Awake or Sleeping? Maybe Both… A Review of Sleep-Related Dissociative States
Abstract
:1. Introduction
2. Physiological States of Consciousness
2.1. Daydreaming
2.1.1. Self-Generated Thoughts, Daydreaming, and Depression
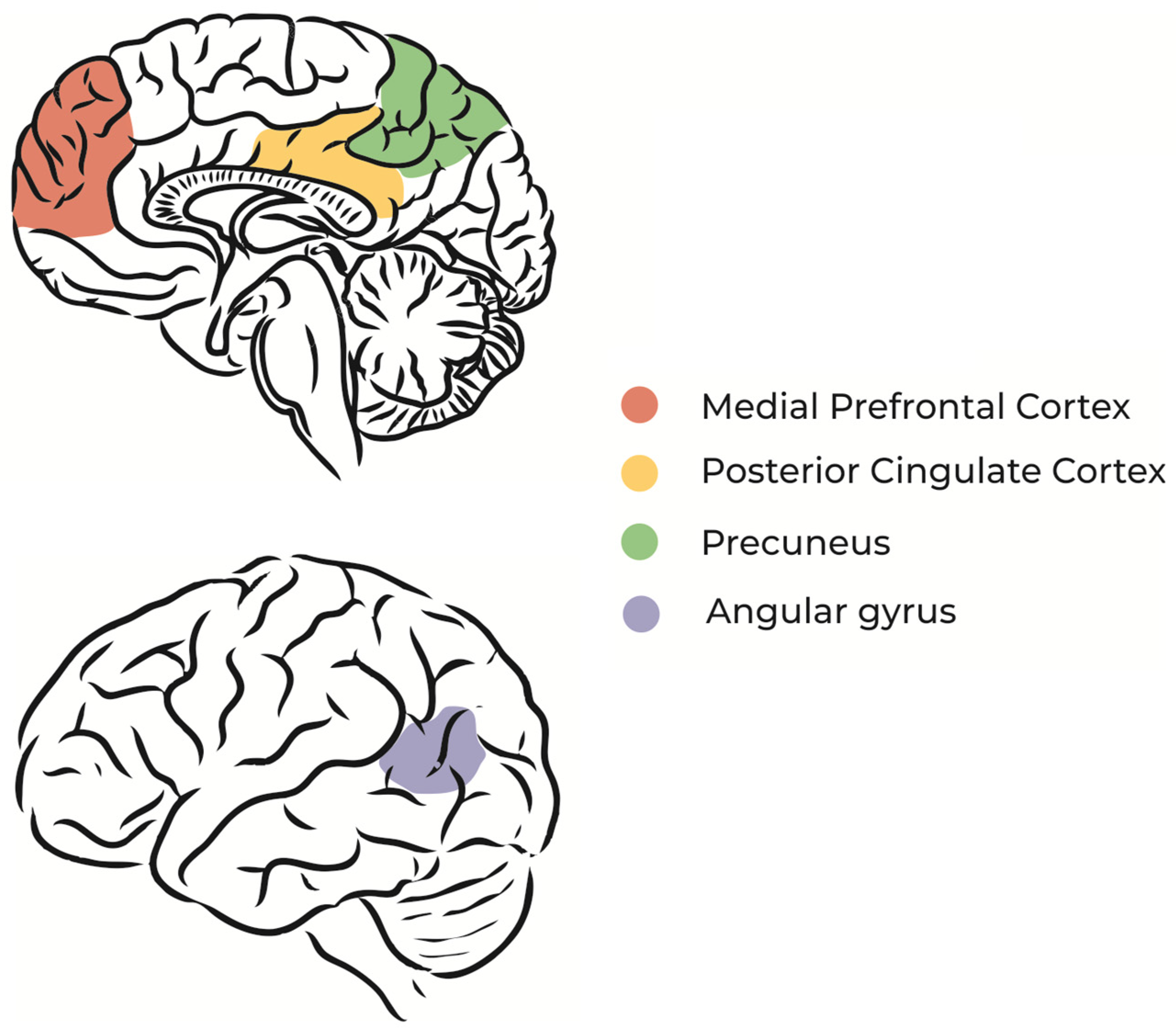
2.1.2. Daydreaming and the Default Mode Network
2.1.3. Daydreaming and Brain Rhythms
2.2. Lucid Dreaming
2.2.1. Lucid Dreaming, REM Sleep, and the Brain Rhythms
2.2.2. Neuroimaging of Non-Lucid and Lucid REM Sleep
2.3. False Awakenings
2.3.1. False Awakenings and REM Sleep
2.3.2. False Awakenings and the Brain Rhythms
3. Pathological States of Consciousness
3.1. Sleep Paralysis
3.1.1. Epidemiology of Sleep Paralysis
3.1.2. Neurobiological Mechanisms of Sleep Paralysis
3.2. Sleepwalking
3.2.1. The Neurobiology and Pathophysiology of Sleepwalking
3.2.2. Treatment of Sleepwalking
3.3. REM Sleep Behavior Disorder
3.3.1. Epidemiology of REM Sleep Behavior Disorder
3.3.2. Neurobiology and Pathophysiology of REM Sleep Behavior Disorder
3.3.3. Evaluation of REM Sleep Behavior Disorder
3.3.4. Management of REM Sleep Behavior Disorder
- (a)
- Environmental modification:
- (b)
- Pharmacotherapy:
- (c)
- Counseling:
4. Altered States of Consciousness
4.1. Hypnosis
4.1.1. Hypnosis, Sleep, and Dreaming
4.1.2. Hypnosis and the Brain
4.2. Anesthesia
4.2.1. Sleep, Anesthesia, and the Brain Rhythms
4.2.2. Neuroimaging Anesthesia
4.3. Psychedelics
4.3.1. Psychedelics and Dreaming
4.3.2. Psychedelics and the Brain
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krueger, J.M.; Nguyen, J.T.; Dykstra-Aiello, C.J.; Taishi, P. Local Sleep. Sleep Med. Rev. 2019, 43, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Scarpelli, S.; Alfonsi, V.; Gorgoni, M. Parasomnias and Disruptive Sleep-Related Disorders: Insights from Local Sleep Findings. J. Clin. Med. 2022, 11, 4435. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Felice Ghilardi, M.; Massimini, M.; Tononi, G. Local Sleep and Learning. Nature 2004, 430, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Vyazovskiy, V.V.; Olcese, U.; Hanlon, E.C.; Nir, Y.; Cirelli, C.; Tononi, G. Local Sleep in Awake Rats. Nature 2011, 472, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Mahowald, M.W.; Cramer Bornemann, M.A.; Schenck, C.H. State Dissociation, Human Behavior, and Consciousness. Curr. Top. Med. Chem. 2011, 11, 2392–2402. [Google Scholar] [CrossRef] [PubMed]
- Singer, J.L.; McCraven, V.G. Some Characteristics of Adult Daydreaming. J. Psychol. 1961, 51, 151–164. [Google Scholar] [CrossRef]
- McMillan, R.L.; Kaufman, S.B.; Singer, J.L. Ode to Positive Constructive Daydreaming. Front. Psychol. 2013, 4, 626. [Google Scholar] [CrossRef] [Green Version]
- Schooler, J.W.; Smallwood, J.; Christoff, K.; Handy, T.C.; Reichle, E.D.; Sayette, M.A. Meta-Awareness, Perceptual Decoupling and the Wandering Mind. Trends Cogn. Sci. 2011, 15, 319–326. [Google Scholar] [CrossRef]
- Ribeiro, S. O Oráculo Da Noite: A História e a Ciência Do Sonho; Editora Companhia das Letras: Rio de Janeiro, Brazil, 2019. [Google Scholar]
- Immordino-Yang, M.H.; Christodoulou, J.A.; Singh, V. Rest Is Not Idleness: Implications of the Brain’s Default Mode for Human Development and Education. Perspect. Psychol. Sci. 2012, 7, 352–364. [Google Scholar] [CrossRef] [Green Version]
- Smallwood, J.; Fitzgerald, A.; Miles, L.K.; Phillips, L.H. Shifting Moods, Wandering Minds: Negative Moods Lead the Mind to Wander. Emotion 2009, 9, 271–276. [Google Scholar] [CrossRef]
- Singer, J.L. The Inner World of Daydreaming, 1st ed.; Harper & Row: New York, NY, USA, 1975; ISBN 978-0-06-013907-0. [Google Scholar]
- Giambra, L.M. Daydreaming across the Life Span: Late Adolescent to Senior Citizen. Int. J. Aging Hum. Dev. 1974, 5, 115–140. [Google Scholar] [CrossRef] [PubMed]
- Giambra, L.M. A Laboratory Method for Investigating Influences on Switching Attention to Task-Unrelated Imagery and Thought. Conscious. Cogn. 1995, 4, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhiyan, T.; Singer, J.L. Daydreaming Styles, Emotionality and the Big Five Personality Dimensions. Imagin. Cogn. Personal. 1997, 16, 399–414. [Google Scholar] [CrossRef]
- Klinger, E. Goal Commitments and the Content of Thoughts and Dreams: Basic Principles. Front. Psychol. 2013, 4, 415. [Google Scholar] [CrossRef] [Green Version]
- Franklin, M.S.; Smallwood, J.; Schooler, J.W. Catching the Mind in Flight: Using Behavioral Indices to Detect Mindless Reading in Real Time. Psychon. Bull. Rev. 2011, 18, 992–997. [Google Scholar] [CrossRef]
- Smallwood, J.; O’Connor, R.C.; Heim, D. Rumination, Dysphoria, and Subjective Experience. Imagin. Cogn. Personal. 2005, 24, 355–367. [Google Scholar] [CrossRef]
- McVay, J.C.; Kane, M.J. Why Does Working Memory Capacity Predict Variation in Reading Comprehension? On the Influence of Mind Wandering and Executive Attention. J. Exp. Psychol. Gen. 2012, 141, 302–320. [Google Scholar] [CrossRef] [Green Version]
- Whitfield-Gabrieli, S.; Ford, J.M. Default Mode Network Activity and Connectivity in Psychopathology. Annu. Rev. Clin. Psychol. 2012, 8, 49–76. [Google Scholar] [CrossRef]
- Giambra, L.M.; Traynor, T.D. Depression and Daydreaming; an Analysis Based on Self-Ratings. J. Clin. Psychol. 1978, 34, 14–25. [Google Scholar] [PubMed]
- Meyer, T.D.; Finucane, L.; Jordan, G. Is Risk for Mania Associated with Increased Daydreaming as a Form of Mental Imagery? J. Affect. Disord. 2011, 135, 380–383. [Google Scholar] [CrossRef]
