Outcome of Out-of-Hospital Cardiac Arrest Patients Stratified by Pre-Clinical Loading with Aspirin and Heparin: A Retrospective Cohort Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Treatment Algorithm
2.2. Measured Data and Investigated Outcomes
2.3. Statistical Analysis
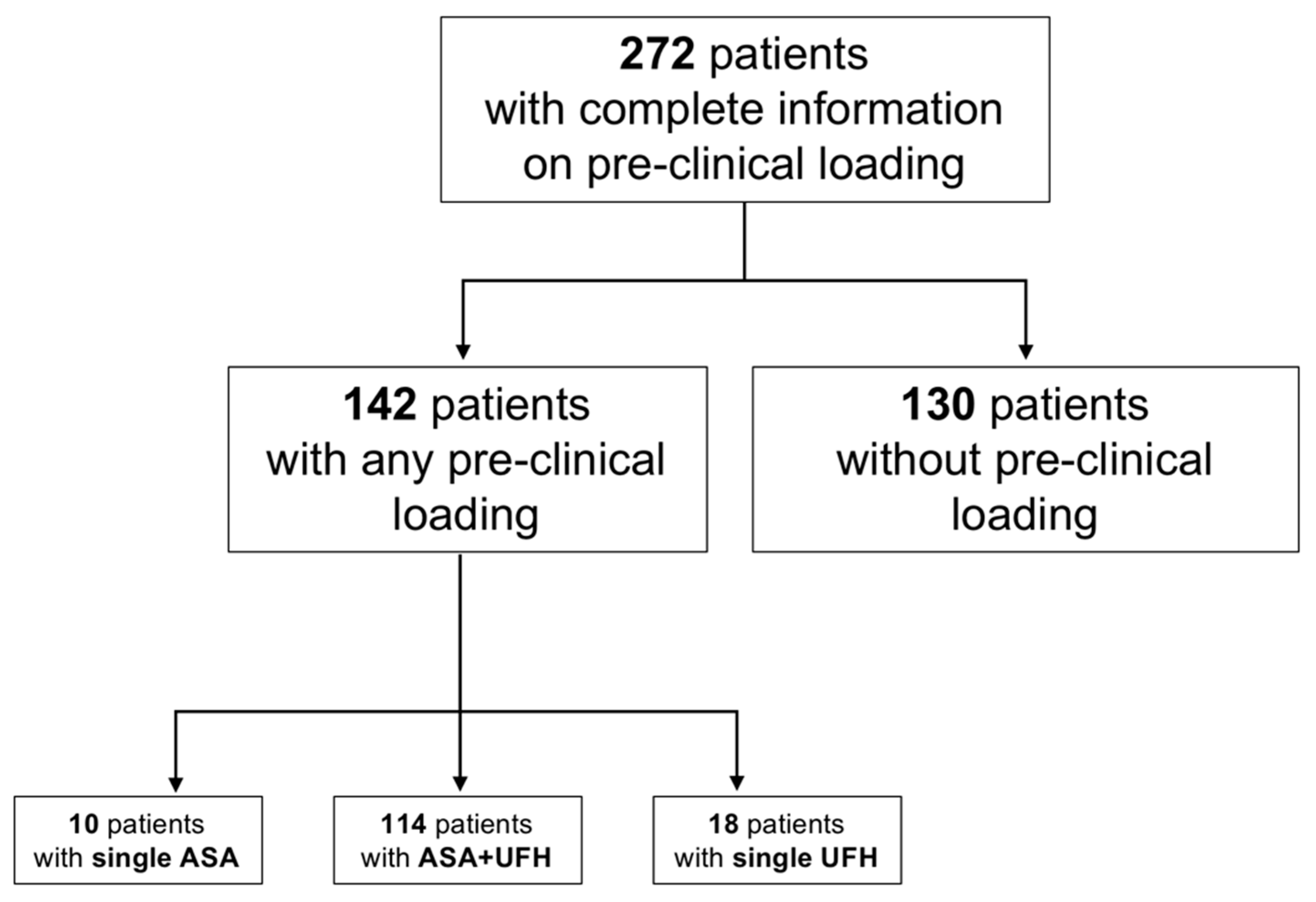
3. Results
3.1. Overall Analysis
3.2. Loading Status
3.3. STEMI Subgroup Analysis
4. Discussion
- ▪
- loading was associated with increased survival to hospital discharge and a more favorable neurological outcome,
- ▪
- the rates for RBC transfusion and intracranial bleeding were not affected by pre-clinical loading,
- ▪
- a considerable number of STEMI patients (33%) were not loaded on scene,
- ▪
- 54% of patients in the loading group had OHCA from non-ischemic cause and had no expected benefit from pretreatment, retrospectively.
