A Systematic Review on Combined [18F]FDG and 68Ga-SSA PET/CT in Pulmonary Carcinoid
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Data Collection
3. Results
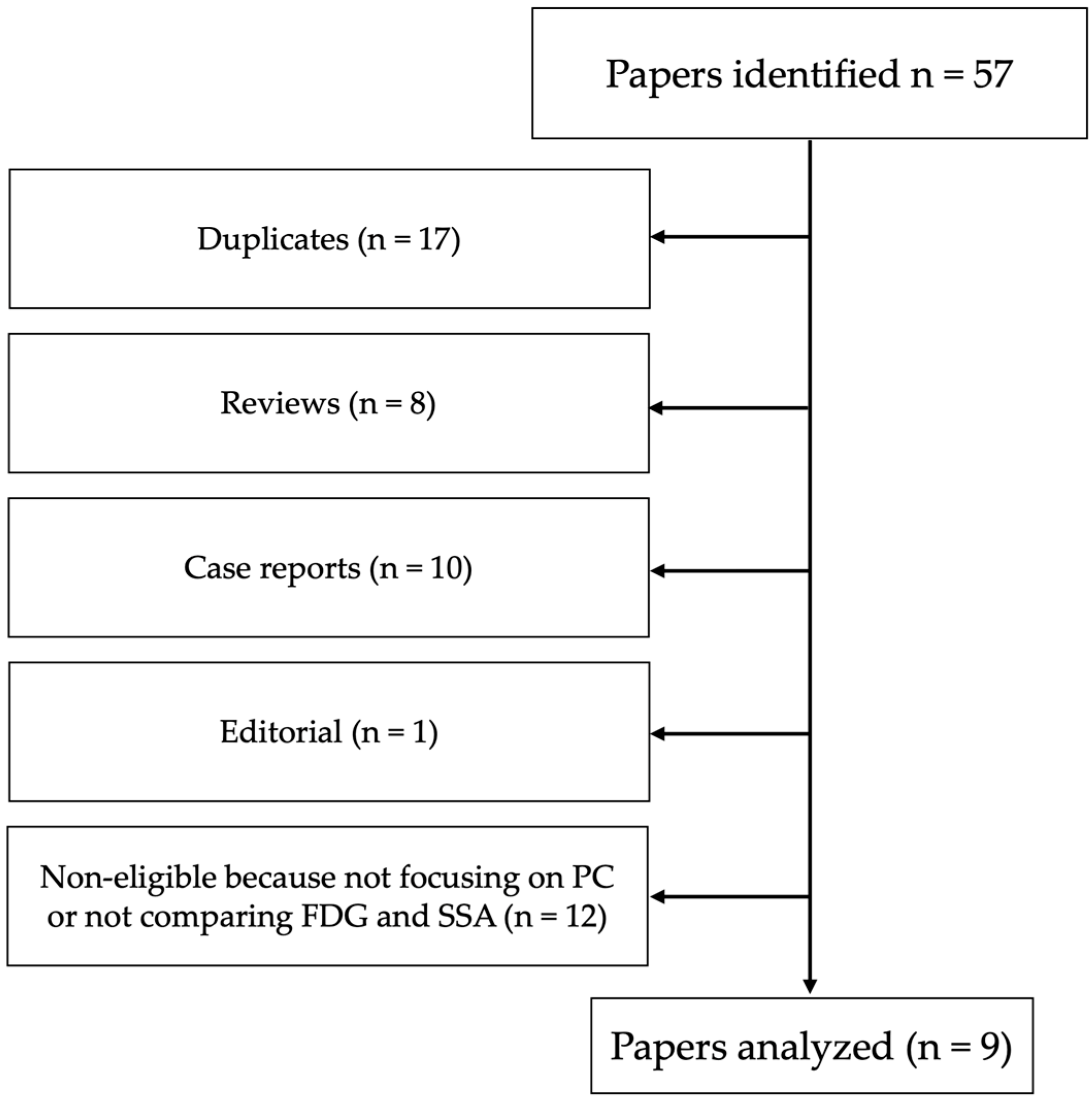
3.1. Search Results
3.2. Radiological Imaging of Pulmonary Carcinoids
3.3. Conventional Somatostatin Receptors Imaging
3.4. [18F]FDG PET/CT Imaging
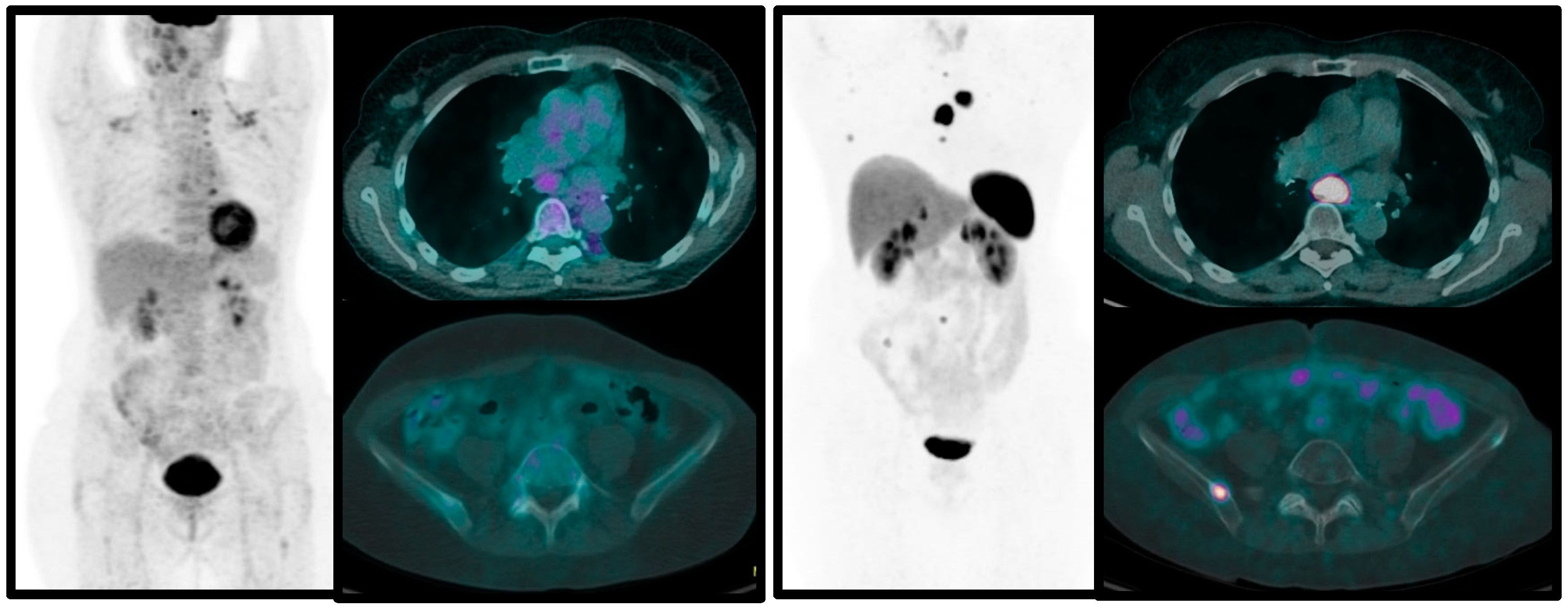
3.5. Combined 68Ga-SSA and [18F]FDG PET/CT in Pulmonary Carcinoids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caplin, M.E.; Baudin, E.; Ferolla, P.; Filosso, P.; Garcia-Yuste, M.; Lim, E.; Oberg, K.; Pelosi, G.; Perren, A.; Rossi, R.E.; et al. Pulmonary neuroendocrine (carcinoid) tumors: European Neuroendocrine Tumor Society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoids. Ann Oncol. 2015, 26, 1604–1620. [Google Scholar] [CrossRef]
- Faggiano, A.; Ferolla, P.; Grimaldi, F.; Campana, D.; Manzoni, M.; Davì, M.V.; Bianchi, A.; Valcavi, R.; Papini, E.; Giuffrida, D.; et al. Natural history of gastro-entero-pancreatic and thoracic neuroendocrine tumors. Data from a large prospective and retrospective Italian epidemiological study: The NET management study. J. Endocrinol. Investig. 2012, 35, 817–823. [Google Scholar]
- Cameselle-Teijeiro, J.M.; Mato Mato, J.A.; Fernández Calvo, O.; García Mata, J. Neuroendocrine Pulmonary Tumors of Low, Interme-diate and High Grade: Anatomopathological Diagnosis-Prognostic and Predictive Factors. Mol. Diagn. Ther. 2018, 22, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Metovic, J.; Barella, M.; Bianchi, F.; Hofman, P.; Hofman, V.; Remmelink, M.; Kern, I.; Carvalho, L.; Pattini, L.; Sonzogni, A.; et al. Morphologic and molecular classification of lung neuroendocrine neoplasms. Virchows Arch. 2021, 478, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Florisson, D.; Telianidis, S.; Yaftian, N.; Lee, J.; Knight, S.; Barnett, S.; Seevanayagam, S.; Antippa, P.; Alam, N.; et al. Pulmonary carcinoid tumours: A multi-centre analysis of survival and predictors of outcome following sublobar, lobar, and extended pulmonary resections. Asian Cardiovasc. Thorac. Ann. 2021, 29, 532–540. [Google Scholar] [CrossRef]
- Evangelista, L.; Ravelli, I.; Bignotto, A.; Cecchin, D.; Zucchetta, P. Ga-68 DOTA-peptides and F-18 FDG PET/CT in patients with neuroendocrine tumor: A review. Clin. Imaging. 2020, 67, 113–116. [Google Scholar] [CrossRef]