- Marchetti, I.; Koster, E.H.W.; De Raedt, R. Mindwandering Heightens the Accessibility of Negative Relative to Positive Thought. Conscious. Cogn. 2012, 21, 1517–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchetti, I.; Van de Putte, E.; Koster, E. Self-Generated Thoughts and Depression: From Daydreaming to Depressive Symptoms. Front. Hum. Neurosci. 2014, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Aldao, A.; Nolen-Hoeksema, S.; Schweizer, S. Emotion-Regulation Strategies across Psychopathology: A Meta-Analytic Review. Clin. Psychol. Rev. 2010, 30, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A Default Mode of Brain Function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domhoff, G.W.; Fox, K.C.R. Dreaming and the Default Network: A Review, Synthesis, and Counterintuitive Research Proposal. Conscious. Cogn. 2015, 33, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; Snyder, A.Z. A Default Mode of Brain Function: A Brief History of an Evolving Idea. NeuroImage 2007, 37, 1083–1090; discussion 1097–1099. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Reidler, J.S.; Sepulcre, J.; Poulin, R.; Buckner, R.L. Functional-Anatomic Fractionation of the Brain’s Default Network. Neuron 2010, 65, 550–562. [Google Scholar] [CrossRef] [Green Version]
- Horovitz, S.G.; Fukunaga, M.; de Zwart, J.A.; van Gelderen, P.; Fulton, S.C.; Balkin, T.J.; Duyn, J.H. Low Frequency BOLD Fluctuations during Resting Wakefulness and Light Sleep: A Simultaneous EEG-FMRI Study. Hum. Brain Mapp. 2008, 29, 671–682. [Google Scholar] [CrossRef]
- Vincent, J.L.; Patel, G.H.; Fox, M.D.; Snyder, A.Z.; Baker, J.T.; Van Essen, D.C.; Zempel, J.M.; Snyder, L.H.; Corbetta, M.; Raichle, M.E. Intrinsic Functional Architecture in the Anaesthetized Monkey Brain. Nature 2007, 447, 83–86. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The Human Brain Is Intrinsically Organized into Dynamic, Anticorrelated Functional Networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [Green Version]
- Hilland, E.; Johannessen, C.; Jonassen, R.; Alnæs, D.; Jørgensen, K.N.; Barth, C.; Andreou, D.; Nerland, S.; Wortinger, L.A.; Smelror, R.E.; et al. Aberrant Default Mode Connectivity in Adolescents with Early-Onset Psychosis: A Resting State FMRI Study. NeuroImage Clin. 2022, 33, 102881. [Google Scholar] [CrossRef] [PubMed]
- Braboszcz, C.; Delorme, A. Lost in Thoughts: Neural Markers of Low Alertness during Mind Wandering. NeuroImage 2011, 54, 3040–3047. [Google Scholar] [CrossRef] [PubMed]
- Oken, B.S.; Salinsky, M.C.; Elsas, S.M. Vigilance, Alertness, or Sustained Attention: Physiological Basis and Measurement. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2006, 117, 1885–1901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabri, M.; Labelle, S.; Gosselin, A.; Campbell, K.B. Effects of Sleep Onset on the Mismatch Negativity (MMN) to Frequency Deviants Using a Rapid Rate of Presentation. Brain Res. Cogn. Brain Res. 2003, 17, 164–176. [Google Scholar] [CrossRef]
- Lang, A.H.; Eerola, O.; Korpilahti, P.; Holopainen, I.; Salo, S.; Aaltonen, O. Practical Issues in the Clinical Application of Mismatch Negativity. Ear Hear. 1995, 16, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Winter, O.; Kok, A.; Kenemans, J.L.; Elton, M. Auditory Event-Related Potentials to Deviant Stimuli during Drowsiness and Stage 2 Sleep. Electroencephalogr. Clin. Neurophysiol. 1995, 96, 398–412. [Google Scholar] [CrossRef]
- La Berge, S.P. Lucid Dreaming as a Learnable Skill: A Case Study. Percept. Mot. Ski. 1980, 51, 1039–1042. [Google Scholar] [CrossRef]
- Mota-Rolim, S.A.; Bulkeley, K.; Campanelli, S.; Lobão-Soares, B.; de Araujo, D.B.; Ribeiro, S. The Dream of God: How Do Religion and Science See Lucid Dreaming and Other Conscious States During Sleep? Front. Psychol. 2020, 11, 555731. [Google Scholar] [CrossRef]
- Ferreira, G.H.; Prata, T.d.A.; Fontenele-Araujo, J.; de Carvalho, F.T.; Mota-Rolim, S.A. I Dream Therefore I Am: A Review on Lucid Dreaming in Western Philosophy. Dreaming 2021, 31, 69–87. [Google Scholar] [CrossRef]
- Dennett, D.C. Are Dreams Experiences? Philos. Rev. 1976, 85, 151. [Google Scholar] [CrossRef]
- Aserinsky, E.; Kleitman, N. Regularly Occurring Periods of Eye Motility, and Concomitant Phenomena, During Sleep. Science 1953, 118, 273–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouvet, M. Research on the neural structures and responsible mechanisms in different phases of physiological sleep. Arch. Ital. Biol. 1962, 100, 125–206. [Google Scholar] [PubMed]
- Dement, W.; Wolpert, E.A. The Relation of Eye Movements, Body Motility, and External Stimuli to Dream Content. J. Exp. Psychol. 1958, 55, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rolim, S.A. On Moving the Eyes to Flag Lucid Dreaming. Front. Neurosci. 2020, 14, 361. [Google Scholar] [CrossRef] [PubMed]
- La Berge, S.P.; Nagel, L.E.; Dement, W.C.; Zarcone, V.P. Lucid Dreaming Verified by Volitional Communication during REM Sleep. Percept. Mot. Ski. 1981, 52, 727–732. [Google Scholar] [CrossRef]
- LaBerge, S.; Levitan, L.; Dement, W.C. Lucid Dreaming: Physiological Correlates of Consciousness during REM Sleep. J. Mind Behav. 1986, 7, 251–258. [Google Scholar]
- Brylowski, A.; Levitan, L.; LaBerge, S. H-Reflex Suppression and Autonomic Activation during Lucid REM Sleep: A Case Study. Sleep 1989, 12, 374–378. [Google Scholar] [CrossRef] [Green Version]
- Stumbrys, T.; Erlacher, D. Lucid Dreaming during NREM Sleep: Two Case Reports. Int. J. Dream Res. 2012, 5, 151–155. [Google Scholar] [CrossRef]