4.1. Bleeding
4.2. Undertreatment of STEMI
4.3. Overtreatment of Non-STEMI OHCA
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gräsner, J.-T.; Wnent, J.; Herlitz, J.; Perkins, G.D.; Lefering, R.; Tjelmeland, I.; Koster, R.W.; Masterson, S.; Rossell-Ortiz, F.; Maurer, H.; et al. Survival after out-of-hospital cardiac arrest in Europe—Results of the EuReCa TWO study. Resuscitation 2020, 148, 218–226. [Google Scholar] [CrossRef]
- Gräsner, J.T.; Lefering, R.; Koster, R.W.; Masterson, S.; Böttiger, B.W.; Herlitz, J.; Wnent, J.; Tjelmeland, I.B.; Ortiz, F.R.; Maurer, H.; et al. EuReCa ONE-27 Nations, ONE Europe, ONE Registry: A prospective one month analysis of out-of-hospital cardiac arrest outcomes in 27 countries in Europe. Resuscitation 2016, 105, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Gräsner, J.-T.; Herlitz, J.; Tjelmeland, I.B.; Wnent, J.; Masterson, S.; Lilja, G.; Bein, B.; Böttiger, B.W.; Rosell-Ortiz, F.; Nolan, J.P.; et al. European Resuscitation Council Guidelines 2021: Epidemiology of cardiac arrest in Europe. Resuscitation 2021, 161, 61–79. [Google Scholar] [CrossRef]
- Kiguchi, T.; Okubo, M.; Nishiyama, C.; Maconochie, I.; Ong, M.E.H.; Kern, K.B.; Wyckoff, M.H.; McNally, B.; Christensen, E.F.; Tjelmeland, I.; et al. Out-of-hospital cardiac arrest across the World: First report from the International Liaison Committee on Resuscitation (ILCOR). Resuscitation 2020, 152, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Gan, Y.; Jiang, N.; Wang, R.; Chen, Y.; Luo, Z.; Zong, Q.; Chen, S.; Lv, C. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: A systematic review and meta-analysis. Crit. Care 2020, 24, 61. [Google Scholar] [CrossRef]
- Reynolds, J.C.; Grunau, B.E.; Rittenberger, J.C.; Sawyer, K.N.; Kurz, M.C.; Callaway, C.W. Association Between Duration of Resuscitation and Favorable Outcome After Out-of-Hospital Cardiac Arrest: Implications for Prolonging or Terminating Resuscitation. Circulation 2016, 134, 2084–2094. [Google Scholar] [CrossRef]
- Kandala, J.; Oommen, C.; Kern, K.B. Sudden cardiac death. Br. Med. Bull. 2017, 122, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Desch, S.; Freund, A.; Akin, I.; Behnes, M.; Preusch, M.R.; Zelniker, T.A.; Skurk, C.; Landmesser, U.; Graf, T.; Eitel, I.; et al. Angiography after Out-of-Hospital Cardiac Arrest without ST-Segment Elevation. N. Engl. J. Med. 2021, 385, 2544–2553. [Google Scholar] [CrossRef]
- Heyne, S.; Macherey, S.; Meertens, M.M.; Braumann, S.; Nießen, F.S.; Tichelbäcker, T.; Baldus, S.; Adler, C.; Lee, S. Coronary angiography after cardiac arrest without ST-elevation myocardial infarction: A network meta-analysis. Eur. Heart J. 2023, 44, 1040–1054. [Google Scholar] [CrossRef]
