Sleep Paralysis and Lucid Dreaming—Between Waking and Dreaming: A Review about Two Extraordinary States
Abstract
1. Introduction
- Intruder hallucinations include the sense of a presence and sensory hallucinations, such as seeing figures, hearing footsteps, and the sensation that something is pulling on the bed sheets.
- Incubus hallucinations include difficulties with breathing, feeling pressure, most often on the chest, feelings of strangulation, choking, and feelings of impending death.
- V-M hallucinations involve feeling as if one is floating, falling, or flying, but also out-of-body experiences (OBEs), which can happen during SP, fall under this category. Autoscopy, which is seeing oneself from an external station point, and illusory motor movements, such as arm movements, sitting up, and moving around can also be experienced during SP.
Lucid Dreams
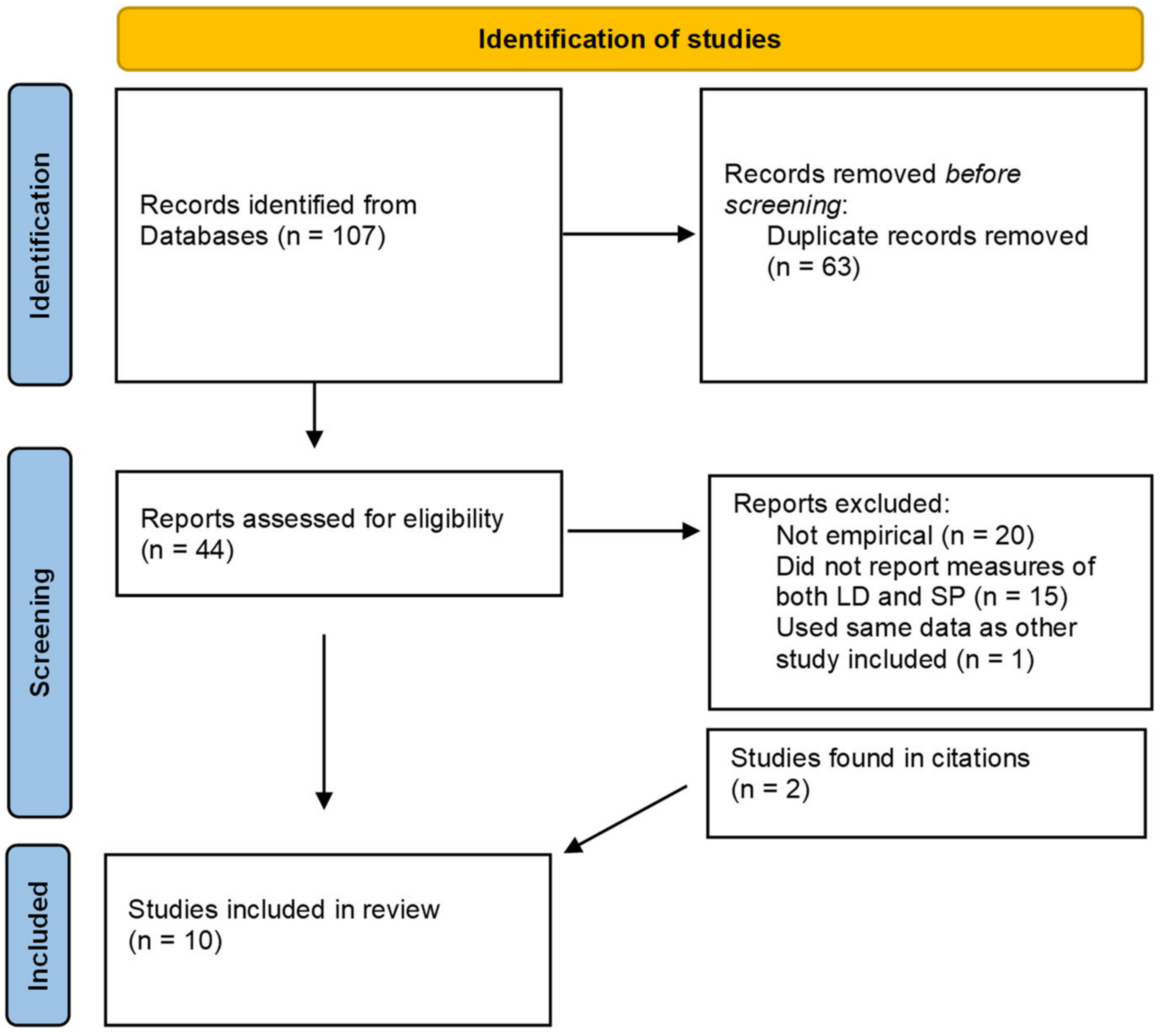
2. Methods
3. Results
3.1. Short Description of the Studies
3.2. Correlation and Cooccurrence of SP and LDs
3.3. Common Factors of Both SP and LDs
4. Discussion
5. Limitations
6. Conclusions and Possible Lines of Future Work
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holzinger, B.; Mayer, L. Sleep Paralysis. In Parasomnia Dreaming: Exploring Other Forms of Sleep Consciousness; Pagel, J.F., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2020; pp. 73–110. [Google Scholar]
- Denis, D. Relationships between sleep paralysis and sleep quality: Current insights. Nat. Sci. Sleep 2018, 10, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Cheyne, J.A.; Rueffer, S.D.; Newby-Clark, I.R. Hypnagogic and Hypnopompic Hallucinations during Sleep Paralysis: Neurological and Cultural Construction of the Night-Mare. Conscious. Cogn. 1999, 8, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Herrero, N.L.; Gallo, F.T.; Gasca, R.M.; Gleiser, P.M.; Forcato, C. Spontaneous and induced out-of-body experiences during sleep paralysis: Emotions, “aura” recognition, and clinical implications. J. Sleep Res. 2022, 32, e13703. [Google Scholar] [CrossRef]
- Cheyne, J.A.; Girard, T.A. Paranoid delusions and threatening hallucinations: A prospective study of sleep paralysis experiences. Conscious. Cogn. 2007, 16, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Lišková, M.; Janečková, D.; Kráčmarová, L.K.; Mladá, K.; Bušková, J. The occurrence and predictive factors of sleep paralysis in university students. Neuropsychiatr. Dis. Treat. 2016, 12, 2957–2962. [Google Scholar] [CrossRef]
- Cheyne, J.A. Situational factors affecting sleep paralysis and associated hallucinations: Position and timing effects. J. Sleep Res. 2002, 11, 169–177. [Google Scholar] [CrossRef]
- Jalal, B.; Romanelli, A.; Hinton, D.E. Cultural Explanations of Sleep Paralysis in Italy: The Pandafeche Attack and Associated Supernatural Beliefs. Culture. Med. Psychiatry 2015, 39, 651–664. [Google Scholar] [CrossRef]
