Early Prediction of Mortality after Birth Asphyxia with the nSOFA
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
- (a)
- documented severe acidosis with a pH ≤ 7.00;
- (b)
- base excess of ≤ −16 mmol/l (blood from umbilical cord or neonate within the first 60 min of life);
- (c)
- Apgar score ≤ 5 at 10 min of life;
- (d)
- prolonged cardiorespiratory support (at least 10 min), consisting of chest compressions, epinephrine, intubation, bag and mask ventilation, or continuous positive airway pressure (CPAP).
- (a)
- encephalopathy (lethargy, stupor, coma) plus at least one of the following signs: (a1) muscular hypotonia, (a2) abnormal reflexes, or (a3) clinical seizures;
- (b)
- post-2020 modified Sarnat score ≥ 5 (hypoxic–ischemic encephalopathy grade II and III);
- (c)
- clinical seizures;
- (d)
- pathologic amplitude integrates electroencephalogram (aEEG, discontinuous normal voltage, low voltage, burst-suppression, flat trace, or electroencephalographic seizures).
2.2. Therapeutic Hypothermia
2.3. Calculation of nSOFA
2.4. Endpoint: Death
2.5. Statistical Analysis
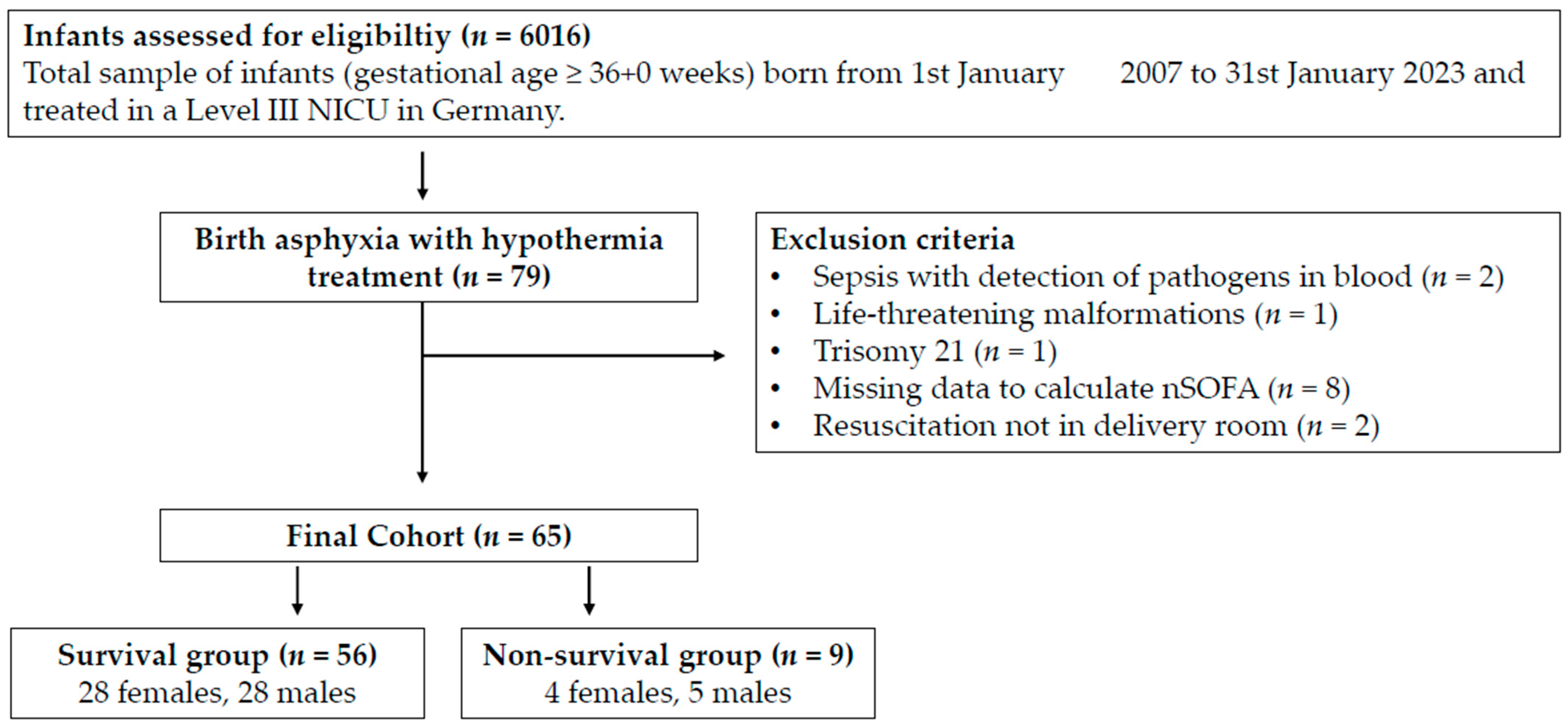
3. Results
3.1. Clinical Characteristics
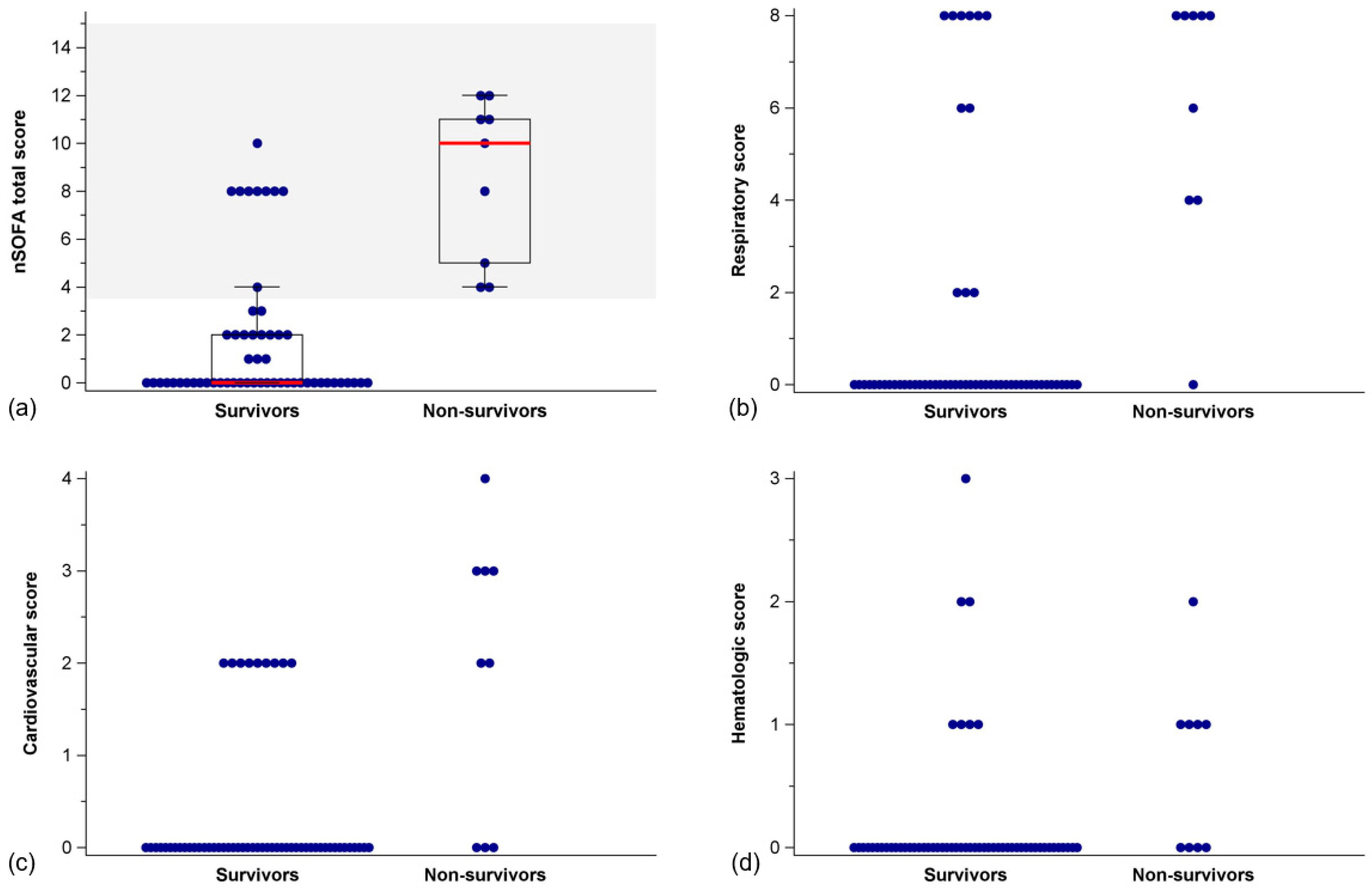
3.2. nSOFA Scores in Survivors and Non-Survivors
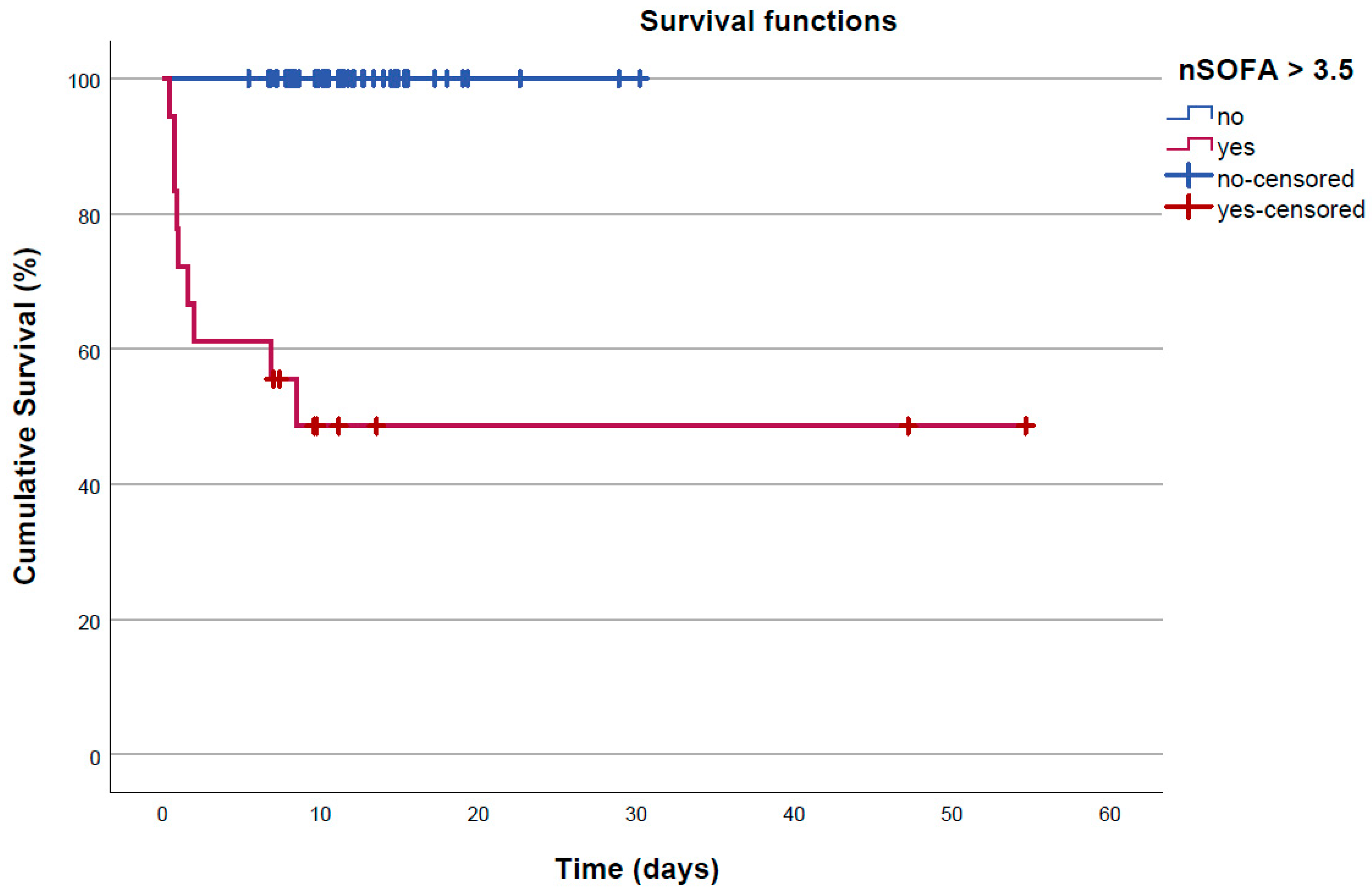
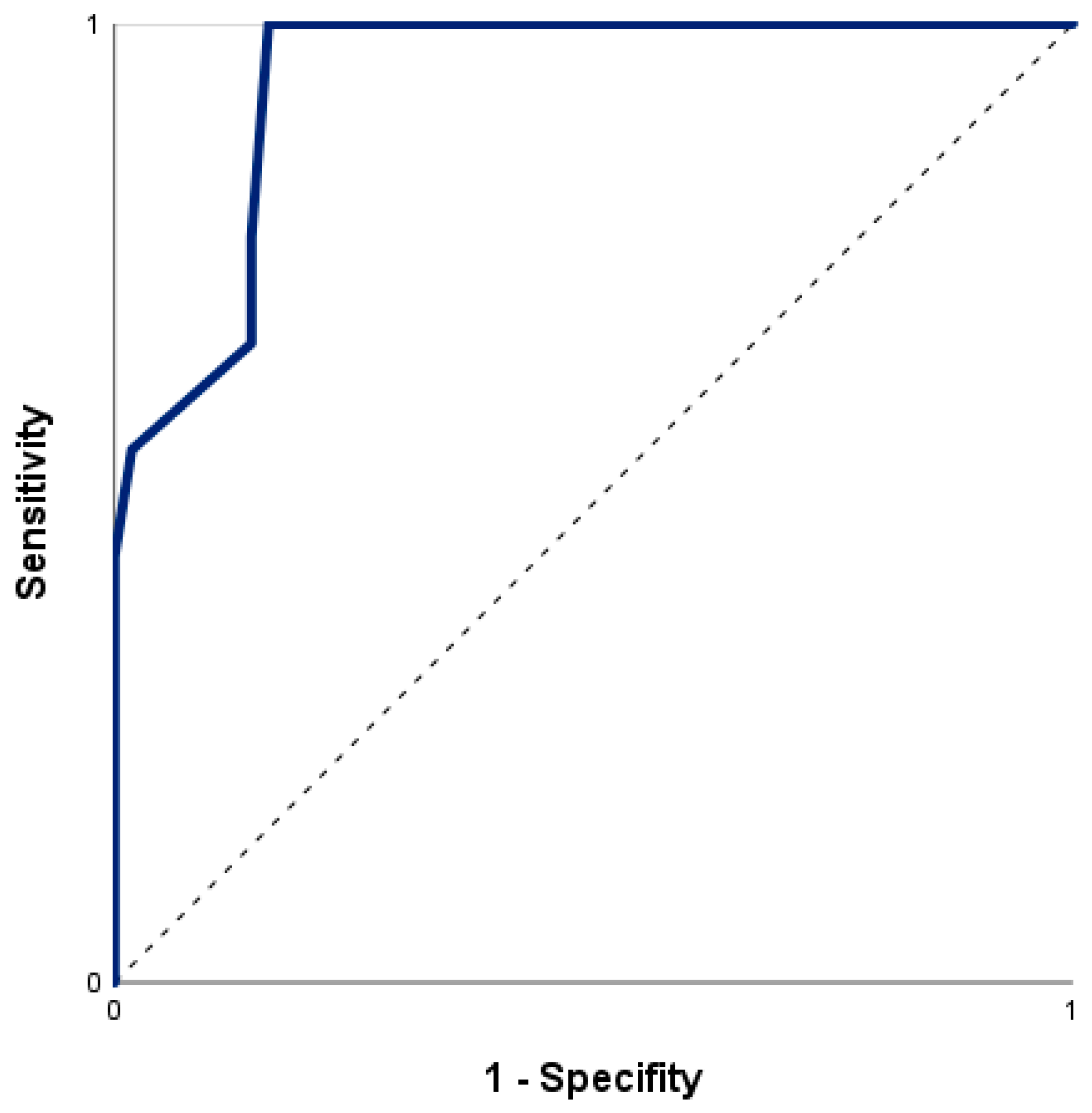
3.3. Prediction of In-Hospital Mortality by nSOFA
4. Discussion
4.1. Prediction of In-Hospital Mortality
4.2. Respiratory and Cardiovascular Sub-Scores
4.3. Hematologic Sub-Score
4.4. Decision Making
4.5. Available Biomarkers and Clinical Assessments
4.6. Strengths and Limitations