- Jindal, T.; Kumar, A.; Venkitaraman, B.; Meena, M.; Kumar, R.; Malhotra, A.; Dutta, R. Evaluation of the role of [18F]FDG-PET/CT and [68Ga]DOTATOC-PET/CT in differentiating typical and atypical pulmonary carcinoids. Cancer Imaging 2011, 11, 70–75. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Kayani, I.; Conry, B.G.; Groves, A.M.; Win, T.; Dickson, J.; Caplin, M.; Bomanji, J.B. A comparison of 68Ga-DOTATATE and 18F-FDG PET/CT in pulmonary neuroendocrine tumors. J. Nucl. Med. 2009, 50, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Venkitaraman, B.; Karunanithi, S.; Kumar, A.; Khilnani, G.C.; Kumar, R. Role of 68Ga-DOTATOC PET/CT in initial evaluation of patients with suspected bronchopulmonary carcinoid. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 856–864. [Google Scholar] [CrossRef]
- Lococo, F.; Perotti, G.; Cardillo, G.; De Waure, C.; Filice, A.; Graziano, P.; Rossi, G.; Sgarbi, G.; Stefanelli, A.; Giordano, A.; et al. Multicenter comparison of 18F-FDG and 68Ga-DOTA-peptide PET/CT for pulmonary carcinoid. Clin. Nucl. Med. 2015, 40, e183–e189. [Google Scholar] [CrossRef]
- Lococo, F.; Rapicetta, C.; Mengoli, M.C.; Filice, A.; Paci, M.; Di Stefano, T.; Coruzzi, C.; Versari, A. Diagnostic performances of 68Ga-DOTATOC versus 18Fluorodeoxyglucose positron emission tomography in pulmonary carcinoid tumours and interrelationship with histological features. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Komek, H.; Can, C.; Urakçi, Z.; Kepenek, F. Comparison of (18F)FDG PET/CT and (68Ga)DOTATATE PET/CT imaging methods in terms of detection of histological subtype and related SUVmax values in patients with pulmonary carcinoid tumors. Nucl. Med. Commun. 2019, 40, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Zidan, L.; Iravani, A.; Kong, G.; Akhurst, T.; Michael, M.; Hicks, R.J. Theranostic implications of molecular imaging phenotype of well-differentiated pulmonary carcinoid based on 68Ga-DOTATATE PET/CT and 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 204–216. [Google Scholar] [CrossRef]
- Deleu, A.L.; Laenen, A.; Decaluwé, H.; Weynand, B.; Dooms, C.; De Wever, W.; Jentjens, S.; Goffin, K.; Vansteenkiste, J.; Van Laere, K.; et al. Value of [68Ga]Ga-somatostatin receptor PET/CT in the grading of pulmonary neuroendocrine (carcinoid) tumours and the detection of disseminated disease: Single-centre pathology-based analysis and review of the literature. EJNMMI Res. 2022, 12, 28. [Google Scholar] [CrossRef]
- Albano, D.; Dondi, F.; Bauckneht, M.; Albertelli, M.; Durmo, R.; Filice, A.; Versari, A.; Morbelli, S.; Berruti, A.; Bertagna, F. The diagnostic and prognostic role of combined [18F]FDG and [68Ga]-DOTA-peptides PET/CT in primary pulmonary carcinoids: A multicentric experience. Eur. Radiol. 2022, 33, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Baudin, E.; Caplin, M.; Garcia-Carbonero, R.; Fazio, N.; Ferolla, P.; Filosso, P.L.; Frilling, A.; De Herder, W.W.; Hörsch, D.; Knigge, U.; et al. Lung and thymic carcinoids: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 439–451. [Google Scholar] [CrossRef]
