Abstract
Intermittent fasting (IF) is an emerging dietetic intervention that has been associated with improved metabolic parameters. Nowadays, the most common IF protocols are Alternate-Day Fasting (ADF) and Time-Restricted Fasting (TRF), but in this review and meta-analysis we have also considered Religious Fasting (RF), which is similar to TRF but against the circadian rhythm. The available studies usually include the analysis of a single specific IF protocol on different metabolic outcomes. Herein, we decided to go further and to conduct a systematic review and meta-analysis on the advantages of different IF protocols for metabolic homeostasis in individuals with different metabolic status, such as with obesity, diabetes mellitus type 2 (T2D) and metabolic syndrome (MetS). Systematic searches (PubMed, Scopus, Trip Database, Web of Knowledge and Embase, published before June 2022) of original articles in peer-review scientific journals focusing on IF and body composition outcomes were performed. Sixty-four reports met the eligibility criteria for the qualitative analysis and forty-seven for the quantitative analysis. Herein, we showed that ADF protocols promoted the major beneficial effects in the improvement of dysregulated metabolic conditions in comparison with TRF and RF protocols. Furthermore, obese and MetS individuals are the most benefited with the introduction of these interventions, through the improvement of adiposity, lipid homeostasis and blood pressure. For T2D individuals, IF impact was more limited, but associated with their major metabolic dysfunctions—insulin homeostasis. Importantly, through the integrated analysis of distinct metabolic-related diseases, we showed that IF seems to differently impact metabolic homeostasis depending on an individual’s basal health status and type of metabolic disease.
1. Introduction
Intermittent fasting (IF) defines eating patterns in which individuals switch between extended periods of fasting and normal eating, on a recurring basis. IF comprises two main dietary regimens, one that includes fasting for entire days (1 to 4 per week), with a 60–100% energy restriction on fasting days (between 300 and 600 kcal) with ad libitum energy consumption on fed days, and another comprising daily time-restricted eating (fasting in a daily window, from 12 to 20 h). Essentially, these two regimens rely in approaches with both less frequent long fasting periods and short-term but frequent fasting periods []. Within these two main IF protocols, it is possible to describe four different types of fasting: (1) Alternate-Day Fasting (ADF) (where no food or energy-containing drinks are consumed for whole days alternating with days with ad libitum eating); (2) modified Alternate-Day Fasting regimens (mADF) (allows consumption of 20 to 25% of energy requirements on fasting days that are interspersed with days without any kind of restriction); (3) Time-Restricted Fasting (commonly TRF) (ad libitum consumption during a certain time frame during the day intercepted with a long period without any energy intake); and (4) religious fasting (RF) (fasting regimens carried out on religious or faith-based grounds). This last group includes some representative IF protocols, such as the Seventh-Day Adventists, which include the long nightly period of fasting, having the last meal of the day in the afternoon, and Ramadan fasting, in which there is no eating or drinking from sunrise to sunset, for one month, and eating occurs during the circadian sleeping phase. Nevertheless, Ramadan protocols (14 to 18 h of fasting per day), like TRF, result in a metabolic shift towards the predominant use of fatty acids as fuel, which lowers body fat [].