5. Future Aspects and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perlman, J.M.; Risser, R. Cardiopulmonary resuscitation in the delivery room. Associated clinical events. Arch. Pediatr. Adolesc. Med. 1995, 149, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Madar, J.; Roehr, C.C.; Ainsworth, S.; Ersdal, H.; Morley, C.; Rudiger, M.; Skare, C.; Szczapa, T.; Te Pas, A.; Trevisanuto, D.; et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 2021, 161, 291–326. [Google Scholar] [CrossRef] [PubMed]
- Rainaldi, M.A.; Perlman, J.M. Pathophysiology of Birth Asphyxia. Clin. Perinatol. 2016, 43, 409–422. [Google Scholar] [CrossRef]
- Aziz, K.; Chadwick, M.; Baker, M.; Andrews, W. Ante- and intra-partum factors that predict increased need for neonatal resuscitation. Resuscitation 2008, 79, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J. Neonatal encephalopathy: An inadequate term for hypoxic-ischemic encephalopathy. Ann. Neurol. 2012, 72, 156–166. [Google Scholar] [CrossRef]
- Sarnat, H.B.; Sarnat, M.S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch. Neurol. 1976, 33, 696–705. [Google Scholar] [CrossRef]
- World Health Organization. Perinatal Asphyxia. Available online: https://www.who.int/teams/maternal-newborn-child-adolescent-health-and-ageing/newborn-health/perinatal-asphyxia (accessed on 26 January 2023).
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000-15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef]
- McIntyre, S.; Nelson, K.B.; Mulkey, S.B.; Lechpammer, M.; Molloy, E.; Badawi, N.; Newborn Brain Society Guidelines and Publications Committee. Neonatal encephalopathy: Focus on epidemiology and underexplored aspects of etiology. Semin. Fetal Neonatal Med. 2021, 26, 101265. [Google Scholar] [CrossRef]
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef]
- Tagin, M.A.; Woolcott, C.G.; Vincer, M.J.; Whyte, R.K.; Stinson, D.A. Hypothermia for neonatal hypoxic ischemic encephalopathy: An updated systematic review and meta-analysis. Arch. Pediatr. Adolesc. Med. 2012, 166, 558–566. [Google Scholar] [CrossRef]