- Lemkes, J.S.; Janssens, G.N.; van der Hoeven, N.W.; Jewbali, L.S.; Dubois, E.A.; Meuwissen, M.; Rijpstra, T.A.; Bosker, H.A.; Blans, M.J.; Bleeker, G.B.; et al. Coronary Angiography after Cardiac Arrest without ST-Segment Elevation. N. Engl. J. Med. 2019, 380, 1397–1407. [Google Scholar] [CrossRef]
- Spirito, A.; Vaisnora, L.; Papadis, A.; Iacovelli, F.; Sardu, C.; Selberg, A.; Bär, S.; Kavaliauskaite, R.; Temperli, F.; Asatryan, B.; et al. Acute Coronary Occlusion in Patients with Non-ST-Segment Elevation Out-of-Hospital Cardiac Arrest. J. Am. Coll. Cardiol. 2023, 81, 446–456. [Google Scholar] [CrossRef]
- Lott, C.; Truhlář, A.; Alfonzo, A.; Barelli, A.; González-Salvado, V.; Hinkelbein, J.; Nolan, J.P.; Paal, P.; Perkins, G.D.; Thies, K.-C.; et al. European Resuscitation Council Guidelines 2021: Cardiac arrest in special circumstances. Resuscitation 2021, 161, 152–219. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Pitts, S.; Niska, R.; Xu, J.; Burt, C. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Natl. Health Stat. Rep. 2008, 6, 1–38. [Google Scholar]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; Conejo, T. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, e368–e454. [Google Scholar] [CrossRef]
- Camaro, C.; Aarts, G.W.; Adang, E.M.M.; van Hout, R.; Brok, G.; Hoare, A.; Rodwell, L.; de Pooter, F.; de Wit, W.; Cramer, G.; et al. Rule-out of non-ST-segment elevation acute coronary syndrome by a single, pre-hospital troponin measurement: A randomized trial. Eur. Heart J. 2023, 44, 1705–1714. [Google Scholar] [CrossRef]
- Stengaard, C.; Sørensen, J.T.; Ladefoged, S.A.; Christensen, E.F.; Lassen, J.F.; Bøtker, H.E.; Terkelsen, C.J.; Thygesen, K. Quantitative point-of-care troponin T measurement for diagnosis and prognosis in patients with a suspected acute myocardial infarction. Am. J. Cardiol. 2013, 112, 1361–1366. [Google Scholar] [CrossRef]
- Braumann, S.; Faber-Zameitat, C.; Macherey-Meyer, S.; Tichelbäcker, T.; Meertens, M.; Heyne, S.; Nießen, F.; Nies, R.J.; Nettersheim, F.; Reuter, H.; et al. Acute Chest Pain—Diagnostic Accuracy and Pre-hospital Use of Anticoagulants and Platelet Aggregation Inhibitors. Dtsch. Arztebl. Int. 2023. [Google Scholar] [CrossRef]
- Eckle, V.S.; Lehmann, S.; Drexler, B. Prehospital management of patients with suspected acute coronary syndrome: Real world experience reflecting current guidelines. Med. Klin. Intensivmed. Notfmed. 2021, 116, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Macherey-Meyer, S.; Braumann, S.; Meertens, M.; Heyne, S.; Nießen, S.F.; Tichelbäcker, T.; Baldus, S.; Lee, S.; Adler, C. The PRELOAD Study—Pre-clinical loading in patients with acute chest pain and suspected or definite acute coronary syndrome: An interim analysis. Clin. Res. Cardiol. 2023, V451. [Google Scholar] [CrossRef]