The ultimate goal of IF regimens is to promote metabolic alterations with increased ketogenesis, promoting broad-spectrum benefits for health conditions, such as obesity, diabetes mellitus, cardiovascular disease, cancers and neurologic disorders, reviewed in []. The mechanisms underlying IF benefits are not solely due to upregulation of enzymes involved in ketogenesis and fatty acid oxidation (FAO), which consequently allows for the efficient utilization of these substrates as an energy source [], but also to an overall increase in FAO associated with other metabolic adaptations []. The reduction in calorie intake in IF interventions also elicit an energy deficit, which consequently leads to weight and fat mass loss over time [,,,,,]. However, IF’s metabolic effects might be independent of weight loss due to FAO, with evidence showing that IF can improve insulin sensitivity, reduce inflammation and promote cellular repair and autophagy, which are mechanisms that help the body to cleanse and repair damaged cells [,]. Nevertheless, there are still contradictory observations on beneficial effects of IF that could be connected with differences between regimens. We have reviewed evidence related to the effects of different IF protocols on the regulation of metabolic homeostasis, both on healthy individuals and subjects with known metabolic dysregulation: diabetes mellitus type 2 (T2D), obesity and metabolic syndrome (MetS). Both T2D and MetS are closely related to obesity or to an excess of body mass index (BMI) and are also known to be prevented and/or improved by diet and lifestyle alterations. MetS is commonly described as a cluster of concomitant conditions, including increased blood pressure, high blood sugar, excess waist fat and abnormal cholesterol or triglyceride levels, increasing the risk of heart disease, stroke and T2D []. This systematic review and meta-analysis go further and analyses not the IF protocols but their effects on metabolic outcomes in individuals that have different metabolic status. Therefore, the primary goal was to identify benefits of IF on diverse parameters related to the optimization of cellular energy status in individuals with different requirements for metabolic homeostasis. Secondary outcomes are related to the assessment of diversity between the effects of IF on different metabolic-related disorders. We hypothesized that IF might impact adiposity, lipid homeostasis, insulin homeostasis and blood pressure differently depending on the basal body metabolic condition. This review and meta-analysis rather than analyzing IF protocols, integrates distinct metabolic disorders, highlighting the relevance of the basal body composition for the positive effects of IF. To our knowledge, this is the first review providing such a comprehensive perspective.
2. Materials and Methods
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement []. This review’s protocol was not registered previously to its submission.
2.1. Search Strategies
A comprehensive literature search was conducted between November 2021 and June 2022 in the following electronic databases: PubMed, Scopus, Trip Database, Web of Knowledge and Embase using the following combination of search keywords: for PubMed—(“fasting” [MeSH Major Topic]) AND ((“obesity” [MeSH Terms]) OR (“overweight” [MeSH Terms]) OR (“metabolic syndrome” [MeSH Terms]) OR (“diabetes mellitus” [MeSH Terms]) OR (“insulin resistance” [MeSH Terms])) and, for Scopus, Trip Database, Web of Knowledge and Embase (“Intermittent fasting”) AND (“obesity” OR “overweight” OR “metabolic syndrome” OR “diabetes” OR “insulin resistance” OR “insulin sensitivity”), from 2000 up to 2022.
2.2. Eligibility
Original articles in peer-review scientific journals focusing on IF and body composition outcomes were retrieved. Studies were included when fulfilling the following criteria: (i) adult participants, regardless of gender; (ii) data related to adiposity (weight, body mass index (BMI), waist circumference); lipid homeostasis (HDL-c, LDL-c, total cholesterol, triglycerides), insulin homeostasis (fasting glucose, fasting insulin, insulin resistance (HOMA-IR)) or blood pressure (Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DBP)); (iii) healthy individuals and/or individuals with metabolic related disorders, such as type 2 diabetes mellitus (T2D), metabolic syndrome (MetS) or obesity; and, (iv) articles presenting pre- and post-intervention measures. For the meta-analysis randomized and non-randomized controlled trials, cohort studies, case-control studies and case series were included.
To avoid significant bias for the systematic review and meta-analysis, studies were excluded when one of the following criteria was observed: (i) non-original articles (systematic reviews, protocols, reviews and book chapters); (ii) articles using animal models; (iii) articles written in languages other than English; (iv) grey literature (conference abstracts, letters to editor); (v) articles where other diets or interventions were mixed, such as IF plus Mediterranean, ketogenic, paleo or vegan diets; (vi) articles on other pathologies or conditions, eating disorders, alcohol abuse, smokers, medications (especially the ones that could affect glucose and lipid profile) and pregnant or breastfeeding women; (vii) results from groups in trials that tested both IF and other diets (such as the ketogenic diet) or that had any other intervention besides IF with or without caloric restriction; (viii) studies with individuals with metabolic related disorders, such as type 2 diabetes mellitus (T2D), metabolic syndrome (MetS) or obesity associated with other severe diseases.