- Simbruner, G.; Mittal, R.A.; Rohlmann, F.; Muche, R.; neo.nEURO.network Trial Participants. Systemic hypothermia after neonatal encephalopathy: Outcomes of neo.nEURO.network RCT. Pediatrics 2010, 126, e771–e778. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, D.V.; Strohm, B.; Edwards, A.D.; Dyet, L.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; Levene, M.; Marlow, N.; Porter, E.; et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 2009, 361, 1349–1358. [Google Scholar] [CrossRef]
- Shankaran, S.; Laptook, A.R.; Ehrenkranz, R.A.; Tyson, J.E.; McDonald, S.A.; Donovan, E.F.; Fanaroff, A.A.; Poole, W.K.; Wright, L.L.; Higgins, R.D.; et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 2005, 353, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Morley, C.J.; Inder, T.E.; Stewart, M.J.; Smith, K.R.; McNamara, P.J.; Wright, I.M.; Kirpalani, H.M.; Darlow, B.A.; Doyle, L.W.; et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: A randomized controlled trial. Arch. Pediatr. Adolesc. Med. 2011, 165, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jary, S.; Cowan, F.; Thoresen, M. Reduced infancy and childhood epilepsy following hypothermia-treated neonatal encephalopathy. Epilepsia 2017, 58, 1902–1911. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Ritter, S.; Brotschi, B.; Werner, H.; Caflisch, J.; Martin, E.; Latal, B. Long-term neurodevelopmental outcome with hypoxic-ischemic encephalopathy. J. Pediatr. 2013, 163, 454–459. [Google Scholar] [CrossRef]
- Ahearne, C.E.; Boylan, G.B.; Murray, D.M. Short and long term prognosis in perinatal asphyxia: An update. World J. Clin. Pediatr. 2016, 5, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; Sepsis Definitions Task Force. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Wynn, J.L.; Polin, R.A. A neonatal sequential organ failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. Pediatr. Res. 2020, 88, 85–90. [Google Scholar] [CrossRef]
