Eltrombopag for Adults and Children with Immune-Refractory Thrombocytopenic Purpura: A Systematic Review
Abstract
1. Introduction
2. Methodology
2.1. Search Strategy
2.2. Selection of Studies
2.3. Data Extraction
2.4. Risk of Bias Assessment
- Generation of randomization sequence: evaluates the methods used to allocate participants in groups, such as random tables, software, and others.
- Allocation secrecy: evaluates the methods used to ensure the implementation of the generated randomization sequence, with telephone exchange, virtual platforms, or others.
- Blinding of the participants and the team: evaluates the methods used to conceal to which group the participants were allocated from other participants and from the care team.
- Blinding of outcome assessors: assesses the methods used to ensure that outcome assessors do not know to which group the participants were allocated.
- Incomplete outcome data: assesses the impact of loss of participants on the results throughout the study.
- Selective reporting of outcomes: evaluates the alignment between the outcomes planned in the study protocol and the outcomes assessed and/or reported.
- Other sources of bias: to ascertain any other source of bias not considered in the previously described domains, such as imbalance between the groups compared at the study baseline, early interruption of the study, or others.
- Low risk of bias: if all domains were judged to have a low risk of bias;
- Some concerns: if at least one domain was judged to raise some concerns and no domain was judged to have a high risk of bias;
- High risk of bias: if at least one domain was judged as having a high risk of bias or if multiple domains were judged to have some concerns, so that confidence in the outcome was substantially reduced.
2.5. Data Analysis and Synthesis of Results
3. Results
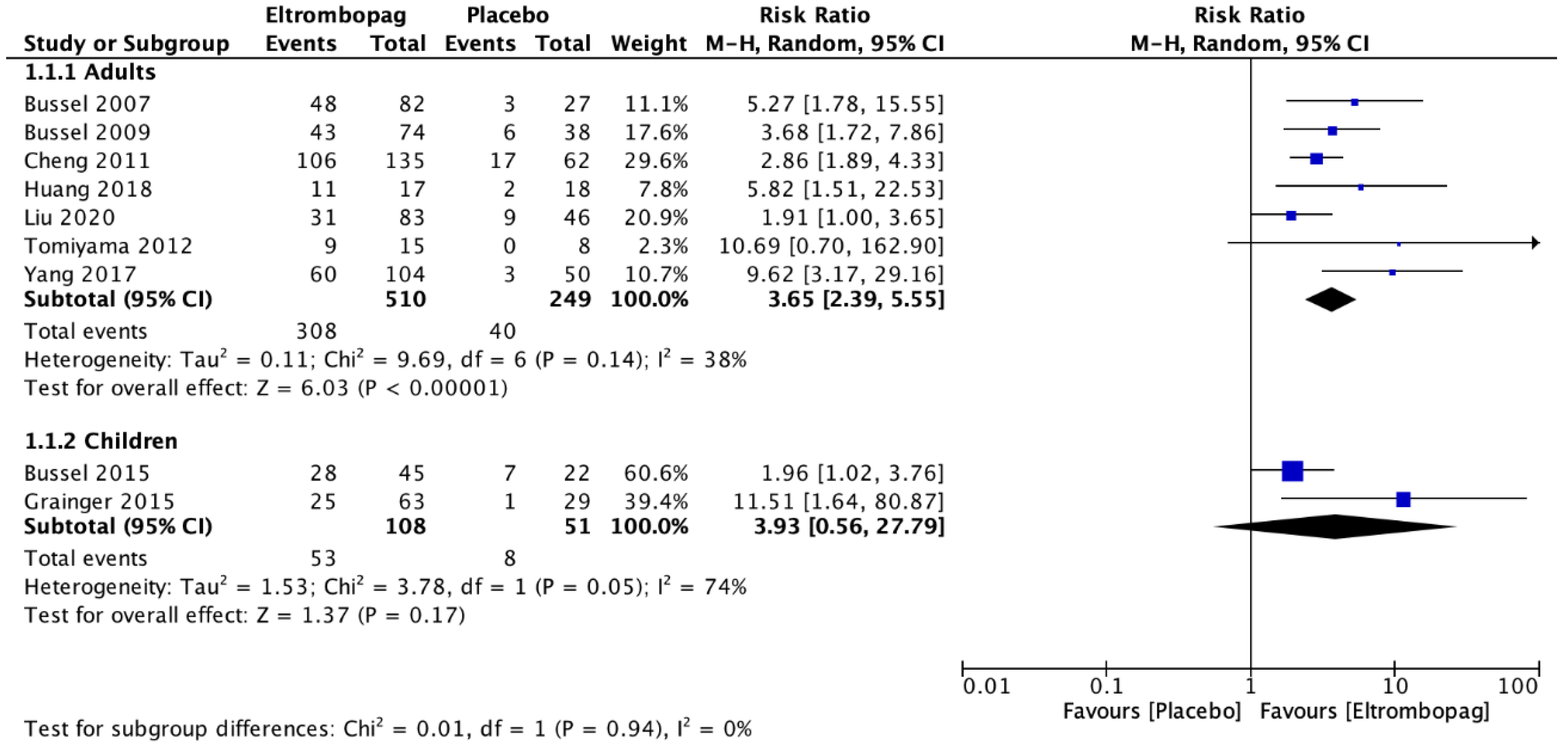
3.1. Analysis of Primary Outcomes
3.2. Analysis of Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzalez-Porras, J.R.; Bastida, J.M. Eltrombopag in immune thrombocytopenia: Efficacy review and update on drug safety. Ther. Adv. Drug. Saf. 2018, 9, 263–285. [Google Scholar] [CrossRef]
- Matzdorff, A.; Meyer, O.; Ostermann, H.; Kiefel, V.; Eberl, W.; Kühne, T.; Pabinger, I.; Rummel, M. Immune thrombocytopenia—Current diagnostics and therapy: Recommendations of a Joint Working Group of DGHO, ÖGHO, SGH, GPOH, and DGTI. Oncol. Res. Treat. 2018, 41 (Suppl. S5), 1–30. [Google Scholar] [CrossRef] [PubMed]
- Ozelo, M.C.; Colella, M.P.; Vinicius de Paula, E.; Virgílio do Nascimento, A.C.K.; Villaça, P.R.; Bernardo, W.M. Guideline on immune thrombocytopenia in adults: Associação Brasileira de Hematologia, Hemoterapia e Terapia Celular. Project guidelines: Associação Médica Brasileira—2018. Hematol. Transfus. Cell Ther. 2018, 40, 50–74. [Google Scholar] [CrossRef] [PubMed]
- Provan, D.; Arnold, D.M.; Bussel, J.B.; Chong, B.H.; Cooper, N.; Gernsheimer, T.; Ghanima, W.; Godeau, B.; González-López, T.J.; Grainger, J.; et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019, 3, 3780–3817. [Google Scholar] [CrossRef]