2.3. Study Selection
After removing duplicates, three researchers (AIS, MD and BSM) independently screened the title and abstract of every citation followed by full-text screening against the inclusion/exclusion criteria. To qualify for inclusion, the authors had to be in agreement.
2.4. Outcomes Measures
The main outcomes evaluated were the impact of IF on weight, BMI, waist circumference, HDL-c, LDL-c, total cholesterol, triglycerides, fasting glucose, fasting insulin, HOMA-IR, SBP and DBP. Although the effects of different IF protocols were not subject of analysis in this systematic review, they were briefly summarized for deeper comprehension and future reference. Different IF regimens were considered but all had to fall into one of two main groups: (1) IF with a daily window where no food or energy containing drinks were ingested or (2) whole days of the week with caloric intake greatly reduced or eliminated, as described on the characterization of the included studies.
2.5. Data Extraction, Management and Synthesis
Detailed data were extracted from each study and compiled in a Microsoft Excel spreadsheet by all authors, and included the following information: name of the article, authors, year of publication, the country in which the study took place, study design (randomized controlled trials (RCT), non-randomized controlled trials (NRCT), cohort studies (CS), case-control studies (CCS), case report series (CRS) and cross-section studies (CSS)), IF protocol, study duration, sample size, participant’s characteristics (gender, age and metabolic status) and outcomes measured. Data from the included studies were synthetized in the Table S1 and a narrative summary of the data is presented in the Section 3.
The meta-analysis was conducted using RevMan, version 5.4.1. Separate meta-analyses were performed for each outcome (weight, BMI, waist circumference, total cholesterol, HDL-c, LDL-c, triglycerides, fasting glucose, fasting insulin, HOMA-IR, SBP and DBP), for each IF protocol and per study group (healthy and/or individuals with type 2 diabetes mellitus (T2D), metabolic syndrome (MetS) or obesity).
Taking the heterogeneity of studies into consideration, a random-effects model in which the summary effect size is the mean difference of a distribution was used to aggregate data and to promote the generality of the results. Mean difference was calculated based on sample size, the mean differences (between baseline and fasting conditions) and effect direction. The 95% confidence interval (CI) and corresponding p values were considered as indicators of statistical significance. In an attempt to evaluate the amount of variation in the effects of included studies, we tested for heterogeneity through the (i) the Cochran’s Q statistic [], for which a significant p-value (<0.05) demonstrates that studies do not share common mean differences (i.e., there is heterogeneity in the effect sizes between studies); and (ii) the I2 statistics that assess the proportion of observed dispersion that is due to real differences in the actual mean differences and is not affected by low statistical power. I2 ranges from 0 to 100%, where it is established that a value of 0% indicates no observed heterogeneity and values of 25, 50 and 75%, respectively, reflect low, moderate and high heterogeneity []. Group analyses were conducted to examine whether the effect of IF on body composition outcomes varied according to disease condition.
2.6. Quality Assessment
The risk of bias was not assessed, as this review included different type of studies. The quality of the articles was carefully assessed, according to: (i) the Critical Appraisal Skills Programme (CASP) [] checklist for RCT, CS and CCS; (ii) the Joanna Briggs [] checklist for CRS and CSS; and (iii) the Methodological Index for Non-Randomized Studies (MINORS) [] checklist for NRCT. Taking into consideration that it would be nearly impossible for patients and staff of the study to be blind, items of the checklist concerning this parameter were not considered for the overall assessment of quality.
3. Results
3.1. Search Results
PubMed, Scopus, Embase, Trip database and Web of Science generated 2365 publications (Figure 1). After the removal of 96 duplicates, 2269 publications were identified as potentially eligible. After screening the title and abstract and checking the full text for detailed information and data extraction, 64 publications were included in the systematic review (Table S1) with 47 [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,] being included in the meta-analysis (Figure 1 and Table 1).
3.2. Characteristics of Included Studies
In the systematic review, the population comprised a total of n = 4052 participants of which n = 2007 were men and n = 1977 women. For 68 participants gender was not disclosed. Of the 64 studies, 19 included participants with T2D [,,,,,,,,,,,,,,,,,,], 35 obese or overweight individuals [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,], 7 participants with MetS [,,,,,,] and 6 included healthy participants without any associated pathology [,,,,,] (Table S1).