- Berka, I.; Korcek, P.; Janota, J.; Stranak, Z. Neonatal Sequential Organ Failure Assessment (nSOFA) Score within 72 Hours after Birth Reliably Predicts Mortality and Serious Morbidity in Very Preterm Infants. Diagnostics 2022, 12, 1342. [Google Scholar] [CrossRef]
- Fleiss, N.; Coggins, S.A.; Lewis, A.N.; Zeigler, A.; Cooksey, K.E.; Walker, L.A.; Husain, A.N.; de Jong, B.S.; Wallman-Stokes, A.; Alrifai, M.W.; et al. Evaluation of the Neonatal Sequential Organ Failure Assessment and Mortality Risk in Preterm Infants with Late-Onset Infection. JAMA Netw. Open 2021, 4, e2036518. [Google Scholar] [CrossRef]
- Shi, S.; Guo, J.; Fu, M.; Liao, L.; Tu, J.; Xiong, J.; Liao, Q.; Chen, W.; Chen, K.; Liao, Y. Evaluation of the neonatal sequential organ failure assessment and mortality risk in neonates with respiratory distress syndrome: A retrospective cohort study. Front. Pediatr. 2022, 10, 911444. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, M.; Kumar, N. Utility of Neonatal Sequential Organ Failure Assessment (nSOFA) Score for Neonatal Mortality prediction. J. Neonatol. 2022, 36, 189–193. [Google Scholar] [CrossRef]
- Polderman, K.H. Mechanisms of action, physiological effects, and complications of hypothermia. Crit. Care Med. 2009, 37, S186–S202. [Google Scholar] [CrossRef]
- Szakmar, E.; Jermendy, A.; El-Dib, M. Correction: Respiratory management during therapeutic hypothermia for hypoxic-ischemic encephalopathy. J. Perinatol. 2019, 39, 891. [Google Scholar] [CrossRef]
- Giannakis, S.; Ruhfus, M.; Markus, M.; Stein, A.; Hoehn, T.; Felderhoff-Mueser, U.; Sabir, H. Mechanical Ventilation, Partial Pressure of Carbon Dioxide, Increased Fraction of Inspired Oxygen and the Increased Risk for Adverse Short-Term Outcomes in Cooled Asphyxiated Newborns. Children 2021, 8, 430. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.; Garnier, Y.; Berger, R. Dynamics of fetal circulatory responses to hypoxia and asphyxia. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 84, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.D.; Baer, V.L.; Yaish, H.M. Thrombocytopenia in late preterm and term neonates after perinatal asphyxia. Transfusion 2015, 55, 187–196. [Google Scholar] [CrossRef]
- Boutaybi, N.; Razenberg, F.; Smits-Wintjens, V.E.; van Zwet, E.W.; Rijken, M.; Steggerda, S.J.; Lopriore, E. Neonatal thrombocytopenia after perinatal asphyxia treated with hypothermia: A retrospective case control study. Int. J. Pediatr. 2014, 2014, 760654. [Google Scholar] [CrossRef] [PubMed]
- Valeri, C.R.; Feingold, H.; Cassidy, G.; Ragno, G.; Khuri, S.; Altschule, M.D. Hypothermia-induced reversible platelet dysfunction. Ann. Surg. 1987, 205, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Barks, J.D.; Bhagat, I.; Donn, S.M. Effects of therapeutic hypothermia on multiorgan dysfunction in asphyxiated newborns: Whole-body cooling versus selective head cooling. J. Perinatol. 2009, 29, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Al Amrani, F.; Racine, E.; Shevell, M.; Wintermark, P. Death after Birth Asphyxia in the Cooling Era. J. Pediatr. 2020, 226, 289–293. [Google Scholar] [CrossRef]
- Murray, D.M.; Boylan, G.B.; Ryan, C.A.; Connolly, S. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics 2009, 124, e459–e467. [Google Scholar] [CrossRef]
- Thayyil, S.; Chandrasekaran, M.; Taylor, A.; Bainbridge, A.; Cady, E.B.; Chong, W.K.; Murad, S.; Omar, R.Z.; Robertson, N.J. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: A meta-analysis. Pediatrics 2010, 125, e382–e395. [Google Scholar] [CrossRef]
- Dorling, J.S.; Field, D.J.; Manktelow, B. Neonatal disease severity scoring systems. Arch. Dis. Childhood Fetal Neonatal Ed. 2005, 90, F11–F16. [Google Scholar] [CrossRef]