- Mota Rolim, S.A.; Brandão, D.S.; Andrade, K.C.; de Queiroz, C.M.T.; Araujo, J.F.; de Araujo, D.B.; Ribeiro, S. Neurophysiological Features of Lucid Dreaming During N1 And N2 Sleep Stages: Two Case Reports. Sleep Sci. 2015, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Mota-Rolim, S.; Targino, Z.; Souza, B.; Blanco, W.; Araujo, J.; Ribeiro, S. Dream Characteristics in a Brazilian Sample: An Online Survey Focusing on Lucid Dreaming. Front. Hum. Neurosci. 2013, 7, 836. [Google Scholar] [CrossRef] [Green Version]
- Ogilvie, R.D.; Hunt, H.T.; Tyson, P.D.; Lucescu, M.L.; Jeakins, D.B. Lucid Dreaming and Alpha Activity: A Preliminary Report. Percept. Mot. Ski. 1982, 55, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Tyson, P.D.; Ogilvie, R.D.; Hunt, H.T. Lucid, Prelucid, and Nonlucid Dreams Related to the Amount of EEG Alpha Activity during REM Sleep. Psychophysiology 1984, 21, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, B.; LaBerge, S.; Levitan, L. Psychophysiological Correlates of Lucid Dreaming. Dreaming 2006, 16, 88–95. [Google Scholar] [CrossRef]
- Voss, U.; Holzmann, R.; Tuin, I.; Hobson, J.A. Lucid Dreaming: A State of Consciousness with Features of Both Waking and Non-Lucid Dreaming. Sleep 2009, 32, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Dodet, P.; Chavez, M.; Leu-Semenescu, S.; Golmard, J.-L.; Arnulf, I. Lucid Dreaming in Narcolepsy. Sleep 2015, 38, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Baird, B.; Mota-Rolim, S.A.; Dresler, M. The Cognitive Neuroscience of Lucid Dreaming. Neurosci. Biobehav. Rev. 2019, 100, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Mota-Rolim, S.A.; Erlacher, D.; Tort, A.B.L.; Araujo, J.F.; Ribeiro, S. Different Kinds of Subjective Experience during Lucid Dreaming May Have Different Neural Substrates. J. Neurosci. 2010, 25, 550–557. [Google Scholar] [CrossRef]
- Baird, B.; Tononi, G.; LaBerge, S. Lucid Dreaming Occurs in Activated Rapid Eye Movement Sleep, Not a Mixture of Sleep and Wakefulness. Sleep 2022, 45, zsab294. [Google Scholar] [CrossRef]
- Braun, A. Regional Cerebral Blood Flow throughout the Sleep-Wake Cycle. An H2(15)O PET Study. Brain 1997, 120, 1173–1197. [Google Scholar] [CrossRef]
- Maquet, P.; Péters, J.; Aerts, J.; Delfiore, G.; Degueldre, C.; Luxen, A.; Franck, G. Functional Neuroanatomy of Human Rapid-Eye-Movement Sleep and Dreaming. Nature 1996, 383, 163–166. [Google Scholar] [CrossRef]
- Hobson, J.A. REM Sleep and Dreaming: Towards a Theory of Protoconsciousness. Nat. Rev. Neurosci. 2009, 10, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Nir, Y.; Tononi, G. Dreaming and the Brain: From Phenomenology to Neurophysiology. Trends Cogn. Sci. 2010, 14, 88–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dresler, M.; Wehrle, R.; Spoormaker, V.I.; Koch, S.P.; Holsboer, F.; Steiger, A.; Obrig, H.; Sämann, P.G.; Czisch, M. Neural Correlates of Dream Lucidity Obtained from Contrasting Lucid versus Non-Lucid REM Sleep: A Combined EEG/FMRI Case Study. Sleep 2012, 35, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Baird, B.; Castelnovo, A.; Gosseries, O.; Tononi, G. Frequent Lucid Dreaming Associated with Increased Functional Connectivity between Frontopolar Cortex and Temporoparietal Association Areas. Sci. Rep. 2018, 8, 17798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filevich, E.; Dresler, M.; Brick, T.R.; Kühn, S. Metacognitive Mechanisms Underlying Lucid Dreaming. J. Neurosci. 2015, 35, 1082–1088. [Google Scholar] [CrossRef] [Green Version]
- Dixon, M.L.; De La Vega, A.; Mills, C.; Andrews-Hanna, J.; Spreng, R.N.; Cole, M.W.; Christoff, K. Heterogeneity within the Frontoparietal Control Network and Its Relationship to the Default and Dorsal Attention Networks. Proc. Natl. Acad. Sci. USA 2018, 115, E1598–E1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The Organization of the Human Cerebral Cortex Estimated by Intrinsic Functional Connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef]
- Blanke, O.; Mohr, C.; Michel, C.M.; Pascual-Leone, A.; Brugger, P.; Seeck, M.; Landis, T.; Thut, G. Linking Out-of-Body Experience and Self Processing to Mental Own-Body Imagery at the Temporoparietal Junction. J. Neurosci. 2005, 25, 550–557. [Google Scholar] [CrossRef] [Green Version]
- Buzzi, G. False Awakenings in Light of the Dream Protoconsciousness Theory: A Study in Lucid Dreamers. Int. J. Dream Res. 2011, 4, 110–116. [Google Scholar] [CrossRef]
- Raduga, M.; Kuyava, O.; Sevcenko, N. Is There a Relation among REM Sleep Dissociated Phenomena, like Lucid Dreaming, Sleep Paralysis, out-of-Body Experiences, and False Awakening? Med. Hypotheses 2020, 144, 110169. [Google Scholar] [CrossRef]
- Mainieri, G.; Maranci, J.-B.; Champetier, P.; Leu-Semenescu, S.; Gales, A.; Dodet, P.; Arnulf, I. Are Sleep Paralysis and False Awakenings Different from REM Sleep and from Lucid REM Sleep? A Spectral EEG Analysis. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2021, 17, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, B.A.; McCarthy, K.S.; Chambless, D.L.; Milrod, B.L.; Khalsa, S.-R.; Barber, J.P. Isolated Sleep Paralysis and Fearful Isolated Sleep Paralysis in Outpatients with Panic Attacksb. J. Clin. Psychol. 2010, 66, 1292–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalal, B.; Hinton, D.E. Rates and Characteristics of Sleep Paralysis in the General Population of Denmark and Egypt. Cult. Med. Psychiatry 2013, 37, 534–548. [Google Scholar] [CrossRef] [PubMed]