- De Sá, J.F.; Mota-Rolim, S.A. Sleep Paralysis in Brazilian Folklore and Other Cultures: A Brief Review. Front. Psychol. 2016, 7, 1294. [Google Scholar] [CrossRef]
- McNally, R.J.; Clancy, S.A. Sleep paralysis, sexual abuse, and space alien abduction. Transcult. Psychiatry 2005, 42, 113–122. [Google Scholar] [CrossRef]
- Raduga, M.; Kuyava, O.; Sevcenko, N. Is there a relation among REM sleep dissociated phenomena, like lucid dreaming, sleep paralysis, out-of-body experiences, and false awakening? Med. Hypotheses 2020, 144, 110169. [Google Scholar] [CrossRef]
- Fukuda, K. Preliminary Study on Kanashibari Phenomenon: A Polygraphic Approach. Jpn. J. Physiol. Psychol. Psychophysiol. 1989, 7, 83–89. [Google Scholar] [CrossRef]
- Paul, F.; Alpers, G.W.; Reinhard, I.; Schredl, M. Nightmares do result in psychophysiological arousal: A multimeasure ambulatory assessment study. Psychophysiology 2019, 56, e13366. [Google Scholar] [CrossRef]
- Munezawa, T.; Kaneita, Y.; Osaki, Y.; Kanda, H.; Ohtsu, T.; Suzuki, H.; Minowa, M.; Suzuki, K.; Higuchi, S.; Mori, J.; et al. Nightmare and sleep paralysis among Japanese adolescents: A nationwide representative survey. Sleep Med. 2010, 12, 56–64. [Google Scholar] [CrossRef]
- Takeuchi, T.; Miyasita, A.; Sasaki, Y.; Inugami, M.; Fukuda, K. Isolated sleep paralysis elicited by sleep interruption. Sleep 1992, 15, 217–225. [Google Scholar] [CrossRef]
- Pizza Moghadam, K.K.; Franceschini, C.; Bisulli, A.; Poli, F.; Ricotta, L.; Vetrugno, R.; Vandi, S.; Montagna, P.; Plazzi, G. Rhythmic movements and sleep paralysis in narcolepsy with cataplexy: A video-polygraphic study. Sleep Med. 2010, 11, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Voss, U.; Frenzel, C.; Koppehele-Gossel, J.; Hobson, A. Lucid dreaming: An age-dependent brain dissociation. J. Sleep Res. 2012, 21, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.T.; Roe, C.A.; Smith, G.; Clegg, H. Lucid dreaming incidence: A quality effects meta-analysis of 50 years of research. Conscious. Cogn. 2016, 43, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Van Eeden, W.F. A study of dreams. Proc. Soc. Psych. Res. 1913, 26, 431–461. [Google Scholar]
- LaBerge, S. Lucid Dreaming; Ballantine: New York, NY, USA, 1985. [Google Scholar]
- Holzinger, B. Lucid dreaming in psychotherapy. In Lucid Dreaming: New Perspectives on Consciousness in Sleep: Science, Psychology, and Education; Religion, Creativity, and Culture; Hurd, R., Bulkeley, K., Eds.; Praeger/ABC-CLIO: Santa Barbara, CA, USA, 2014; pp. 37–61. [Google Scholar]
- Holzinger, B.; Klösch, G.; Saletu, B. Studies with lucid dreaming as addon therapy to Gestalt therapy. Acta Neurol Scand. 2015, 131, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, B.; Saletu, B.; Klösch, G. Cognitions in Sleep: Lucid Dreaming as an Intervention for Nightmares in Patients With Posttraumatic Stress Disorder. Front. Psychol. 2020, 11, 1826. [Google Scholar] [CrossRef]