- Garg, B.; Sharma, D.; Farahbakhsh, N. Assessment of sickness severity of illness in neonates: Review of various neonatal illness scoring systems. J. Matern. Fetal Neonatal Med. 2018, 31, 1373–1380. [Google Scholar] [CrossRef]
- Mrelashvili, A.; Russ, J.B.; Ferriero, D.M.; Wusthoff, C.J. The Sarnat score for neonatal encephalopathy: Looking back and moving forward. Pediatr. Res. 2020, 88, 824–825. [Google Scholar] [CrossRef]
| Component | nSOFA Scores | ||||
|---|---|---|---|---|---|
| Respiratory score | 0 | 2 | 4 | 6 | 8 |
| Not intubated or intubated SpO2/FiO2 ratio ≥ 300 | Intubated SpO2/FiO2 ratio < 300 | Intubated SpO2/FiO2 ratio < 200 | Intubated SpO2/FiO2 ratio < 150 | Intubated SpO2/FiO2 ratio < 100 | |
| Cardiovascular score | 0 | 1 | 2 | 3 | 4 |
| No systemic corticosteroids and no inotropes | Systemic corticosteroid treatment but no inotropes | 1 inotrope and no systemic corticosteroids | ≥2 inotropes or 1 inotrope and systemic corticosteroids | ≥2 inotropes and systemic corticosteroids | |
| Hematologic score | 0 | 1 | 2 | 3 | |
| Platelet count ≥150/nL | Platelet count 100–149/nL | Platelet count <100/nL | Platelet count <50/nL | ||
| Infants (n = 65) | |
|---|---|
| Block 1 Birth asphyxia criteria | |
| (a) Severe acidosis, pH ≤ 7.00 a | 56 (91.8) c |
| (b) Base excess ≤ −16 mmol/L a | 42 (68.9) c |
| (c) Apgar 10 min ≤ 5 | 22 (34.4) d |
| (d) Prolonged delivery room resuscitation b | 59 (96.7) c |
| Number of criteria block 1: | |
| One | 6 |
| Two | 17 |
| >two | 42 |
| Block 2 Hypoxic–ischemic encephalopathy criteria | |
| (a) Encephalopathy (lethargy, stupor, coma) plus | 60 (92.3) |
| (a1) Muscular hypotonia | 51 (78.5) |
| (a2) Abnormal reflexes | 58 (89.2) |
| (a3) Clinical seizures | 3 (4.6) |
| (b) Sarnat score ≥ 5 | 34 (52.3) |
| (c) Clinical seizures | 3 (4.6) |
| (d) Pathologic aEEG | 40 (70.0) e |