- Adler, C.; Paul, C.; Michels, G.; Pfister, R.; Sabashnikov, A.; Hinkelbein, J.; Braumann, S.; Djordjevic, L.; Blomeyer, R.; Krings, A.; et al. One year experience with fast track algorithm in patients with refractory out-of-hospital cardiac arrest. Resuscitation 2019, 144, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Seewald, S.; Ristau, P.; Fischer, M.; Gräsner, J.T.; Brenner, S.; Wnent, J.; Bein, B. Öffentlicher Jahresbericht 2021 des Deutschen Reanimationsregisters: Cardiac Arrest Center. Anästh. Intensivmed. 2021, 62, V128–V130. [Google Scholar]
- Blom, M.T.; Oving, I.; Berdowski, J.; van Valkengoed, I.G.M.; Bardai, A.; Tan, H.L. Women have lower chances than men to be resuscitated and survive out-of-hospital cardiac arrest. Eur. Heart J. 2019, 40, 3824–3834. [Google Scholar] [CrossRef]
- Shin, J.; Kim, K.; Lim, Y.S.; Lee, H.J.; Lee, S.J.; Jung, E.; Kim, J.; Yang, H.J.; Kim, J.J.; Hwang, S.Y. Incidence and clinical features of intracranial hemorrhage causing out-of-hospital cardiac arrest: A multicenter retrospective study. Am. J. Emerg. Med. 2016, 34, 2326–2330. [Google Scholar] [CrossRef]
- Cocchi, M.N.; Lucas, J.M.; Salciccioli, J.; Carney, E.; Herman, S.; Zimetbaum, P.; Donnino, M.W. The role of cranial computed tomography in the immediate post-cardiac arrest period. Intern. Emerg. Med. 2010, 5, 533–538. [Google Scholar] [CrossRef]
- Gelber, J.; Montgomery, M.E.; Singh, A. A prospective study of the incidence of intracranial hemorrhage in survivors of out of hospital cardiac arrest. Am. J. Emerg. Med. 2021, 41, 70–72. [Google Scholar] [CrossRef]
- Agrawal, A.; Cardinale, M.; Frenia, D.; Mukherjee, A. Cerebellar Haemorrhage Leading to Sudden Cardiac Arrest. J. Crit. Care Med. 2020, 6, 71–73. [Google Scholar] [CrossRef]
- Nguyen, M.-L.; Gause, E.; Mills, B.; Tonna, J.E.; Alvey, H.; Saczkowski, R.; Grunau, B.; Becker, L.B.; Gaieski, D.F.; Youngquist, S.; et al. Traumatic and hemorrhagic complications after extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest. Resuscitation 2020, 157, 225–229. [Google Scholar] [CrossRef]
- García, J.; Jiménez-Brítez, G.; Flores-Umanzor, E.; Mendieta, G.; Freixa, X.; Sabaté, M. Thrombotic and Bleeding Events after Percutaneous Coronary Intervention in Out-of-Hospital Cardiac Arrest with and without Therapeutic Hypothermia. Rev. Esp. Cardiol. (Engl. Ed.) 2019, 72, 433–435. [Google Scholar] [CrossRef]
- Yannopoulos, D.; Bartos, J.; Raveendran, G.; Walser, E.; Connett, J.; Murray, T.A.; Collins, G.; Zhang, L.; Kalra, R.; Kosmopoulos, M.; et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial. Lancet 2020, 396, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Belohlavek, J.; Smalcova, J.; Rob, D.; Franek, O.; Smid, O.; Pokorna, M.; Horák, J.; Mrazek, V.; Kovarnik, T.; Zemanek, D.; et al. Effect of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment on Functional Neurologic Outcome in Refractory Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA 2022, 327, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Gall, E.; Lafont, A.; Varenne, O.; Dumas, F.; Cariou, A.; Picard, F. Balancing thrombosis and bleeding after out-of-hospital cardiac arrest related to acute coronary syndrome: A literature review. Arch. Cardiovasc. Dis. 2021, 114, 667–679. [Google Scholar] [CrossRef]