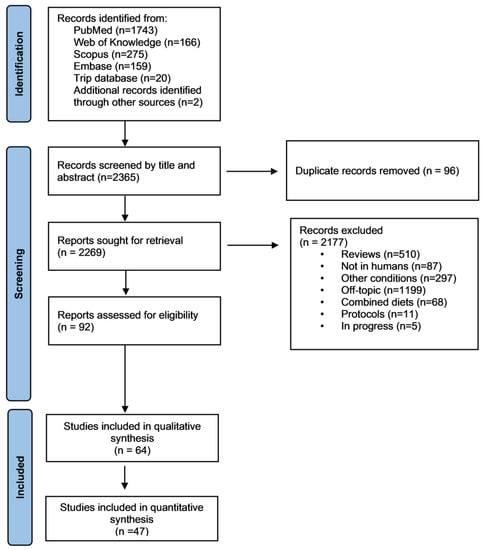
Figure 1.
Flow chart of the methodology followed during the literature search.
Individual characteristics of the 47 studies included in the meta-analyses are summarized in Table 1, which comprises 18 randomized controlled trials (RCT), 1 non-randomized controlled trial (NRCT), 4 cohort studies (CS), 2 case control studies (CCS), 1 cross-sectional study (CSS) and 21 case-report series (CRS). The studies were conducted in distinct countries. Considering the IF protocols, we categorized them in Alternate-Day Fasting (ADF), Time-Restricted Fasting (TRF) and Religious Fasting (RF) as previously described []. The first group-ADF-included the following feeding + fasting protocols: 1 d + 1 d [,,,,], 5 d + 2 d [,,,], 4 d + 3 d [,,] and 1-week fasting []. TRF involved time restricted fasting + feeding protocols: 12 h + 12 h [], 14 h + 10 h [,], 15 h + 9 h [], 16 h + 8 h [,,,,,,], 18 h + 6 h [,], while the RF included exclusively Ramadan fasting [,,,,,,,,,,,,,,,,,,,,]. Importantly, ADF protocols also include the modified Alternate-Day Fasting regimens (mADF), since they may or may not include the possibility of consumption of up to 25% of the caloric needs on fasting days (between 300–600 Kcal).
3.3. Quality Assessment
Table S2 shows the detailed classification of each study quality according to the CASP checklist for RCT, CS and CCS; the Joanna Briggs checklist for CRS and CSS; and the MINORS checklist for NRCT. Briefly, a score of 0 points was attributed when a parameter was not met (red color), 2 points when all the criteria were fulfilled (green color) and 1 for intermediate fulfillment of criteria (yellow color). Articles evaluated with CASP checklists scoring between 0 and 10 points were considered of low quality (“weak”), from 11 to 15 of “moderate” quality, and from 16 to 20 of “good” quality. The same method as before was used the Joanna Briggs checklist for CRS. For CSS, quality was considered weak from 0 to 8, moderate from 9 to 12 and good from 13 to 16. For MINORS checklist, after eliminating the parameter assessing the blindness of the experiment, a total of 11 parameters remained. Parameters were as usual classified with a punctuation between 0 and 2 points, for a total score of 22 points. Studies ranging from 0 to 11 points were classified as low quality, between 12 and 16 of moderate quality and of good quality when a total of 17 points or more were attributed.

Table 1.
Characterization of the studies included in the quantitative analysis synthesis.
Table 1.