- de Sá, J.F.R.; Mota-Rolim, S.A. Sleep Paralysis in Brazilian Folklore and Other Cultures: A Brief Review. Front. Psychol. 2016, 7, 1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, K.; Miyasita, A.; Inugami, M.; Ishihara, K. High Prevalence of Isolated Sleep Paralysis: Kanashibari Phenomenon in Japan. Sleep 1987, 10, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassaniti, J.L.; Luhrmann, T.M. The Cultural Kindling of Spiritual Experiences. Curr. Anthropol. 2014, 55, S333–S343. [Google Scholar] [CrossRef] [Green Version]
- Sharpless, B.A.; Doghramji, K. Sleep Paralysis: Historical, Psychological, and Medical Perspectives; Oxford University Press: New York, NY, USA, 2015; pp. xiii, 287. ISBN 978-0-19-931380-8. [Google Scholar]
- McNally, R.J.; Clancy, S.A. Sleep Paralysis, Sexual Abuse, and Space Alien Abduction. Transcult. Psychiatry 2005, 42, 113–122. Available online: https://journals.sagepub.com/doi/10.1177/1363461505050715 (accessed on 18 March 2023). [CrossRef]
- Cheyne, J.A.; Rueffer, S.D.; Newby-Clark, I.R. Hypnagogic and Hypnopompic Hallucinations during Sleep Paralysis: Neurological and Cultural Construction of the Night-Mare. Conscious. Cogn. 1999, 8, 319–337. [Google Scholar] [CrossRef] [Green Version]
- Hinton, D.E.; Hufford, D.J.; Kirmayer, L.J. Culture and Sleep Paralysis. Transcult. Psychiatry 2005, 42, 5–10. [Google Scholar] [CrossRef]
- Denis, D.; French, C.C.; Gregory, A.M. A Systematic Review of Variables Associated with Sleep Paralysis. Sleep Med. Rev. 2018, 38, 141–157. [Google Scholar] [CrossRef]
- Dement, W.; Kleitman, N. The Relation of Eye Movements during Sleep to Dream Activity: An Objective Method for the Study of Dreaming. J. Exp. Psychol. 1957, 53, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouvet, M. Locus Coeruleus et Sommeil Paradoxal. Comptes Rendus Séances Société Biol. Ses Fil. 1965, 159, 895–899. [Google Scholar]
- Brooks, P.L.; Peever, J.H. Identification of the Transmitter and Receptor Mechanisms Responsible for REM Sleep Paralysis. J. Neurosci. 2012, 32, 9785–9795. [Google Scholar] [CrossRef] [PubMed]
- Dahlitz, M.; Parkes, J.D. Sleep Paralysis. Lancet 1993, 341, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Jalal, B.; Ramachandran, V.S. Sleep Paralysis and “the Bedroom Intruder”: The Role of the Right Superior Parietal, Phantom Pain and Body Image Projection. Med. Hypotheses 2014, 83, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Umanath, S.; Sarezky, D.; Finger, S. Sleepwalking through History: Medicine, Arts, and Courts of Law. J. Hist. Neurosci. 2011, 20, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Grunstein, R.R. Treatments for Somnambulism in Adults: Assessing the Evidence. Sleep Med. Rev. 2009, 13, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Idir, Y.; Oudiette, D.; Arnulf, I. Sleepwalking, Sleep Terrors, Sexsomnia and Other Disorders of Arousal: The Old and the New. J. Sleep Res. 2022, 31, e13596. [Google Scholar] [CrossRef]
- Zadra, A.; Desautels, A.; Petit, D.; Montplaisir, J. Somnambulism: Clinical Aspects and Pathophysiological Hypotheses. Lancet Neurol. 2013, 12, 285–294. [Google Scholar] [CrossRef]
- Busby, K.A.; Mercier, L.; Pivik, R.T. Ontogenetic Variations in Auditory Arousal Threshold during Sleep. Psychophysiology 1994, 31, 182–188. [Google Scholar] [CrossRef]
- Plazzi, G.; Vetrugno, R.; Provini, F.; Montagna, P. Sleepwalking and Other Ambulatory Behaviours during Sleep. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2005, 26 (Suppl. S3), s193–s198. [Google Scholar] [CrossRef] [PubMed]
- Mahowald, M.W.; Schenck, C.H. Insights from Studying Human Sleep Disorders. Nature 2005, 437, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Palombini, L.; Pelayo, R.; Chervin, R.D. Sleepwalking and Sleep Terrors in Prepubertal Children: What Triggers Them? Pediatrics 2003, 111, e17–e25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, J.L.; Kaemingk, K.L.; Fregosi, R.F.; Rosen, G.M.; Morgan, W.J.; Smith, T.; Quan, S.F. Parasomnias and Sleep Disordered Breathing in Caucasian and Hispanic Children—The Tucson Children’s Assessment of Sleep Apnea Study. BMC Med. 2004, 2, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petit, D.; Pennestri, M.-H.; Paquet, J.; Desautels, A.; Zadra, A.; Vitaro, F.; Tremblay, R.E.; Boivin, M.; Montplaisir, J. Childhood Sleepwalking and Sleep Terrors: A Longitudinal Study of Prevalence and Familial Aggregation. JAMA Pediatr. 2015, 169, 653–658. [Google Scholar] [CrossRef] [Green Version]
- Remulla, A.; Guilleminault, C. Somnambulism (Sleepwalking). Expert Opin. Pharmacother. 2004, 5, 2069–2074. [Google Scholar] [CrossRef]
- Lam, S.-P.; Fong, S.Y.-Y.; Yu, M.W.-M.; Li, S.X.; Wing, Y.-K. Sleepwalking in Psychiatric Patients: Comparison of Childhood and Adult Onset. Aust. N. Z. J. Psychiatry 2009, 43, 426–430. Available online: https://journals.sagepub.com/doi/10.1080/00048670902817703 (accessed on 19 March 2023). [CrossRef] [PubMed]
- Guilleminault, C.; Kirisoglu, C.; da Rosa, A.C.; Lopes, C.; Chan, A. Sleepwalking, a Disorder of NREM Sleep Instability. Sleep Med. 2006, 7, 163–170. [Google Scholar] [CrossRef]
- Masand, P.; Popli, A.P.; Weilburg, J.B. Sleepwalking. Am. Fam. Physician 1995, 51, 649–654. [Google Scholar]
- Wills, L.; Garcia, J. Parasomnias: Epidemiology and Management. CNS Drugs 2002, 16, 803–810. [Google Scholar] [CrossRef]