- Bisdas, T.; Beutel, G.; Warnecke, G.; Hoeper, M.M.; Kuehn, C.; Haverich, A.; Teebken, O.E. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann. Thorac. Surg. 2011, 92, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.E.; Andrew, E.; Nehme, Z.; Dinh, D.T.; Fernando, H.; Shi, W.Y.; Vriesendorp, P.; Nanayakarra, S.; Dawson, L.P.; Brennan, A.; et al. Pre-hospital heparin use for ST-elevation myocardial infarction is safe and improves angiographic outcomes. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 1140–1147. [Google Scholar] [CrossRef]
- Zijlstra, F.; Ernst, N.; de Boer, M.-J.; Nibbering, E.; Suryapranata, H.; Hoorntje, J.C.; Dambrink, J.-H.; Hof, A.W.V.; Verheugt, F.W. Influence of prehospital administration of aspirin and heparin on initial patency of the infarct-related artery in patients with acute ST elevation myocardial infarction. J. Am. Coll. Cardiol. 2002, 39, 1733–1737. [Google Scholar] [CrossRef]
- Emilsson, O.E.; Bergman, S.; Mohammad, M.M.; Olivecrona, G.O.; Götberg, M.; Erlinge, D.; Koul, S. Pretreatment with heparin in patients with ST-segment elevation myocardial infarction: A report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). EuroIntervention 2022, 18, 709–718. [Google Scholar] [CrossRef]
- Arnaout, M.; Mongardon, N.; Deye, N.; Legriel, S.; Dumas, F.; Sauneuf, B.; Malissin, I.; Charpentier, J.; Pène, F.; Baud, F.; et al. Out-of-hospital cardiac arrest from brain cause: Epidemiology, clinical features, and outcome in a multicenter cohort. Crit. Care Med. 2015, 43, 453–460. [Google Scholar] [CrossRef]
- Baldi, E.; Schnaubelt, S.; Caputo, M.L.; Klersy, C.; Clodi, C.; Bruno, J.; Compagnoni, S.; Benvenuti, C.; Domanovits, H.; Burkart, R.; et al. Association of Timing of Electrocardiogram Acquisition after Return of Spontaneous Circulation with Coronary Angiography Findings in Patients with Out-of-Hospital Cardiac Arrest. JAMA Netw. Open 2021, 4, e2032875. [Google Scholar] [CrossRef]
- Beygui, F.; Castren, M.; Brunetti, N.D.; Rosell-Ortiz, F.; Christ, M.; Zeymer, U.; Huber, K.; Folke, F.; Svensson, L.; Bueno, H.; et al. Pre-hospital management of patients with chest pain and/or dyspnoea of cardiac origin. A position paper of the Acute Cardiovascular Care Association (ACCA) of the ESC. Eur. Heart J. Acute Cardiovasc. Care 2020, 9 (Suppl. 1), 59–81. [Google Scholar] [CrossRef]
- Thiele, H.; Bauersachs, J.; Mehilli, J.; Möllmann, H.; Landmesser, U.; Jobs, A. Kommentar zu den 2020er Leitlinien der Europäischen Gesellschaft für Kardiologie (ESC) zum Management des akuten Koronarsyndroms bei Patienten ohne persistierende ST-Strecken-Hebung. Der Kardiol. 2021, 15, 19–31. [Google Scholar] [CrossRef]