- MacMahon, H.; Naidich, D.P.; Goo, J.M.; Lee, K.S.; Leung, A.N.C.; Mayo, J.R.; Mehta, A.C.; Ohno, Y.; Powell, C.A.; Prokop, M.; et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017, 284, 228–243. [Google Scholar] [CrossRef]
- Sundin, A.; Vullierme, M.P.; Kaltsas, G.; Plöckinger, U. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Radiological, Nuclear Medicine & Hybrid Imaging. Neuroendocrinology 2017, 105, 212–244. [Google Scholar]
- Bombardieri, E.; Ambrosini, V.; Aktolun, C.; Baum, R.P.; Bishof-Delaloye, A.; Del Vecchio, S.; Maffioli, L.; Mortelmans, L.; Oyen, W.; Pepe, G.; et al. Oncology Committee of the EANM. 111In-pentetreotide scintigraphy: Procedure guidelines for tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 441–448. [Google Scholar]
- Castaldi, P.; Rufini, V.; Treglia, G.; Bruno, I.; Perotti, G.; Stifano, G.; Barbaro, B.; Giordano, A. Impact of 111In-DTPA-octreotide SPECT/CT fusion images in the management of neuroendocrine tumors. Radiol. Med. 2008, 113, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.; Decristoforo, C.; Maina, T.; Nock, B.; von Guggenberg, E.; Cordopatis, P.; Moncayo, R. 99mTc-N4-[Tyr3]Octreotate Versus 99mTc-EDDA/ HYNIC-[Tyr3]Octreotide: An intrapatient comparison of two novel Technetium-99m labeled tracers for somatostatin receptor scintigraphy. Cancer Biother. Radiopharm. 2004, 19, 73–79. [Google Scholar] [CrossRef]
- Pavlovic, S.; Artiko, V.; Sobic-Saranovic, D.; Damjanovic, S.; Popovic, B.; Jakovic, R.; Petrasinovic, Z.; Jaksic, E.; Todorovic-Tirnanic, M.; Saranovic, D.; et al. The utility of 99mTc-EDDA/HYNICTOC scintigraphy for assessment of lung lesions in patients with neuroendocrine tumors. Neoplasma 2010, 57, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Czepczyński, R.; Parisella, M.G.; Kosowicz, J.; Mikołajczak, R.; Ziemnicka, K.; Gryczyńska, M.; Sowiński, J.; Signore, A. Somatostatin receptor scintigraphy using 99mTc-EDDA/HYNIC-TOC in patients with medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, M.; Decristoforo, C.; Kendler, D.; Dobrozemsky, G.; Heute, D.; Uprimny, C.; Kovács, P.; Von Guggenberg, E.; Bale, R.; Virgolini, I.J. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptors scintigraphy and CT. J. Nucl. Med. 2007, 48, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Barrio, M.; Czernin, J.; Fanti, S.; Ambrosini, V.; Binse, I.; Du, L.; Eiber, M.; Herrmann, K.; Fendler, W.P. The Impact of Somatostatin Receptor-Directed PET/CT on the Management of Patients with Neuroendocrine Tumor: A Systematic Review and Meta-Analysis. J. Nucl. Med. 2017, 58, 756–761. [Google Scholar] [CrossRef]