- Kistangari, G.; McCrae, K.R. Immune thrombocytopenia. Hematol. Oncol. Clin. N. Am. 2013, 27, 495–520. [Google Scholar] [CrossRef] [PubMed]
- Tumaini Massaro, J.; Chen, Y.; Ke, Z. Efficacy and safety of thrombopoietin receptor agonists in children with chronic immune thrombocytopenic purpura: Meta-analysis. Platelets 2019, 30, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Bussel, J.B.; Garcia de Miguel, P.; Despotovic, J.M.; Grainger, J.D.; Sevilla, J.; Blanchette, V.S.; Krishnamurti, L.; Connor, P.; David, M.; Boayue, K.B.; et al. Eltrombopag for the treatment of children with persistent and chronic immune thrombocytopenia (PETIT): A randomised, multicentre, placebo-controlled study. Lancet Haematol. 2015, 2, e315–e325. [Google Scholar] [CrossRef] [PubMed]
- Grainger, J.D.; Locatelli, F.; Chotsampancharoen, T.; Donyush, E.; Pongtanakul, B.; Komvilaisak, P.; Sosothikul, D.; Drelichman, G.; Sirachainan, N.; Holzhauer, S.; et al. Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): A randomised, multicentre, placebo-controlled trial. Lancet 2015, 386, 1649–1658, Erratum in Lancet 2015, 386, 1630. [Google Scholar] [CrossRef]
- Raymond, S.M.; Wong, R.M.S.; Saleh, M.N.; Khelif, A.; Salama, A.; Portella, M.S.O.; Burgess, P.; Bussel, J.B. Safety and efficacy of long-term treatment of chronic/persistent ITP with Eltrombopag: Final results of the EXTEND study. Blood 2017, 130, 2527–2536. [Google Scholar]
- Siegal, D.; Crowther, M.; Cuker, A. Thrombopoietin receptor agonists in primary immune thrombocytopenia. Semin. Hematol. 2013, 50 (Suppl. S1), S18–S21. [Google Scholar] [CrossRef]
- Kim, T.O.; Despotovic, J.; Lambert, M.P. Eltrombopag for use in children with immune thrombocytopenia. Blood Adv. 2018, 2, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, L.A.; Kwitkowski, V.E.; Reaman, G.; Ko, C.W.; Nie, L.; Pazdur, R.; Farrell, A.T. U.S. Food and Drug Administration approval summary: Eltrombopag for the treatment of pediatric patients with chronic immune (idiopathic) thrombocytopenia. Pediatr. Blood Cancer. 2017, 64, e26657. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, Z.; Chen, X.P.; Zhang, H.Y.; Yang, N.; Wang, F.Y.; Guan, L.X.; Gu, Z.Y.; Zhao, S.S.; Luo, L.; et al. Efficacy and safety of thrombopoietin receptor agonists in patients with primary immune thrombocytopenia: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 39003. [Google Scholar] [CrossRef]
- Elgebaly, A.S.; Ashal, G.E.; Elfil, M.; Menshawy, A. Tolerability and efficacy of eltrombopag in chronic immune thrombocytopenia: Meta-analysis of randomized controlled trials. Clin. Appl. Thromb. Hemost. 2017, 23, 928–937. [Google Scholar] [CrossRef]
- Kolanis, S.; Vasileiou, E.; Hatzipantelis, E.; Economou, M.; Tragiannidis, A. Safety and Efficacy of Eltrombopag in Children and Adults with Immune Thrombocytopenia: A Systematic Review and Meta-Analysis. Cardiovasc. Hematol. Agents Med. Chem. 2021, 19, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Cooper, N.; Kruse, A.; Kruse, C.; Watson, S.; Morgan, M.; Provan, D.; Ghanima, W.; Arnold, D.M.; Tomiyama, Y.; Santoro, C.; et al. Immune thrombocytopenia (ITP) World Impact Survey (I-WISh): Impact of ITP on health-related quality of life. Am. J. Hematol. 