- Stallman, H.M.; Kohler, M.; White, J. Medication Induced Sleepwalking: A Systematic Review. Sleep Med. Rev. 2018, 37, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Stallman, H.M.; Kohler, M. Prevalence of Sleepwalking: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0164769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, H.-C.; Zhang, F.; Adamantidis, A.; Stuber, G.D.; Bonci, A.; de Lecea, L.; Deisseroth, K. Phasic Firing in Dopaminergic Neurons Is Sufficient for Behavioral Conditioning. Science 2009, 324, 1080–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenck, C.H.; Mahowald, M.W. REM Sleep Behavior Disorder: Clinical, Developmental, and Neuroscience Perspectives 16 Years After Its Formal Identification in SLEEP. Sleep 2002, 25, 120–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenck, C.H.; Bundlie, S.R.; Ettinger, M.G.; Mahowald, M.W. Chronic Behavioral Disorders of Human REM Sleep: A New Category of Parasomnia. Sleep 1986, 9, 293–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahowald, M.W.; Schenck, C.H. Dissociated States of Wakefulness and Sleep. Neurology 1992, 42, 44–51; discussion 52. [Google Scholar]
- Schenck, C.; Högl, B.; Videnovic, A. (Eds.) Rapid-Eye-Movement Sleep Behavior Disorder; Springer: Cham, Switzerland, 2019; ISBN 978-3-319-90152-7. [Google Scholar]
- Cicero, C.E.; Giuliano, L.; Luna, J.; Zappia, M.; Preux, P.-M.; Nicoletti, A. Prevalence of Idiopathic REM Behavior Disorder: A Systematic Review and Meta-Analysis. Sleep 2021, 44, zsaa294. [Google Scholar] [CrossRef]
- Galbiati, A.; Verga, L.; Giora, E.; Zucconi, M.; Ferini-Strambi, L. The Risk of Neurodegeneration in REM Sleep Behavior Disorder: A Systematic Review and Meta-Analysis of Longitudinal Studies. Sleep Med. Rev. 2019, 43, 37–46. [Google Scholar] [CrossRef]
- Haba-Rubio, J.; Frauscher, B.; Marques-Vidal, P.; Toriel, J.; Tobback, N.; Andries, D.; Preisig, M.; Vollenweider, P.; Postuma, R.; Heinzer, R. Prevalence and Determinants of Rapid Eye Movement Sleep Behavior Disorder in the General Population. Sleep 2018, 41, zsx197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.S.; Lam, S.P.; Zhang, J.; Wing, Y.K. Instruments for Screening, Diagnosis and Assessment of RBD Severity and Monitoring Treatment Outcome; Springer International Publishing, Part of Springer Nature: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-319-90151-0. [Google Scholar]
- Mateo-Montero, R.C.; Pedrera-Mazarro, A.; Martín-Palomeque, G.; del Mar Moreno-Galera, M.; Valera-Dávila, C.; Gómez-Ansede, A.; Braun, J.; Jiménez-Escrig, A. Clinical and Genetical Study of a Familial Form of REM Sleep Behavior Disorder. Clin. Neurol. Neurosurg. 2018, 175, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Valencia Garcia, S.; Libourel, P.-A.; Lazarus, M.; Grassi, D.; Luppi, P.-H.; Fort, P. Genetic Inactivation of Glutamate Neurons in the Rat Sublaterodorsal Tegmental Nucleus Recapitulates REM Sleep Behaviour Disorder. Brain J. Neurol. 2017, 140, 414–428. [Google Scholar] [CrossRef] [Green Version]
- Valencia Garcia, S.; Brischoux, F.; Clément, O.; Libourel, P.-A.; Arthaud, S.; Lazarus, M.; Luppi, P.-H.; Fort, P. Ventromedial Medulla Inhibitory Neuron Inactivation Induces REM Sleep without Atonia and REM Sleep Behavior Disorder. Nat. Commun. 2018, 9, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, M.; Avidan, A.Y.; Foldvary-Schaefer, N.; Malkani, R.G.; During, E.H.; Roland, J.P.; McCarter, S.J.; Zak, R.S.; Carandang, G.; Kazmi, U.; et al. Management of REM Sleep Behavior Disorder: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2022, 19, 769–810. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Howell, M.J. Rapid Eye Movement Sleep Behavior Disorder: Overview and Current Perspective. Curr. Sleep Med. Rep. 2016, 2, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Hauri, P.J.; Silber, M.H.; Boeve, B.F. The Treatment of Parasomnias with Hypnosis: A 5-Year Follow-up Study. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2007, 3, 369–373. [Google Scholar] [CrossRef] [Green Version]
- McGrane, I.R.; Leung, J.G.; St. Louis, E.K.; Boeve, B.F. Melatonin Therapy for REM Sleep Behavior Disorder: A Critical Review of Evidence. Sleep Med. 2015, 16, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Stefani, A.; Mozersky, J.; Kotagal, V.; Högl, B.; Ingravallo, F.; Ju, Y.-E.S.; Avidan, A.; Sharp, R.; Videnovic, A.; Schenck, C.H.; et al. Ethical Aspects of Prodromal Synucleinopathy Prognostic Counseling. Semin. Neurol. 2023, 43, 166–177. [Google Scholar] [CrossRef]
- Hammond, D.C. A Review of the History of Hypnosis through the Late 19th Century. Am. J. Clin. Hypn. 2013, 56, 174–191. [Google Scholar] [CrossRef]
- Matthews, W.J.; Lankton, S.; Lankton, C. An Ericksonian Model of Hypnotherapy. In Handbook of Clinical Hypnosis; American Psychological Association: Washington, DC, USA, 1993; pp. 187–214. ISBN 978-1-55798-440-1. [Google Scholar]
- Kirsch, I.; Lynn, S.J.; Rhue, J.W. Introduction to Clinical Hypnosis. In Handbook of Clinical Hypnosis; American Psychological Association: Washington, DC, USA, 1993; pp. 3–22. ISBN 978-1-55798-440-1. [Google Scholar]
- Montgomery, G.H.; DuHamel, K.N.; Redd, W.H. A Meta-Analysis of Hypnotically Induced Analgesia: How Effective Is Hypnosis? Int. J. Clin. Exp. Hypn. 2000, 48, 138–153. [Google Scholar] [CrossRef]
- Evans, F.J. Sleep and Hypnosis: Accessibility of Altered States of Consciousness. In Advances in Physiological Science; Adam, G., Meszaros, I., Banyai, E.I., Eds.; Elsevier: Amsterdam, The Netherlands, 1981; pp. 17–453. [Google Scholar]
- Spiegel, D.; Cardeña, E. Disintegrated Experience: The Dissociative Disorders Revisited. J. Abnorm. Psychol. 1991, 100, 366–378. [Google Scholar] [CrossRef]