- Lee, I.; Paeng, J.C.; Lee, S.J.; Shin, C.S.; Jang, J.Y.; Cheon, G.J.; Lee, D.S.; Chung, J.K.; Kang, K.W. Comparison of Diagnostic Sensitivity and Quantitative Indices between 68Ga-DOTATOC PET/CT and 111In-Pentetreotide SPECT/CT in Neuroendocrine Tumors: A Preliminary Report. Nucl. Med. Mol. Imaging 2015, 49, 284–290. [Google Scholar] [CrossRef]
- Van Binnebeek, S.; Vanbilloen, B.; Baete, K.; Terwinghe, C.; Koole, M.; Mottaghy, F.M.; Clement, P.M.; Mortelmans, L.; Bogaerts, K.; Haustermans, K.; et al. Comparison of diagnostic accuracy of (111)In-pentetreotide SPECT and (68)Ga-DOTATOC PET/CT: A lesion-by-lesion analysis in patients with metastatic neuroendocrine tumours. Eur. Radiol. 2016, 26, 900–909. [Google Scholar] [CrossRef]
- Ambrosini, V.; Kunikowska, J.; Baudin, E.; Bodei, L.; Bouvier, C.; Capdevila, J.; Cremonesi, M.; de Herder, W.W.; Dromain, C.; Falconi, M.; et al. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur. J. Cancer 2021, 146, 56–73. [Google Scholar] [CrossRef]
- Rinzivillo, M.; Prosperi, D.; Bartolomei, M.; Panareo, S.; Iannicelli, E.; Magi, L.; Panzuto, F. Efficacy of Lutetium-Peptide Receptor Radionuclide Therapy in Inducing Prolonged Tumour Regression in Small-Bowel Neuroendocrine Tumours: A Case of Favourable Response to Retreatment after Initial Objective Response. Oncol. Res. Treat. 2021, 44, 276–280. [Google Scholar] [CrossRef]
- Rivinzillo, M.; Panzuto, F.; Esposito, G.; Lahner, E.; Signore, A.; Annibale, B. Usefulness of 68-Gallium PET in Type I Gastric Neuroendocrine Neoplasia: A Case Series. J. Clin. Med. 2022, 11, 1641. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, M.F.; Virgolini, I.; Balogova, S.; Beheshti, M.; Rubello, D.; Decristoforo, C.; Ambrosini, V.; Kjaer, A.; Delgado-Bolton, R.; Kunikowska, J.; et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1588–1601. [Google Scholar] [CrossRef]
- Cives, M.; Pellè, E.; Rinzivillo, M.; Prosperi, D.; Tucci, M.; Silvestris, F.; Panzuto, F. Bone Metastases in Neuroendocrine Tumors: Molecular Pathogenesis and Implications in Clinical Practice. Neuroendocrinology 2021, 111, 207–216. [Google Scholar] [CrossRef]
- Briganti, V.; Cuccurullo, V.; Berti, V.; Di Stasio, G.D.; Linguanti, F.; Mungai, F.; Mansi, L. 99mTc-EDDA/HYNIC-TOC is a New Opportunity in Neuroendocrine Tumors of the Lung (and in other Malignant and Benign Pulmonary Diseases). Curr. Radiopharm. 2020, 13, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Carideo, L.; Prosperi, D.; Panzuto, F.; Magi, L.; Pratesi, M.S.; Rinzivillo, M.; Annibale, B.; Signore, A. Role of Combined [68Ga]Ga-DOTA-SST Analogues and [18F]FDG PET/CT in the Management of GEP-NENs: A Systematic Review. J. Clin. Med. 2019, 8, 1032. [Google Scholar] [CrossRef]