- Spoormaker, V.I.; Van Den Bout, J. Lucid dreaming treatment for nightmares: A pilot study. Psychother. Psychosom. 2006, 75, 389–394. [Google Scholar] [CrossRef] [PubMed]
- LaBerge, S. Induction of lucid dreams. Sleep Res. 1980, 9, 138. [Google Scholar]
- Raduga, M. Detecting lucid dreams only by submentalis electromyography. Sleep Med. 2021, 88, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Aviram, L.; Soffer-Dudek, N. Lucid Dreaming: Intensity, But Not Frequency, Is Inversely Related to Psychopathology. Front. Psychol. 2018, 9, 384. [Google Scholar] [CrossRef]
- Baird, B.; Mota-Rolim, S.A.; Dresler, M. The cognitive neuroscience of lucid dreaming. Neurosci. Biobehav. Rev. 2019, 100, 305–323. [Google Scholar] [CrossRef]
- Voss, U.; Holzmann, R.; Tuin, I.; Hobson, A.J. Lucid dreaming: A state of consciousness with features of both waking and non-lucid dreaming. Sleep 2009, 32, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, B.; Mayer, L. Lucid Dreaming Brain Network Based on Tholey’s 7 Klartraum Criteria. Front. Psychol. 2020, 11, 1885. [Google Scholar] [CrossRef]
- Baird, B.; Tononi, G.; LaBerge, S. Lucid dreaming occurs in activated rapid eye movement sleep, not a mixture of sleep and wakefulness. Sleep 2022, 45, 1. [Google Scholar] [CrossRef]
- Green, C.E. Waking dreams and other metachoric experiences. Psychiatr. J. Univ. Ott. Rev. Psychiatr. L’Universite D’Ottawa 1990, 15, 123–128. [Google Scholar]
- Raduga, M.; Shashkov, A.; Zhunusova, Z. Emulating alien and UFO encounters in REM sleep. Int. J. Dream Res. 2021, 14, 247–256. [Google Scholar]
- Mota-Rolim, S.A.; Bulkeley, K.; Campanelli, S.; Lobao-Soares, B.; de Araujo, D.B.; Ribeiro, S. The Dream of God: How Do Religion and Science See Lucid Dreaming and Other Conscious States During Sleep? Front. Psychol. 2020, 11, 555731. [Google Scholar] [CrossRef]
- Aspy, D.J.; Delfabbro, P.; Proeve, M.; Mohr, P. Reality testing and the mnemonic induction of lucid dreams: Findings from the national Australian lucid dream induction study. Dreaming 2017, 27, 206. [Google Scholar] [CrossRef]
- Blagrove, M.; Hartnell, S.J. Lucid dreaming: Associations with internal locus of control, need for cognition and creativity. Personal. Individ. Differ. 2000, 28, 41–47. [Google Scholar] [CrossRef]
- Zink, N.; Pietrowsky, R. Relationship between lucid dreaming, creativity and dream characteristics. Int. J. Dream Res. 2013, 6, 98–103. [Google Scholar]
- D’Anselmo, A.; Agnoli, S.; Filardi, M.; Pizza, F.; Mastria, S.; Corazza, G.E.; Plazzi, G. Creativity in Narcolepsy Type 1: The Role of Dissociated REM Sleep Manifestations. Nat. Sci. Sleep 2020, 12, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Lacaux, C.; Izabelle, C.; Santantonio, G.; De Villèle, L.; Frain, J.; Lubart, T.; Pizza, F.; Plazzi, G.; Arnulf, I.; Oudiette, D. Increased creative thinking in narcolepsy. Brain A J. Neurol. 2019, 142, 1988–1999. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021, 372, n71. [Google Scholar] [CrossRef]