| Number of criteria block 2: | |
| One | 15 |
| Two | 31 |
| >two | 20 |
| Survivors (n = 56) | Non-Survivors (n = 9) | |
|---|---|---|
| Gestational age, weeks | 39 + 3 [36 + 0–41 + 4] | 38 + 6 [37 + 2–41 + 2] |
| Weight at birth, grams | 3170 [1745–4400] | 3380 [2600–4400] |
| SGA, n (%) | 12 (21.4) | 1 (11.1) |
| Height at birth, cm | 50.0 [42.0–58.0] | 52.5 [50.0–56.0] f |
| Head circumference at birth, cm | 34.0 [30.0–38.0] g | 34.8 [31.5–38.0] f |
| Female, n (%) | 28 (50.0) | 4 (44.4) |
| Multiple birth, n (%) | 1 (1.8) | 0 (0) |
| C-section, n (%) | 28 (50) | 8 (88.9) |
| Perinatal sentinel event a, n (%) | 22 (39.3) | 7 (77.8) |
| Umbilical cord pH (SD) | 6.98 [6.61–7.28] h | 7.05 [6.59–7.29] i |
| First pH from infant b | 6.93 [6.75–7.19] j | 6.74 [6.41–7.23] |
| Umbilical cord base excess | −15.25 [−33.90–−4.20] k | −16.70 [−23.00–−5.00] l |
| First base excess from infant b | −17.90 [−28.00–−9.10] m | −25.15 [−31.70–−8.70] f |
| Apgar 1 min | 2 [0–6] g | 0 [0–1] |
| Apgar 5 min | 5 [0–8] g | 0 [0–4] |
| Apgar 10 min | 7 [1–10] g | 0 [0–4] |
| Delivery room resuscitation c, n (%) | 13 (23.2) | 7 (77.7) |
| Respiratory support d, n (%) | 55 (98.2) | 9 (100) |
| Intubation, n (%) | 23 (41.0) | 9 (100) |
| Mask ventilation via T-piece, n (%) | 28 (50.0) | 0 (0) |
| CPAP, n (%) | 3 (5.4) | 0 (0) |
| Chest compressions, n (%) | 3 (5.3) | 0 (0) |
| Epinephrine, n (%) | 4 (7.3) g | 6 (66.7) |
| Sarnat score e | 11 [5–15] n | 20.5 [18–23] o |
| Sarnat score ≥ 5 (reconstructed) e, n (%) | 25 (45.5) g | 9 (100) |
| Seizure activity in aEEG, n (%) Before TH, n (%) | 2 (3.6) g | 0 (0) |
| During TH, n (%) | 16 (29.1) g | 5 (62.5) f |
| Time to death or discharge, days | 11.29 [5.43–54.66] | 0.97 [0.42–8.50] |
| Survivors (n = 56) | Non-Survivors (n = 9) | p-Value | |
|---|---|---|---|
| nSOFA total score | 0 [0–2] | 10 [4.5–11.5] | <0.001 |