- Ambrosini, V.; Castellucci, P.; Rubello, D.; Nanni, C.; Musto, A.; Allegri, V.; Montini, G.C.; Mattioli, S.; Grassetto, G.; Al-Nahhas, A.; et al. 68Ga-DOTA-NOC: A new PET tracer for evaluating patients with bronchial carcinoid. Nucl. Med. Commun. 2008, 30, 281–286. [Google Scholar] [CrossRef]
- Jiang, Y.; Hou, G.; Cheng, W. The utility of 18F-FDG and 68Ga-DOTA-Peptide PET/TC. In the evaluation of primary pulmonary carcinoid A systematic review and meta-analysis. Medicine 2019, 98, e14769. [Google Scholar] [CrossRef]
- Wei, L.; Ren, X.; Zhao, Y.; Wang, L.; Zhao, Y. Facilitative glucose transporters: Expression, distribution and the relationship to diseases. Sheng Li Xue Bao 2019, 71, 350–360. [Google Scholar] [PubMed]
- Mamede, M.; Higashi, T.; Kitaichi, M.; Ishizu, K.; Ishimori, T.; Nakamoto, Y.; Yanagihara, K.; Li, M.; Tanaka, F.; Wada, H.; et al. [18F]FDG uptake and PCNA, Glut-1, and hexokinase-II expressions in cancers and inflammatory lesions of the lung. Neoplasia 2005, 7, 369–379. [Google Scholar] [CrossRef]
- Prosperi, D.; Gentiloni Silveri, G.; Panzuto, F.; Faggiano, A.; Russo, V.M.; Caruso, D.; Polici, M.; Lauri, C.; Filice, A.; Laghi, A.; et al. Nuclear Medicine and Radiological Imaging of Pancreatic Neuroendocrine Neoplasms: A Multidisciplinary Update. J. Clin. Med. 2022, 11, 6836. [Google Scholar] [CrossRef]
- Magi, L.; Prosperi, D.; Lamberti, G.; Marasco, M.; Ambrosini, V.; Rinzivillo, M.; Campana, D.; Gentiloni, G.; Annibale, B.; Signore, A.; et al. Role of 18-F -FDG PET/CT in the managment of G1 gastenteropancreatic neuroendocrine tumors. Endocrine 2022, 76, 484–490. [Google Scholar] [CrossRef]
- Binderup, T.; Knigge, U.; Loft, A.; Federspiel, B.; Kjaer, A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin. Cancer Res. 2010, 16, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Anzola, L.; Lauri, C.; Granados, C.; Laganà, B.; Signore, A. Uptake pattern of [68Ga]Ga-DOTA-NOC in tissues: Implications for inflammatory diseases. Q. J. Nucl. Med. Mol. Imaging 2022, 66, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.; Anzola Fuentes, L.C.; Chianelli, M. Somatostatin receptors Scintigraphy in Inflammation and infection imaging. In Somatostatin Analogues: From Research to Clinical Practice; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Del Gobbo, A.; Pellegrinelli, A.; Gaudioso, G.; Castellani, M.; Zito Marino, F.; Franco, R.; Palleschi, A.; Nosotti, M.; Bosari, S.; Vaira, V.; et al. Analysis of NSCLC tumour heterogeneity, proliferative and 18F-FDG PET indices reveals Ki67 prognostic role in adenocarcinomas. Histopathology 2016, 68, 746–751. [Google Scholar] [CrossRef] [PubMed]