- Biehl, J. Foods and substances influencing (lucid) dreams. Int. J. Dream Res. 2022, 15, 224–234. [Google Scholar]
- Conesa, J. Isolated Sleep Paralysis and lucid dreaming: Ten-year longitudinal case study and related dream frequencies, types, and categories. Sleep Hypn. 2002, 4, 132–142. [Google Scholar]
- Denis, D.; Poerio, G.L. Terror and bliss? Commonalities and distinctions between sleep paralysis, lucid dreaming, and their associations with waking life experiences. J. Sleep Res. 2017, 26, 38–47. [Google Scholar] [CrossRef]
- Dodet, P.; Chavez, M.; Leu-Semenescu, S.; Golmard, J.-L.; Arnulf, I. Lucid dreaming in narcolepsy. Sleep J. Sleep Sleep Disord. Res. 2015, 38, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Drinkwater, K.G.; Denovan, A.; Dagnall, N. Lucid dreaming, nightmares, and sleep paralysis: Associations with reality testing deficits and paranormal experience/belief. Front. Psychol. 2020, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Kliková, M.; Sharpless, B.A.; Bušková, J. Could sleep paralysis be pleasant? J. Sleep Res. 2021, 30, e13154. [Google Scholar] [CrossRef]
- LaBerge, S. Substances that Enhance Recall and Lucidity during Dreaming. U.S. Patent 10/604,138, 30 December 2004. [Google Scholar]
- Mainieri, G.; Maranci, J.-B.; Champetier, P.; Leu-Semenescu, S.; Gales, A.; Dodet, P.; Arnulf, I. Are sleep paralysis and false awakenings different from REM sleep and from lucid REM sleep? A spectral EEG analysis. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2021, 17, 719–727. [Google Scholar] [CrossRef]
- Solomonova, E.; Nielsen, T.; Stenstrom, P. Lucid dreaming is associated with sleep paralysis but not nightmares. In Proceedings of the 4th Conference of the Canadian Sleep Society, Toronto Marriott Downtown Eaton Centre, Toronto, ON, Canada, 26–28 April 2009. [Google Scholar]
- Keyes, K.M.; Hatzenbuehler, M.L.; Grant, B.F.; Hasin, D.S. Stress and alcohol: Epidemiologic evidence. Alcohol Res. Curr. Rev. 2012, 34, 391–400. [Google Scholar]
- Brooks Holliday, S.; Pedersen, E.R.; Leventhal, A.M. Depression, posttraumatic stress, and alcohol misuse in young adult veterans: The transdiagnostic role of distress tolerance. Drug Alcohol Depend. 2016, 161, 348–355. [Google Scholar] [CrossRef]
- Fluharty, M.; Taylor, A.E.; Grabski, M.; Munafò, M.R. The Association of Cigarette Smoking with Depression and Anxiety: A Systematic Review. Nicotine Tob. Res. Off. J. Soc. Res. Nicotine Tob. 2017, 19, 3–13. [Google Scholar] [CrossRef]
- Doll, E.; Gittler, G.; Holzinger, B. Dreaming, lucid dreaming and personality. Int. J. Dream Res. 2009, 2, 52–57. [Google Scholar]
- Hsieh, S.-W.; Lai, C.-L.; Liu, C.-K.; Lan, S.-H.; Hsu, C.-Y. Isolated sleep paralysis linked to impaired nocturnal sleep quality and health-related quality of life in Chinese-Taiwanese patients with obstructive sleep apnea. Qual. Life Res. 2010, 19, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, B.A.; McCarthy, K.S.; Chambless, D.L.; Milrod, B.L.; Khalsa, S.-R.; Barber, J.P. Isolated sleep paralysis and fearful isolated sleep paralysis in outpatients with panic attacks. J. Clin. Psychol. 2010, 66, 1292–1306. [Google Scholar] [CrossRef]