- Windt, J.M. The Immersive Spatiotemporal Hallucination Model of Dreaming. Phenomenol. Cogn. Sci. 2010, 9, 295–316. [Google Scholar] [CrossRef]
- Kubie, L.S.; Margolin, S. The Process of Hypnotism and the Nature of the Hypnotic State. Am. J. Psychiatry 1944, 100, 611–622. [Google Scholar] [CrossRef]
- Malinowski, J.E.; Horton, C.L. Metaphor and Hyperassociativity: The Imagination Mechanisms behind Emotion Assimilation in Sleep and Dreaming. Front. Psychol. 2015, 6, 1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kihlstrom, J.F. Memory, Abuse, and Science. Am. Psychol. 1997, 52, 994–995. [Google Scholar] [CrossRef]
- Hobson, J.A.; Hoffman, S.A.; Helfand, R.; Kostner, D. Dream Bizarreness and the Activation-Synthesis Hypothesis. Hum. Neurobiol. 1987, 6, 157–164. [Google Scholar] [PubMed]
- Lifshitz, M.; Cusumano, E.P.; Raz, A. Hypnosis as Neurophenomenology. Front. Hum. Neurosci. 2013, 7, 469. [Google Scholar] [CrossRef] [Green Version]
- Chamine, I.; Atchley, R.; Oken, B.S. Hypnosis Intervention Effects on Sleep Outcomes: A Systematic Review. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2018, 14, 271–283. [Google Scholar] [CrossRef]
- Murray-Jobsis, J. The Borderline Patient and the Psychotic Patient. In Handbook of Clinical Hypnosis; American Psychological Association: Washington, DC, USA, 1993; pp. 425–451. ISBN 978-1-55798-440-1. [Google Scholar]
- Barrett, D. The Hypnotic Dream: Its Relation to Nocturnal Dreams and Waking Fantasies. J. Abnorm. Psychol. 1979, 88, 584–591. [Google Scholar] [CrossRef]
- Zamore, N.; Barrett, D. Hypnotic Susceptibility and Dream Characteristics. Psychiatr. J. Univ. Ott. 1989, 14, 572–574. [Google Scholar] [PubMed]
- Redefining Hypnosis: Theory, Methods and Integration—Gruzelier—2000—Contemporary Hypnosis—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/ch.193 (accessed on 20 March 2023).
- Landry, M.; Lifshitz, M.; Raz, A. Brain Correlates of Hypnosis: A Systematic Review and Meta-Analytic Exploration. Neurosci. Biobehav. Rev. 2017, 81, 75–98. [Google Scholar] [CrossRef]
- Vanhaudenhuyse, A.; Laureys, S.; Faymonville, M.-E. Neurophysiology of Hypnosis. Neurophysiol. Clin. Clin. Neurophysiol. 2014, 44, 343–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, M.P.; Adachi, T.; Hakimian, S. Brain Oscillations, Hypnosis, and Hypnotizability. Am. J. Clin. Hypn. 2015, 57, 230–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terhune, D.B.; Cardeña, E.; Lindgren, M. Differential Frontal-Parietal Phase Synchrony during Hypnosis as a Function of Hypnotic Suggestibility. Psychophysiology 2011, 48, 1444–1447. [Google Scholar] [CrossRef] [PubMed]
- De Pascalis, V. EEG Spectral Analysis during Hypnotic Induction, Hypnotic Dream and Age Regression. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 1993, 15, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Lendner, J.; Helfrich, R. How Can I Run Sleep and Anesthesia Studies with Intracranial EEG? PsyArXiv 2022. [Google Scholar] [CrossRef]
- Huang, Z.; Tarnal, V.; Vlisides, P.E.; Janke, E.L.; McKinney, A.M.; Picton, P.; Mashour, G.A.; Hudetz, A.G. Asymmetric Neural Dynamics Characterize Loss and Recovery of Consciousness. NeuroImage 2021, 236, 118042. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, V.; Staquet, C.; Montupil, J.; Defresne, A.; Kirsch, M.; Martial, C.; Vanhaudenhuyse, A.; Chatelle, C.; Larroque, S.K.; Raimondo, F.; et al. General Anesthesia: A Probe to Explore Consciousness. Front. Syst. Neurosci. 2019, 13, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klinzing, J.G.; Niethard, N.; Born, J. Mechanisms of Systems Memory Consolidation during Sleep. Nat. Neurosci. 2019, 22, 1598–1610. [Google Scholar] [CrossRef]
- Zhang, H.; Fell, J.; Axmacher, N. Electrophysiological Mechanisms of Human Memory Consolidation. Nat. Commun. 2018, 9, 4103. [Google Scholar] [CrossRef] [Green Version]
- Banks, M.I.; Krause, B.M.; Endemann, C.M.; Campbell, D.I.; Kovach, C.K.; Dyken, M.E.; Kawasaki, H.; Nourski, K.V. Cortical Functional Connectivity Indexes Arousal State during Sleep and Anesthesia. NeuroImage 2020, 211, 116627. [Google Scholar] [CrossRef] [PubMed]
- Zimmern, V. Why Brain Criticality Is Clinically Relevant: A Scoping Review. Front. Neural Circuits 2020, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Toker, D.; Pappas, I.; Lendner, J.D.; Frohlich, J.; Mateos, D.M.; Muthukumaraswamy, S.; Carhart-Harris, R.; Paff, M.; Vespa, P.M.; Monti, M.M.; et al. Consciousness Is Supported by Near-Critical Slow Cortical Electrodynamics. Proc. Natl. Acad. Sci. USA 2022, 119, e2024455119. [Google Scholar] [CrossRef] [PubMed]
- Scheinin, A.; Kantonen, O.; Alkire, M.; Långsjö, J.; Kallionpää, R.E.; Kaisti, K.; Radek, L.; Johansson, J.; Sandman, N.; Nyman, M.; et al. Foundations of Human Consciousness: Imaging the Twilight Zone. J. Neurosci. Off. J. Soc. Neurosci. 2021, 41, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Clavé, E.; Soler, J.; Elices, M.; Pascual, J.C.; Álvarez, E.; de la Fuente Revenga, M.; Friedlander, P.; Feilding, A.; Riba, J. Ayahuasca: Pharmacology, Neuroscience and Therapeutic Potential. Brain Res. Bull. 2016, 126, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Reiff, C.M.; Richman, E.E.; Nemeroff, C.B.; Carpenter, L.L.; Widge, A.S.; Rodriguez, C.I.; Kalin, N.H.; McDonald, W.M.; The Work Group on Biomarkers and Novel Treatments; A Division of the American Psychiatric Association Council of Research. Psychedelics and Psychedelic-Assisted Psychotherapy. Am. J. Psychiatry 2020, 177, 391–410. [Google Scholar] [CrossRef]