| Total Cohort N = 272 | Pre-Clinical Loading N = 142 | No Loading N = 130 | p-Value * | |
|---|---|---|---|---|
| Age, mean [SD] | 62.7 [±15.5] | 61.5 [±14.9] | 64.1 [±16.4] | 0.168 |
| Gender male (%) | 196 (72.1) | 108 (76) | 88 (67.7) | 0.125 |
| Pre-emergency status (%) | 0.255 | |||
| No prior diseases | 50 (18.4) | 31 (21.8) | 19 (14.6) | |
| Diseases without limitations in daily living | 134 (49.3) | 72 (50.7) | 62 (47.7) | |
| Diseases with limitation in daily living | 41 (15.1) | 17 (12) | 24 (18.5) | |
| No independent daily living | 4 (1.5) | 1 (0.7) | 3 (2.3) | |
| Unknown status | 43 (15.8) | 21 (14.8) | 22 (16.9) | |
| Pre-hospital characteristics | ||||
| Witnessed arrest | 211 | 116 | 95 | 0.037 |
| Bystander CPR | 170 (62.5) | 97 (68.3) | 73 (56.2) | 0.039 |
| No-flow time, min | 2.2 [±4] | 2.1 [±4.1] | 2.4 [±4] | 0.624 |
| Shockable rhythm | 174 | 99 | 75 | 0.114 |
| Shocks, n | 2.99 [±3.5] | 3.1 [±3.7] | 2.9 [±3.3] | 0.649 |
| Epinephrine use, n | 214 | 109 | 105 | 0.460 |
| Amiodarone use, n | 108 | 49 | 59 | 0.008 |
| Achieving ROSC before hospital arrival § | 190 | 100 | 90 | 0.914 |
| Achieving ROSC after hospital arrival § | 55 | 28 | 27 | |
| Never ROSC achieved § | 25 | 14 | 11 | |
| Time until ROSC, min | 26.7 [±22.9] | 26.4 [±25.5] | 27.2 [±19.8] | 0.795 |
| EMS transport with mechanical cardiopulmonary resuscitation device | 75 | 40 | 35 | 0.854 |
| Presenting arterial blood gases, means | ||||
| Initial arterial O2, mm Hg | 182.5 [±106.5] | 176 [±88.3] | 190.4 [±125.7] | 0.419 |
| Initial arterial CO2, mm Hg | 59.8 [±27.9] | 58.7 [±25.2] | 60.6 [±88.3] | 0.829 |
| Initial lactate, mmol/L | 7.98 [±6] | 7.36 [±5.9] | 8.67 [±6.0] | 0.073 |
| Initial pH | 7.15 [±0.2] | 7.17 [±0.2] | 7.12 [±0.2] | 0.166 |
| Initial hemoglobin g/dL | 14.3 [±2.9] | 15.1 [±2.3] | 13.5 [±3.3] | 0.135 |
| In-hospital treatment | ||||
| Coronary angiography performed (%) | 229 (84.2) | 129 (90.8) | 100 (76.9) | 0.002 |
| Mechanical circulatory support implantation (%) | ||||
| ECMO | 33 (12.1) | 16 (11.3) | 17 (13.1) | 0.631 |
| Axial flow pump (Impella©) | 8 (2.9) | 6 (4.2) | 2 (1.5) | 0.286 |
| IABP | 7 (2.6) | 4 (2.8) | 3 (2.3) | 1.000 |
| Target temperature management | 89 (32.7) | 48 (33.8) | 41 (31.5) | 0.691 |
| PTT, s | 50.2 [±31.7] | 57 [±31.4] | 45.1 [±31.1] | 0.014 |
| Aspiration pneumonia, n | 110 | 55 | 55 | 0.565 |
| Hypoxic ischemic encephalopathy (%) | 64 (23.5) | 24 (16.9) | 40 (30.8) | 0.007 |
| Ejection fraction, EF (%) | 0.101 | |||
| Preserved EF (≥50%) | 83 (30.5) | 42 (29.6) | 41 (31.5) | |
| Mildly reduced EF (41 to 49%) | 42 (15.4) | 27 (19) | 15 (11.5) | |
| Reduced EF (≤40%) | 86 (31.7) | 48 (33.8) | 38 (29.2) | |
| Not estimated | 61 (22.5) | 25 (17.6) | 36 (27.7) | |
| Cause of non-traumatic cardiac arrest (%) | 0.019 | |||