2021, 96, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Rodeghiero, F.; Michel, M.; Gernsheimer, T.; Ruggeri, M.; Blanchette, V.; Bussel, J.B.; Cines, D.B.; Cooper, N.; Godeau, B.; Greinacher, A.; et al. Standardization of bleeding assessment in immune thrombocytopenia: Report from the International Working Group. Blood 2013, 121, 2596–2606. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Updated March 2011. The Cochrane Collabora-tion. 2011. Available online: http://www.handbook.cochrane.org (accessed on 18 March 2022).
- GRADE Guidelines—Journal of Clinical Epidemiology Series. Available online: http://www.jclinepi.com/content/jce-GRADE-Series (accessed on 5 May 2022).
- Cheng, G.; Saleh, M.N.; Marcher, C.; Vasey, S.; Mayer, B.; Aivado, M.; Arning, M.; Stone, N.L.; Bussel, J.B. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): A 6-month, randomised, phase 3 study. Lancet 2011, 377, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Bussel, J.B.; Provan, D.; Shamsi, T.; Cheng, G.; Psaila, B.; Kovaleva, L.; Salama, A.; Jenkins, J.M.; Roychowdhury, D.; Mayer, B.; et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: A randomised, double-blind, placebo-controlled trial. Lancet 2009, 373, 641–648. [Google Scholar] [CrossRef]
- Bussel, J.B.; Cheng, G.; Saleh, M.N.; Psaila, B.; Kovaleva, L.; Meddeb, B.; Kloczko, J.; Hassani, H.; Mayer, B.; Stone, N.L.; et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N. Engl. J. Med. 2007, 357, 2237–2247. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hou, M.; Li, J.; Jin, J.; Huang, M.; Yu, Z.; Xu, X.; Zhang, X.; Yang, R. Efficacy and safety of eltrombopag in Chinese patients with chronic immune thrombocytopenia: Stage 2 results from a multicenter phase III study. Platelets 2022, 33, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, J.; Jin, J.; Huang, M.; Yu, Z.; Xu, X.; Zhang, X.; Hou, M. Multicentre, randomised phase III study of the efficacy and safety of eltrombopag in Chinese patients with chronic immune thrombocytopenia. Br. J. Haematol. 2017, 176, 101–110. [Google Scholar] [CrossRef]
- Tomiyama, Y.; Miyakawa, Y.; Okamoto, S.; Katsutani, S.; Kimura, A.; Okoshi, Y.; Ninomiya, H.; Kosugi, H.; Nomura, S.; Ozaki, K.; et al. A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. J. Thromb. Haemost. 2012, 10, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Liu, X.F.; Chen, Y.F.; Fu, R.F.; Liu, W.; Zhang, L.; Yang, R.C. The efficacy and safety of eltrombopag in Chinese patients with chronic immune thrombocytopenia. Zhonghua Xue Ye Xue Za Zhi 2018, 39, 32–36. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.A.W.; Masoud, A.T.; Han, J.; Adel Sofy, A.; Saeed Ahmed, A.; Abdesattart, A.T.; Drokow, E.K.; Sun, K. Eltrombopag effectiveness and tolerability in chronic immune thrombocytopenia: A meta-analysis. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211005555. [Google Scholar] [CrossRef] [PubMed]






| Quality of the Study | Number of Events | Effect: RR 4 (95% CI) | Quality | |||||
|---|---|---|---|---|---|---|---|---|
| No. of Studies | Risk of Bias 1 | Inconsistency 2 | Imprecision 3 | Intervention Group | Control Group | |||
| Platelets ≥ 50.000/mm3 | ||||||||