| Reference | Patients | Research Type | Histology | Radiopharmaceuticals |
|---|---|---|---|---|
| Kayani I et al. [9] | 13 | Retrospective | 11 TC, 2 AC | 68Ga-DOTA-TATE and [18F]FDG |
| Jindal T et al. [7] | 20 | Retrospective | 13 TC, 7 AC | 68Ga-DOTA-TOC and [18F]FDG |
| Venkitaraman B et al. [10] | 26 | Prospective | 21 TC, 5 AC | 68Ga-DOTA-TOC and [18F]FDG |
| Lococo F et al. [11] | 33 | Retrospective | 23 TC, 10 AC | 68Ga-DOTA-TATE/NOC/TOC and [18F]FDG |
| Lococo F et al. [12] | 62 | Retrospective | 55 TC, 7 AC | 68Ga-DOTA-TOC and [18F]FDG |
| Komek H et al. [13] | 20 | Retrospective | 13 TC, 7 AC | 68Ga-DOTA-TATE and [18F]FDG |
| Zidan L et al. [14] | 56 | Retrospective | 22 TC, 34 AC | 68Ga-DOTA-TATE and [18F]FDG |
| Deleu AL et al. [15] | 64 | Retrospective | 52 TC, 12 AC | 68Ga-DOTA-TATE/TOC and [18F]FDG |
| Albano D et al. [16] | 61 | Retrospective | 35 TC, 26 AC | 68Ga-DOTA-TATE and [18F]FDG |
| Title | Comments and Conclusions | Reference |
|---|---|---|
| A comparison of 68Ga-DOTA-TATE and [18F]FDG in pulmonary neuroendocrine tumors | Role of combined imaging with 68Ga-DOTA-TATE and [18F]FDG in PCs, correlating uptake values with metabolic grade and histology (TCs vs. ACs) | Kayani I et al. [9] |
| Evaluation of the role of [18F]FDG and 68Ga-DOTA-TOC in differentiating typical and atypical pulmonary carcinoids | The analysis of tumor uptake parameters on [18F]FDG and 68Ga-DOTA-TOC was able to differentiate between typical and atypical carcinoids | Baudin E et al. [7] |
| The diagnostic and prognostic role of combined [18F]FDG and 68Ga-DOTA-peptides in primary pulmonary carcinoids: a multicentric experience | Importance of dual tracer approach with 68Ga-DOTA-TATE and [18F]FDG to stratify patients in order to predict histological results (TC or AC) and give prognostic information | Albano D et al. [16] |
| Multicenter comparison of [18F]FDG and 68Ga-DOTA-peptide for pulmonary carcinoid | DR evaluation of 68Ga-DOTA-peptide and [18F]FDG in PC, with proposal of some semi-quantitative parameters to distinguish between TCs and ACs | Lococo F et al. [11] |
| Diagnostic performances of 68Ga-DOTA-TOC versus 18Fluorodeoxyglucose positron emission tomography in pulmonary carcinoid tumors and interrelationship with histological features | Diagnostic ability of [18F]FDG and 68Ga-DOTA-TOC in PCs and correlation of the results with histopathology after surgery | Lococo F et al. [12] |
| Comparison of [18F]FDG and 68Ga-DOTA-TATE imaging methods in terms of detection of histological subtype and related SUVmax values in patients with pulmonary carcinoid tumors | Combined use of 1[18F]FDG and 68Ga-DOTA-TATE in patients with PCs; DR analysis with a focus on histological prediction (TC vs. AC) | Komek H et al. [13] |