- Abrams, M.P.; Mulligan, A.D.; Carleton, R.N.; Asmundson, G.J. Prevalence and correlates of sleep paralysis in adults reporting childhood sexual abuse. J. Anxiety Disord. 2008, 22, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Schadow, C.; Schredl, M.; Rieger, J.; Göritz, A.S. The relationship between lucid dream frequency and sleep quality: Two cross-sectional studies. Int. J. Dream Res. 2018, 11, 154–159. [Google Scholar]
- Munezawa, T.; Kaneita, Y.; Yokoyama, E.; Suzuki, H.; Ohida, T. Epidemiological study of nightmare and sleep paralysis among Japanese adolescents. Sleep Biol. Rhythm. 2009, 7, 201–210. [Google Scholar] [CrossRef]
- Schredl, M.; Göritz, A.S. Nightmare Themes: An Online Study of Most Recent Nightmares and Childhood Nightmares. J. Clin. Sleep Med. 2018, 14, 465–471. [Google Scholar] [CrossRef]
- Tholey, P.A. Model for lucidity training as a means of self-healing and psychological growth. In Conscious Mind, Sleeping Brain. Perspectives on Lucid Dreaming; Gackenbach, J., LaBerge, S., Eds.; Plenum Press: New York, NY, USA, 1988; pp. 263–287. [Google Scholar]
- De la Brena, D.E.M.; Schoenmann, C. Lucid dreaming as a method for living otherwise. Sociol. Technosci. 2021, 11, 125–151. [Google Scholar]
- Hishikawa, Y.; Shimizu, T. Physiology of REM sleep, cataplexy, and sleep paralysis. Adv. Neurol. 1995, 67, 245–271. [Google Scholar]
- Schredl, M.; Bulkeley, K. Lucid nightmares: An exploratory online study. Int. J. Dream Res. 2020, 13, 215–219. [Google Scholar]
- Wong, S.-S.; Yu, C.K.-C. Lucid nightmare as a state midway between nightmare and lucid dream. Dreaming 2022, 32, 63–74. [Google Scholar] [CrossRef]
| Study | Design | Sample-Size | Measures | Main Results |
|---|---|---|---|---|
| Biehl, 2022 [41] | Online Survey | 436 (45.7% female) | Frequency of LD, SP, and other dream phenomena. Intake of food and substances. Personality | LD and SP correlated with r = 0.276, p < 0.001, and were both connected to other dream phenomena. |
| Conesa, 2002 [42] | Case Study | 1 | Noted dreams and dream related phenomena | LD and SP correlated with r = 0.31. Both were further connected to flying dreams |
| Online Survey | 92 (not reported) | Not reported | 16.3% report experiencing both LD and SP | |
| Denis, & Poerio, 2017 [43] | Online Survey | 1928 (53% female) | Frequency and intensity of SP, LD, and hallucination types of SP. Various other measures | LD correlated with SP r = 0.15, p < 0.001, intruder hallucinations, and V-M hallucinations. Both were also connected to various other measures |