| Adults | 7 | No risk of serious bias | Without serious inconsistency | Without serious imprecision | 308 | 40 | 3.65 (2.39–5.55) | ⊗ ⊗ ⊗ High |
| Children | 2 | Without risk of serious bias | Serious inconsistency | Serious imprecision | 53 | 8 | 3.93 (0.56–27.79) | ⊗ Low |
| Bleeding (WHO classification > 2) | ||||||||
| Adults | 6 | Without risk of serious bias | Without serious inconsistency | Serious imprecision | 106 | 67 | 0.80 (0.52–1.22) | ⊗ ⊗ Moderate |
| Children | 2 | Without risk of serious bias | Without serious inconsistency | Serious imprecision | 15 | 15 | 0.47 (0.27–0.83) | ⊗ ⊗ Moderate |
| Use of rescue therapy | ||||||||
| Adults | 5 | Without risk of serious bias | Without serious inconsistency | Serious imprecision | 57 | 72 | 0.40 (0.29–0.56) | ⊗ ⊗ Moderate |
| Children | 1 | Without risk of serious bias | Without risk of serious inconsistency | Serious imprecision | 28 | 7 | 1.96 (1.02–3.76) | ⊗ ⊗ Moderate |
| Lasting response | ||||||||
| Adults | 2 | Without risk of serious bias | Serious inconsistency | Serious imprecision | 82 | 13 | 3.17 (1.07–9.43) | ⊗ Low |
| Children | 1 | Without risk of serious bias | Without serious inconsistency | Serious imprecision | 13 | 1 | 6.36 (0.89–45.53) | ⊗ ⊗ Moderate |
| Adverse events | ||||||||
| Adults | 5 | Without risk of serious bias | Without serious inconsistency | Serious imprecision | 38 | 18 | 0.99 (0.55–1.78) | ⊗ ⊗ Moderate |
| Children | 2 | Without risk of serious bias | Without serious inconsistency | Serious imprecision | 10 | 8 | 0.59 (0.25–1.41) | ⊗ ⊗ Moderate |
| Platelets ≥ 50,000/mm3 | ||||||||
| Adults | 7 | Without risk of serious bias | Without risk of serious bias 1 | Without serious imprecision | 308 | 40 | 3.65 (2.39–5.55) | ⊗ ⊗ ⊗ High |
| Children | 2 | Without risk of serious bias | Serious inconsistency | Serious imprecision | 53 | 8 | 3.93 (0.56–27.79) | ⊗ Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Barros Torelli, D.F.H.; Oliveira, C.B.S.; Nai, G.A.; Trindade, E.M.; Prestes-Carneiro, L.E. Eltrombopag for Adults and Children with Immune-Refractory Thrombocytopenic Purpura: A Systematic Review. J. Clin. Med. 2023, 12, 3872. https://doi.org/10.3390/jcm12123872
de Barros Torelli DFH, Oliveira CBS, Nai GA, Trindade EM, Prestes-Carneiro LE. Eltrombopag for Adults and Children with Immune-Refractory Thrombocytopenic Purpura: A Systematic Review. Journal of Clinical Medicine. 2023; 12(12):3872. https://doi.org/10.3390/jcm12123872
Chicago/Turabian Stylede Barros Torelli, Danielle Francisco Honorato, Crystian Bitencourt Soares Oliveira, Gisele Alborghetti Nai, Evelinda Marramon Trindade, and Luiz Euribel Prestes-Carneiro. 2023. "Eltrombopag for Adults and Children with Immune-Refractory Thrombocytopenic Purpura: A Systematic Review" Journal of Clinical Medicine 12, no. 12: 3872. https://doi.org/10.3390/jcm12123872
APA Stylede Barros Torelli, D. F. H., Oliveira, C. B. S., Nai, G. A., Trindade, E. M., & Prestes-Carneiro, L. E. (2023). Eltrombopag for Adults and Children with Immune-Refractory Thrombocytopenic Purpura: A Systematic Review. Journal of Clinical Medicine, 12(12), 3872. https://doi.org/10.3390/jcm12123872