| Value of 68Ga-somatostatin in the grading of pulmonary neuroendocrine (carcinoid) tumors and the detection of disseminated disease: single-center pathology-based analysis and review of the literature | Importance of dual tracer approach to differentiate between ACs and TCs, highlighting the excellent performance of SSTR PET/CT to detect nodal and distant metastases confirmed by pathological analysis | Deleu AL et al. [15] |
| Role of 68Ga-DOTA-TOC in initial evaluation of patients with suspected bronchopulmonary carcinoid | Showing the superior diagnostic performance of 68Ga-DOTA-TOC compared to [18F]FDG in patients with suspect PC, and correlation with histopathology | Venkitaraman B et al. [10] |
| Theranostic implications of molecular imaging phenotype of well-differentiated pulmonary carcinoid based on 68Ga-DOTA-TATE and [18F]FDG | Introducing some scores to define tumor heterogeneity thanks to the combined approach with [18F]FDG and 68Ga-DOTA-TATE, in order to have the optimal selection of patients with PC suitable for PRRT | Zidan L et al. [14] |
| SSTR | Positive Human Tumors | Cell Line | Affinity for 68Ga-SSA |
|---|---|---|---|
| SSTR 1 | Breast carcinoma, Paragangliomas, Prostate cancer, Sarcomas, Inactive pituitary adenomas, GEP-NET, Pheochromocytomas, Gastric cancer, and Ependymomas | Colon cancer (with neuroendocrine features), and Gastric cancer | Low |
| SSTR 2 | Neuroblastomas, Meningiomas, Medulloblastomas, Breast cancer, Lymphomas, Renal cell carcinomas, Paragangliomas, Small cell lung cancer, Hepatomas Sarcomas, Inactive pituitary adenomas, GH-secreting adenomas, GEP-NET, Pheochromocytomas, and Gastric cancer | Breast cancer, Colon cancer, Gastric cancer, and Glioblastoma | Very high |
| SSTR 3 | Paragangliomas (low density), Inactive pituitary adenomas, GH-secreting adenomas (low density), and GEP-NET | Lung cancer (squamous), and Colon cancer (with neuroendocrine features) | High |
| SSTR 4 | Sarcomas | Monoblastic leukemia, and Breast cancer | Low |
| SSTR 5 | Lymphomas (low density), Prostate cancer (low density), Inactive pituitary adenomas, GH-secreting adenomas, GEP-NET, Pheochromocytomas (low density), Gastric cancer, and Ependymomas | Breast cancer, Gastric cancer, and Colon cancer | High |
| GLUT | Tissue Distribution | Sub-Cellular Localization | Substrate Selectivity | Up-Regulated Expression |
|---|---|---|---|---|
| GLUT 1 | Widely distributed, and highly expressed especially in erythrocytes, brain, testis and kidney | Cell membrane | Glucose, dehydriascorbic acid | Tumor cells, endothelial cells, renal cells, skeletal muscle cells, adipocytes, hepatocytes, and brain |