| Acute coronary syndrome - STEMI - NSTE-ACS | 103 (37.9) 60 43 | 65 (45.8) # 40 25 | 38 (29.2) # 20 18 | 0.005 # |
| Arrhythmia | 76 (27.9) | 32 (22.5) | 44 (33.8) | |
| Asphyxia | 28 (10.3) | 11 (7.7) | 17 (13.1) | |
| Other | 65 (23.9) | 34 (23.9) | 31 (23.9) | |
| Pre-Clinical Loading N = 142 | No Loading N = 130 | p-Value * | |
| Patients with bleeding complication, n (%) | 38 (26.8) | 41 (31.5) | 0.740 |
| RBC transfusion, n patients (%) Mean number of RBC transfusion | 36 (25.4) 2.4 [±7] | 37 (28.5) 2.7 [±7] | 0.587 0.722 |
| Intracranial bleeding, n | 4 | 7 | 0.553 |
| ICU stay, mean | 6.3 [±5.5] | 7 [±5.8] | 0.557 |
| Hospital stay, mean | 14 [±14.5] | 11.1 [±10.8] | 0.07 |
| Survival to hospital discharge, % total group | 56.3 | 40.3 | 0.008 |
| Favorable neurological outcome at hospital discharge, % of survivors | 80.7 | 62.6 | 0.003 |
| Pre-Clinical Loading N = 40 | No Loading N = 20 | p-Value * | |
|---|---|---|---|
| Patients with bleeding complication, n (%) | 10 (25) | 11 (55) | 0.212 |
| RBC transfusion, n patients Mean number of RBC transfusion | 10 (25) 2 [±6.1] | 10 (50) 4.8 [±8.1] | 0.053 0.139 |
| Intracranial bleeding, n | 0 | 1 | 0.429 |
| ICU stay, mean | 6.8 [±3.3] | 12.2 [±9.8] | 0.327 |
| Hospital stay, mean | 13.9 [±9.4] | 14.9 [±12.7] | 0.725 |
| Survival to hospital discharge, % | 77.5 | 60 | 0.156 |
| Favorable neurological outcome at hospital discharge, % of survivors | 94.1 | 82.4 | 0.318 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macherey-Meyer, S.; Heyne, S.; Meertens, M.M.; Braumann, S.; Niessen, S.F.; Baldus, S.; Lee, S.; Adler, C. Outcome of Out-of-Hospital Cardiac Arrest Patients Stratified by Pre-Clinical Loading with Aspirin and Heparin: A Retrospective Cohort Analysis. J. Clin. Med. 2023, 12, 3817. https://doi.org/10.3390/jcm12113817
Macherey-Meyer S, Heyne S, Meertens MM, Braumann S, Niessen SF, Baldus S, Lee S, Adler C. Outcome of Out-of-Hospital Cardiac Arrest Patients Stratified by Pre-Clinical Loading with Aspirin and Heparin: A Retrospective Cohort Analysis. Journal of Clinical Medicine. 2023; 12(11):3817. https://doi.org/10.3390/jcm12113817
Chicago/Turabian StyleMacherey-Meyer, Sascha, Sebastian Heyne, Max M. Meertens, Simon Braumann, Stephan F. Niessen, Stephan Baldus, Samuel Lee, and Christoph Adler. 2023. "Outcome of Out-of-Hospital Cardiac Arrest Patients Stratified by Pre-Clinical Loading with Aspirin and Heparin: A Retrospective Cohort Analysis" Journal of Clinical Medicine 12, no. 11: 3817. https://doi.org/10.3390/jcm12113817
APA StyleMacherey-Meyer, S., Heyne, S., Meertens, M. M., Braumann, S., Niessen, S. F., Baldus, S., Lee, S., & Adler, C. (2023). Outcome of Out-of-Hospital Cardiac Arrest Patients Stratified by Pre-Clinical Loading with Aspirin and Heparin: A Retrospective Cohort Analysis. Journal of Clinical Medicine, 12(11), 3817. https://doi.org/10.3390/jcm12113817