- Palhano-Fontes, F.; Mota-Rolim, S.; Lobão-Soares, B.; Galvão-Coelho, N.; Maia-Oliveira, J.P.; Araújo, D.B. Recent Evidence on the Antidepressant Effects of Ayahuasca. In Ayahuasca Healing and Science; Labate, B.C., Cavnar, C., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 21–41. ISBN 978-3-030-55687-7. [Google Scholar]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef] [Green Version]
- Vollenweider, F.X.; Preller, K.H. Psychedelic Drugs: Neurobiology and Potential for Treatment of Psychiatric Disorders. Nat. Rev. Neurosci. 2020, 21, 611–624. [Google Scholar] [CrossRef]
- Wießner, I.; Falchi, M.; Palhano-Fontes, F.; Feilding, A.; Ribeiro, S.; Tófoli, L.F. LSD, Madness and Healing: Mystical Experiences as Possible Link between Psychosis Model and Therapy Model. Psychol. Med. 2023, 53, 1151–1165. [Google Scholar] [CrossRef]
- Kraehenmann, R. Dreams and Psychedelics: Neurophenomenological Comparison and Therapeutic Implications. Curr. Neuropharmacol. 2017, 15, 1032–1042. [Google Scholar] [CrossRef] [Green Version]
- Friedrichs, H.; Passie, T.; Dierssen, O. Die Psychologie des Meskalinrausches; Bewusstsein, Kognition, Erleben; VWB, Verl. für Wiss. und Bildung: Berlin, Germany, 2009; ISBN 978-3-86135-206-8. [Google Scholar]
- Hobson, J.A.; Pace-Schott, E.F.; Stickgold, R. Dreaming and the brain: Toward a cognitive neuroscience of conscious states. Behav. Brain Sci. 2000, 23, 793–842. [Google Scholar] [CrossRef]
- Sanz, C.; Zamberlan, F.; Erowid, E.; Erowid, F.; Tagliazucchi, E. The Experience Elicited by Hallucinogens Presents the Highest Similarity to Dreaming within a Large Database of Psychoactive Substance Reports. Front. Neurosci. 2018, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Millière, R. Looking for the Self: Phenomenology, Neurophysiology and Philosophical Significance of Drug-Induced Ego Dissolution. Front. Hum. Neurosci. 2017, 11, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, L.I.; Orme-Johnson, D. Transcendental Consciousness Wakes up in Dreaming and Deep Sleep. Int. J. Dream Res. 2010, 3, 28–32. [Google Scholar] [CrossRef]
- Hobson, J.A.; Hong, C.C.-H.; Friston, K.J. Virtual Reality and Consciousness Inference in Dreaming. Front. Psychol. 2014, 5, 1133. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.; Levin, R. Nightmares: A New Neurocognitive Model. Sleep Med. Rev. 2007, 11, 295–310. [Google Scholar] [CrossRef]
- van Elk, M.; Yaden, D.B. Pharmacological, Neural, and Psychological Mechanisms Underlying Psychedelics: A Critical Review. Neurosci. Biobehav. Rev. 2022, 140, 104793. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Leech, R.; Williams, T.M.; Erritzoe, D.; Abbasi, N.; Bargiotas, T.; Hobden, P.; Sharp, D.J.; Evans, J.; Feilding, A.; et al. Implications for Psychedelic-Assisted Psychotherapy: Functional Magnetic Resonance Imaging Study with Psilocybin. Br. J. Psychiatry 2012, 200, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Wießner, I.; Olivieri, R.; Falchi, M.; Palhano-Fontes, F.; Oliveira Maia, L.; Feilding, A.; Araujo, D.B.; Ribeiro, S.; Tófoli, L.F. LSD, Afterglow and Hangover: Increased Episodic Memory and Verbal Fluency, Decreased Cognitive Flexibility. Eur. Neuropsychopharmacol. 2022, 58, 7–19. [Google Scholar] [CrossRef]
- Catlow, B.J.; Song, S.; Paredes, D.A.; Kirstein, C.L.; Sanchez-Ramos, J. Effects of Psilocybin on Hippocampal Neurogenesis and Extinction of Trace Fear Conditioning. Exp. Brain Res. 2013, 228, 481–491. [Google Scholar] [CrossRef]
- Zhang, G.; Ásgeirsdóttir, H.N.; Cohen, S.J.; Munchow, A.H.; Barrera, M.P.; Stackman, R.W. Stimulation of Serotonin 2A Receptors Facilitates Consolidation and Extinction of Fear Memory in C57BL/6J Mice. Neuropharmacology 2013, 64, 403–413. [Google Scholar] [CrossRef] [Green Version]
- Barrett, F.S.; Carbonaro, T.M.; Hurwitz, E.; Johnson, M.W.; Griffiths, R.R. Double-Blind Comparison of the Two Hallucinogens Psilocybin and Dextromethorphan: Effects on Cognition. Psychopharmacology 2018, 235, 2915–2927. [Google Scholar] [CrossRef] [PubMed]
- Wießner, I.; Falchi, M.; Palhano-Fontes, F.; Oliveira Maia, L.; Feilding, A.; Ribeiro, S.; Bezerra Mota, N.; Araujo, D.B.; Tófoli, L.F. Low-Dose LSD and the Stream of Thought: Increased Discontinuity of Mind, Deep Thoughts and Abstract Flow. Psychopharmacology 2022, 239, 1721–1733. [Google Scholar] [CrossRef] [PubMed]
- Wießner, I.; Falchi, M.; Maia, L.O.; Daldegan-Bueno, D.; Palhano-Fontes, F.; Mason, N.L.; Ramaekers, J.G.; Gross, M.E.; Schooler, J.W.; Feilding, A.; et al. LSD and Creativity: Increased Novelty and Symbolic Thinking, Decreased Utility and Convergent Thinking. J. Psychopharmacol. 2022, 36, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.J.; Mednick, S.A.; Harrison, E.M.; Kanady, J.C.; Mednick, S.C. REM, Not Incubation, Improves Creativity by Priming Associative Networks. Proc. Natl. Acad. Sci. USA 2009, 106, 10130–10134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stickgold, R.; Scott, L.; Rittenhouse, C.; Hobson, J.A. Sleep-Induced Changes in Associative Memory. J. Cogn. Neurosci. 1999, 11, 182–193. [Google Scholar] [CrossRef]
- Wagner, U.; Gais, S.; Haider, H.; Verleger, R.; Born, J. Sleep Inspires Insight. Nature 2004, 427, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.P.; Liston, C.; Hobson, J.A.; Stickgold, R. Cognitive Flexibility across the Sleep–Wake Cycle: REM-Sleep Enhancement of Anagram Problem Solving. Cogn. Brain Res. 2002, 14, 317–324. [Google Scholar] [CrossRef]