| GLUT 2 | Widely distributed, and highly expressed in erythrocytes, brain, testis and kidney | Cell membrane | Glucose, and fructose | Unclear |
| GLUT 3 | Widely distributed, and highly expressed in erythrocytes, brain, testis and kidney | Cell membrane | Glucose | Tumor cell, and pancreatic cells |
| GLUT 4 | Adipose tissue, skeletal, and cardiac muscle | Cell membrane | Insulin | Skeletal muscle cells |
| GLUT 5 | Small intestine, kidney, testis, and adipose cell | Cell membrane | Fructose | Tumor cells |
| GLUT 6 | Brain, spleen, immune cells, and adipose cell | Cell membrane | Glucose | Brain |
| GLUT 7 | Small intestine and colon, testis and prostate | Cell membrane | Glucose and fructose | Small intestine tissue |
| GLUT 8 | Brain (hippocampus), testis, epencephalon, and adrenal glands | Cell membrane, and endoplasmic reticulum | Glucose, fructose and trehalose | Neuronal cells and tumor cells |
| GLUT 9 | Liver, kidneys, small intestine, leukocytes, and chondrocytes | Cell membrane | Uric acid | Ovarian granular cells |
| GLUT 10 | Liver and pancreas | Cell membrane, endoplasmic reticulum | Glucose | Brain tissue |
| GLUT 11 | Skeletal muscles, heart, kidneys, adipose tissue, placenta, and pancreas | Cell membrane | Glucose | Unclear |
| GLUT 12 | Skeletal muscles, heart, placenta, and prostate | Cell membrane and intracellular organelles | Glucose, fructose and galactose | Mammary tumor cells |
| GLUT 13 | Cerebrum, adipose tissue, and kidneys | Cell membrane and vesicles | Inositol | Unclear |
| GLUT 14 | Testis | Cell membrane | Unclear | Unclear |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prosperi, D.; Carideo, L.; Russo, V.M.; Meucci, R.; Campagna, G.; Lastoria, S.; Signore, A. A Systematic Review on Combined [18F]FDG and 68Ga-SSA PET/CT in Pulmonary Carcinoid. J. Clin. Med. 2023, 12, 3719. https://doi.org/10.3390/jcm12113719
Prosperi D, Carideo L, Russo VM, Meucci R, Campagna G, Lastoria S, Signore A. A Systematic Review on Combined [18F]FDG and 68Ga-SSA PET/CT in Pulmonary Carcinoid. Journal of Clinical Medicine. 2023; 12(11):3719. https://doi.org/10.3390/jcm12113719
Chicago/Turabian StyleProsperi, Daniela, Luciano Carideo, Vincenzo Marcello Russo, Rosaria Meucci, Giuseppe Campagna, Secondo Lastoria, and Alberto Signore. 2023. "A Systematic Review on Combined [18F]FDG and 68Ga-SSA PET/CT in Pulmonary Carcinoid" Journal of Clinical Medicine 12, no. 11: 3719. https://doi.org/10.3390/jcm12113719
APA StyleProsperi, D., Carideo, L., Russo, V. M., Meucci, R., Campagna, G., Lastoria, S., & Signore, A. (2023). A Systematic Review on Combined [18F]FDG and 68Ga-SSA PET/CT in Pulmonary Carcinoid. Journal of Clinical Medicine, 12(11), 3719. https://doi.org/10.3390/jcm12113719