| Dodet, Chavez, Leu-Semenescu, Golmard, & Arnulf, 2015 [44] | Case-Control Study using Interviews | 53 + 53 = 106 (41.5% and 43.3% female) | In-Person interviews about nighttime sleep characteristics, such as SP, LD and others | Participants with narcolepsy had both LD and SP more often than those without narcolepsy (58.5% vs. 17% and 58.5% vs. 15.1%) |
| Drinkwater, Denovan, & Dagnall, 2020 [45] | Online Survey | 455 (76% female) | Frequency of LD. Overall recall of SP, Nightmares, Nightmare distress, reality testing deficits, paranormal experience and belief | LD and SP correlated with r = 0.23, p < 0.01. Both were connected to Nightmare frequency, paranormal experience, delusional thinking, and reality testing |
| Kliková, Sharpless, & Bušková, 2021 [46] | Online Survey | 172 (68% female) | Frequency of SP. Occurrence of LD, Ability to induce LD, Questions on pleasant SP, for example occurrence and frequency. Trauma, life satisfaction, Personality | Positive association between pleasant SP and LD: X2 (1, N = 172) = 8.414, p = 0.004, φ = 0.22 and pleasant SP and the ability to induce LD: X2 (1, N = 134) = 9.327, p = 0.002, φ = 0.26 |
| LaBerge, 2004 [47] | Randomized trial | 10 (30% female) | Dream content, other self-reported measures of nightly sleep and dreams | Donepezil was associated with both LD and SP |
| Mainieri et al., 2021 [48] | Observational EEG study | 5 (80% female) | EEG, EOG, EMG | All episodes of SP happened during REM sleep. Increased alpha rhythms in SP compared to LD but less enhanced muscle tone. |
| Raduga, Kuyava, & Sevcenko, 2020 [11] | Live Survey | 974 (54% female) | Frequency of LD, SP, dream recall frequency, false awakenings, OBE. Sleep duration, overall awareness of practices such as LD | Positive association between LD and SP frequency, X2 (25, N = 974) = 126.767, p < 0.001, φ = 0.36 Both connected to dream recall frequency, false awakenings, OBE. |
| Solomonova, Nielsen, & Stenstrom, 2009 [49] | Online Survey | 245 (58% female) | Frequency of LD, SP, nightmares. SP-, and Nightmare distress | LD correlated with SP r = 0.24, p < 0.001, and SP r = 0.21, p = 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ableidinger, S.; Holzinger, B. Sleep Paralysis and Lucid Dreaming—Between Waking and Dreaming: A Review about Two Extraordinary States. J. Clin. Med. 2023, 12, 3437. https://doi.org/10.3390/jcm12103437
Ableidinger S, Holzinger B. Sleep Paralysis and Lucid Dreaming—Between Waking and Dreaming: A Review about Two Extraordinary States. Journal of Clinical Medicine. 2023; 12(10):3437. https://doi.org/10.3390/jcm12103437
Chicago/Turabian StyleAbleidinger, Severin, and Brigitte Holzinger. 2023. "Sleep Paralysis and Lucid Dreaming—Between Waking and Dreaming: A Review about Two Extraordinary States" Journal of Clinical Medicine 12, no. 10: 3437. https://doi.org/10.3390/jcm12103437
APA StyleAbleidinger, S., & Holzinger, B. (2023). Sleep Paralysis and Lucid Dreaming—Between Waking and Dreaming: A Review about Two Extraordinary States. Journal of Clinical Medicine, 12(10), 3437. https://doi.org/10.3390/jcm12103437