- Kraehenmann, R.; Pokorny, D.; Vollenweider, L.; Preller, K.H.; Pokorny, T.; Seifritz, E.; Vollenweider, F.X. Dreamlike Effects of LSD on Waking Imagery in Humans Depend on Serotonin 2A Receptor Activation. Psychopharmacology 2017, 234, 2031–2046. [Google Scholar] [CrossRef] [Green Version]
- McGinty, D.T. Serotonin and Sleep: Molecular, Functional, and Clinical Aspects. Sleep 2009, 32, 699–700. [Google Scholar] [CrossRef] [Green Version]
- Dudysová, D.; Janků, K.; Šmotek, M.; Saifutdinova, E.; Kopřivová, J.; Bušková, J.; Mander, B.A.; Brunovský, M.; Zach, P.; Korčák, J.; et al. The Effects of Daytime Psilocybin Administration on Sleep: Implications for Antidepressant Action. Front. Pharmacol. 2020, 11, 602590. [Google Scholar] [CrossRef] [PubMed]
- Muzio, J.N.; Roffwarg, H.P.; Kaufman, E. Alterations in the Nocturnal Sleep Cycle Resulting from LSD. Electroencephalogr. Clin. Neurophysiol. 1966, 21, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.L.; Muthukumaraswamy, S.; Roseman, L.; Kaelen, M.; Droog, W.; Murphy, K.; Tagliazucchi, E.; Schenberg, E.E.; Nest, T.; Orban, C.; et al. Neural Correlates of the LSD Experience Revealed by Multimodal Neuroimaging. Proc. Natl. Acad. Sci. USA 2016, 113, 4853–4858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Araujo, D.B.; Ribeiro, S.; Cecchi, G.A.; Carvalho, F.M.; Sanchez, T.A.; Pinto, J.P.; de Martinis, B.S.; Crippa, J.A.; Hallak, J.E.C.; Santos, A.C. Seeing with the Eyes Shut: Neural Basis of Enhanced Imagery Following Ayahuasca Ingestion. Hum. Brain Mapp. 2012, 33, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.G.; Osório, F.L.; Crippa, J.A.S.; Hallak, J.E.C. Classical Hallucinogens and Neuroimaging: A Systematic Review of Human Studies. Neurosci. Biobehav. Rev. 2016, 71, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The Brain’s Default Network: Anatomy, Function, and Relevance to Disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desseilles, M.; Dang-Vu, T.T.; Sterpenich, V.; Schwartz, S. Cognitive and Emotional Processes during Dreaming: A Neuroimaging View. Conscious. Cogn. 2011, 20, 998–1008. [Google Scholar] [CrossRef] [Green Version]
- Vogt, B.A.; Laureys, S. Posterior Cingulate, Precuneal and Retrosplenial Cortices: Cytology and Components of the Neural Network Correlates of Consciousness. Prog. Brain Res. 2005, 150, 205–217. [Google Scholar]
- Dolder, P.C.; Schmid, Y.; Müller, F.; Borgwardt, S.; Liechti, M.E. LSD Acutely Impairs Fear Recognition and Enhances Emotional Empathy and Sociality. Neuropsychopharmacology 2016, 41, 2638–2646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraehenmann, R.; Preller, K.H.; Scheidegger, M.; Pokorny, T.; Bosch, O.G.; Seifritz, E.; Vollenweider, F.X. Psilocybin-Induced Decrease in Amygdala Reactivity Correlates with Enhanced Positive Mood in Healthy Volunteers. Biol. Psychiatry 2015, 78, 572–581. [Google Scholar] [CrossRef]
- Alonso, J.F.; Romero, S.; Mañanas, M.À.; Riba, J. Serotonergic Psychedelics Temporarily Modify Information Transfer in Humans. Int. J. Neuropsychopharmacol. 2015, 18, pyv039. [Google Scholar] [CrossRef] [Green Version]
- Muthukumaraswamy, S.D.; Carhart-Harris, R.L.; Moran, R.J.; Brookes, M.J.; Williams, T.M.; Errtizoe, D.; Sessa, B.; Papadopoulos, A.; Bolstridge, M.; Singh, K.D.; et al. Broadband Cortical Desynchronization Underlies the Human Psychedelic State. J. Neurosci. 2013, 33, 15171–15183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliazucchi, E.; Carhart-Harris, R.; Leech, R.; Nutt, D.; Chialvo, D.R. Enhanced Repertoire of Brain Dynamical States during the Psychedelic Experience: Enhanced Repertoire of Brain Dynamical States. Hum. Brain Mapp. 2014, 35, 5442–5456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenberg, E.E.; Alexandre, J.F.M.; Filev, R.; Cravo, A.M.; Sato, J.R.; Muthukumaraswamy, S.D.; Yonamine, M.; Waguespack, M.; Lomnicka, I.; Barker, S.A.; et al. Acute Biphasic Effects of Ayahuasca. PLoS ONE 2015, 10, e0137202. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.; Golembiewski, S.; Erlacher, D.; Dresler, M. Extending Mental Practice to Sleep: Enhancing Motor Skills through Lucid Dreaming. Med. Hypotheses 2023, 174, 111066. [Google Scholar] [CrossRef]

| Physiological | Altered | Pathological |
|---|---|---|
| Daydreaming | Hypnosis | Sleep paralysis |
| Lucid dreaming | Anesthesia | Sleepwalking |
| False awakening | Psychedelics | REM behavior disorder |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sodré, M.E.; Wießner, I.; Irfan, M.; Schenck, C.H.; Mota-Rolim, S.A. Awake or Sleeping? Maybe Both… A Review of Sleep-Related Dissociative States. J. Clin. Med. 2023, 12, 3876. https://doi.org/10.3390/jcm12123876
Sodré ME, Wießner I, Irfan M, Schenck CH, Mota-Rolim SA. Awake or Sleeping? Maybe Both… A Review of Sleep-Related Dissociative States. Journal of Clinical Medicine. 2023; 12(12):3876. https://doi.org/10.3390/jcm12123876
Chicago/Turabian StyleSodré, Maria Eduarda, Isabel Wießner, Muna Irfan, Carlos H. Schenck, and Sergio A. Mota-Rolim. 2023. "Awake or Sleeping? Maybe Both… A Review of Sleep-Related Dissociative States" Journal of Clinical Medicine 12, no. 12: 3876. https://doi.org/10.3390/jcm12123876
APA StyleSodré, M. E., Wießner, I., Irfan, M., Schenck, C. H., & Mota-Rolim, S. A. (2023). Awake or Sleeping? Maybe Both… A Review of Sleep-Related Dissociative States. Journal of Clinical Medicine, 12(12), 3876. https://doi.org/10.3390/jcm12123876