| Respiratory score | 0 [0–0] | 8 [4–8] | <0.001 |
| Intubation, n (%) | 23 (41.1%) | 9 (100.0%) | <0.001 |
| SpO2/FiO2 ratio | 447.6 [246.9–461.9] a | 79.0 [55.0–178.3] | <0.001 |
| Cardiovascular score | 0 [0–0] | 2 [2,3] | <0.001 |
| One inotrope, n (%) | 9 (16.1%) | 2 (22.2%) | 0.642 |
| Two or more inotropes, n (%) | 0 (0%) | 4 (44.4%) | <0.001 |
| Systemic steroids, n (%) | 0 (0%) | 1 (11.1%) | 0.138 |
| Hematologic score | 0 [0–0] | 1 [1] | 0.003 |
| Platelets/nL | 237 [180–279] | 132 [110–242] | 0.077 |
| Survivors | Non-Survivors | Total | ||
|---|---|---|---|---|
| nSOFA at least 3.5 | no | 47 | 0 | 47 |
| yes | 9 | 9 | 18 | |
| 56 | 9 | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dathe, A.-K.; Stein, A.; Bruns, N.; Craciun, E.-D.; Tuda, L.; Bialas, J.; Brasseler, M.; Felderhoff-Mueser, U.; Huening, B.M. Early Prediction of Mortality after Birth Asphyxia with the nSOFA. J. Clin. Med. 2023, 12, 4322. https://doi.org/10.3390/jcm12134322
Dathe A-K, Stein A, Bruns N, Craciun E-D, Tuda L, Bialas J, Brasseler M, Felderhoff-Mueser U, Huening BM. Early Prediction of Mortality after Birth Asphyxia with the nSOFA. Journal of Clinical Medicine. 2023; 12(13):4322. https://doi.org/10.3390/jcm12134322
Chicago/Turabian StyleDathe, Anne-Kathrin, Anja Stein, Nora Bruns, Elena-Diana Craciun, Laura Tuda, Johanna Bialas, Maire Brasseler, Ursula Felderhoff-Mueser, and Britta M. Huening. 2023. "Early Prediction of Mortality after Birth Asphyxia with the nSOFA" Journal of Clinical Medicine 12, no. 13: 4322. https://doi.org/10.3390/jcm12134322
APA StyleDathe, A.-K., Stein, A., Bruns, N., Craciun, E.-D., Tuda, L., Bialas, J., Brasseler, M., Felderhoff-Mueser, U., & Huening, B. M. (2023). Early Prediction of Mortality after Birth Asphyxia with the nSOFA. Journal of Clinical Medicine, 12(13), 4322. https://doi.org/10.3390/jcm12134322







