic valve calcification, AU | 2328 [1464; 3239] | 2124 [1342; 3358] | 0.556 |
| Calcium density, AU/cm2 | 271 [108; 571] | 301 [117; 630] | 0.452 |
| Variable | PORTICO | NAVITOR | p Value |
|---|---|---|---|
| n = 139 | n = 137 | ||
| Procedural parameter | |||
| Procedural duration, min | 50.0 [40.0; 60.0] | 45.0 [40.0; 55.0] | 0.016 |
| Contrast agent, mL | 127.0 [98.0; 158.5] | 120.0 [99.5; 161.5] | 0.864 |
| Pre-dilatation, % | 127 (91.4%) | 122 (90.4%) | 0.939 |
| Post-dilatation, % | 48 (35.6%) | 33 (25.0%) | 0.081 |
| Depth NCC, mm | 4.0 [3.0; 6.0] | 4.00 [2.0; 5.0] | 0.052 |
| Depth LCC, mm | 4.0 [2.0; 5.0] | 3.0 [1.0; 5.0] | 0.088 |
| Echocardiographic outcome | |||
| LV ejection fraction, % | 60.5 [53.0; 65.0] | 60.0 [54.0; 65.0] | 0.859 |
| Mean gradient, mmHg | 7.0 [6.0; 9.0] | 8.0 [6.0; 10.5] | 0.121 |
| AVA, cm2 | 1.9 [1.7; 2.1] | 2.0 [1.7; 2.2] | 0.235 |
| Relevant PVL (>mild/trace or SAVR/ViV due to PVL) | 10 (7.2%) | 2 (1.5%) | 0.041 |
| Severe PPM | 3 (2.7%) | 1 (0.8%) | 0.353 |
| Clinical and procedural outcome | |||
| Technical success | 124 (89.2%) | 130 (94.9%) | 0.128 |
| Device success at 30 days | 111 (79.9%) | 119 (86.9%) | 0.162 |
| Early safety at 30 days | 82 (59.0%) | 95 (69.3%) | 0.096 |
| In-hospital death | 3 (2.2%) | 5 (3.7%) | 0.497 |
| Periprocedural death (in-hospital and up to 30 days) | 6 (4.3%) | 7 (5.2%) | 0.968 |
| Conversion to sternotomy | 0 (0.0%) | 1 (0.7%) | 0.496 |
| Multiple valves (ViV) | 5 (3.6%) | 2 (1.5%) | 0.447 |
| Device migration/embolization | 8 (5.8%) | 3 (2.2%) | 0.218 |
| Major vascular complication | 8 (5.8%) | 1 (0.7%) | 0.036 |
| Severe bleeding (type 2–4) | 38 (27.3%) | 18 (13.1%) | 0.005 |
| Major cardiac structural complication | 4 (2.9%) | 4 (2.9%) | 1.000 |
| All stroke (overt CNS injury) | 5 (3.6%) | 3 (2.2%) | 0.723 |
| AKI (type 2–4) | 5 (3.6%) | 4 (2.9%) | 1.000 |
| New permanent pacemaker 2 | 19 (15.3%) | 24 (20.9%) | 0.271 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckel, C.E.; Kim, W.-K.; Grothusen, C.; Tiyerili, V.; Elsässer, A.; Sötemann, D.; Schlüter, J.; Choi, Y.-H.; Charitos, E.I.; Renker, M.; et al. Comparison of the New-Generation Self-Expanding NAVITOR Transcatheter Heart Valve with Its Predecessor, the PORTICO, in Severe Native Aortic Valve Stenosis. J. Clin. Med. 2023, 12, 3999. https://doi.org/10.3390/jcm12123999
Eckel CE, Kim W-K, Grothusen C, Tiyerili V, Elsässer A, Sötemann D, Schlüter J, Choi Y-H, Charitos EI, Renker M, et al. Comparison of the New-Generation Self-Expanding NAVITOR Transcatheter Heart Valve with Its Predecessor, the PORTICO, in Severe Native Aortic Valve Stenosis. Journal of Clinical Medicine. 2023; 12(12):3999. https://doi.org/10.3390/jcm12123999
Chicago/Turabian StyleEckel, Clemens Enno, Won-Keun Kim, Christina Grothusen, Vedat Tiyerili, Albrecht Elsässer, Dagmar Sötemann, Judith Schlüter, Yeong-Hoon Choi, Efstratios I. Charitos, Matthias Renker, and et al. 2023. "Comparison of the New-Generation Self-Expanding NAVITOR Transcatheter Heart Valve with Its Predecessor, the PORTICO, in Severe Native Aortic Valve Stenosis" Journal of Clinical Medicine 12, no. 12: 3999. https://doi.org/10.3390/jcm12123999
APA StyleEckel, C. E., Kim, W.-K., Grothusen, C., Tiyerili, V., Elsässer, A., Sötemann, D., Schlüter, J., Choi, Y.-H., Charitos, E. I., Renker, M., Hamm, C. W., Dohmen, G., Möllmann, H., & Blumenstein, J. (2023). Comparison of the New-Generation Self-Expanding NAVITOR Transcatheter Heart Valve with Its Predecessor, the PORTICO, in Severe Native Aortic Valve Stenosis. Journal of Clinical Medicine, 12(12), 3999. https://doi.org/10.3390/jcm12123999