Characterization of the studies included in the quantitative analysis synthesis.
| Study Type | Control Conditions | Experimental Conditions | IF Protocol | Duration (Days) | Age (Mean ± SD) | Participants (n and Gender) | Results Overview | |
|---|---|---|---|---|---|---|---|---|
| 1. Guo I, et al., 2021 [] | RCT | MetS | MetS | ADF 5 + 2 (NC) | 56 | CTL = 42.7 ± 4.1 EG = 36.6 ± 5.7 | CTL—11 M/7 W EG—10 M/12 W | Weight, waist circumference, BMI, triglycerides, insulin resistance and fasting insulin were significantly improved after IF. CTL comprises individuals with MetS maintained their habitual diet and lifestyle. |
| 2. Parvaresh A, et al., 2019 [] | RCT | - | MetS | ADF 4 + 3 (NC) | 56 | EG = 44.6 ± 9.8 | EG—21 M/14 W | mADF diet is an effective option for managing body weight, waist circumference, systolic blood pressure and fasting plasma glucose when compared with common calorie restriction. CTL comprises individuals with MetS that consumed 75% of their energy needs each day. |
| 3. Gabel K, et al., 2019 [] | RCT | - | Obesity | ADF 1 + 1 (NC) | 365 | CTL = 43 ± 1 EG = 43 ± 3 | CTL—4 M/11 W EG—9 M/2 W | ADF may produce greater reductions in fasting insulin and insulin resistance compared with caloric restriction in insulin-resistant participants despite similar decreases in body weight. CTL comprises obese individuals that maintained their usual eating and activity habits. |
| 4. Cho A-Ra, et al., 2019 [] | RCT | - | Overweight | ADF 4 + 3 (NC) | 56 | CTL = 42.6 ± 10.6 EG = 33.5 ± 5 | CTL—3 M/2 W EG—3 M/6 W | Exercise, with or without ADF, improves cholesterol metabolism (serum sterol signatures) and increased physical activity has a greater effect on cholesterol biosynthesis than weight reduction or calorie restriction. CTL comprises overweight individuals that maintained their usual eating and activity habits. |
| 5. Corley BT, et al., 2018 [] | RCT | - | T2D | ADF 5 + 2 (C) 5 + 2 (NC) | 84 | 62 (44 to 77) * 58 (42 to 74) * | 11 M/7 W 11 M/8 W | IF in a 5 + 2 protocol (both consecutive and non-consecutive) increases the rate of hypoglycaemia in patients with T2D, even after education on this topic and medication adjustments. Yet it improves weight, HbA1c, fasting glucose and quality of life (both protocols). |
| 6. Hutchison AT, et al., 2018 [] | RCT | Overweight or Obesity | Overweight or Obesity | ADF 4 + 3 (NC) | 70 | CTL 1 = 49 ± 3 CTL 2 = 49 ± 2 EG-IF100 = 51 ± 2 EG-IF70 = 51 ± 2 | CTL 1—11 W CTL 2—11 W EG-IF100—22 W EG-IF70—22 W | IF combined with a 30% caloric restriction is better at improving weight, fat mass, HOMA-IR, fasting glucose, non-esterified fatty acids, total cholesterol, LDL-cholesterol and triglycerides than energy-matched continuous caloric restriction. CTL comprises overweight or obese women maintained under continuous food intake at 100% of baseline energy requirements. |
| 7. Sundfør TM, et al., 2018 [] | RCT | - | Overweight and MetS | ADF 5 + 2 (NC) | 365 | EG = 49.9 ± 10.1 | EG—28 M/26 W | Both intermittent and continuous energy restriction promoted similar weight loss, maintenance and improvement in cardiovascular risk factors after one year. However, the feelings of hunger, appears to be more pronounced during IF. |
| 8. Trepanowski JF, et al., 2018 [] | RCT | - | Obesity | ADF 1 + 1 (NC) | 168 | CTL = 44 ± 2 EG = 46 ± 2 | CTL—4 M/21 W EG—3 M/22 W | ADF and CR similarly increased the fat-free mass:total-mass ratio, decreased circulating leptin and did not affect the visceral adipose tissue:subcutaneous adipose tissue ratio or other measured adipokines. Weight loss, rather than the pattern of energy restriction, appeared to be the main driver of these changes. CTL comprises obese individuals that maintained their usual diet. |
| 9. Conley M, et al., 2018 [] | RCT | - | Obesity | ADF 5 + 2 (NC) | 180 | EG = 68 ± 2.7 | EG—11 M | The 5:2 diet is a feasible weight loss strategy in this older male population. Furthermore, it also indicates that participants were able to follow the diet sufficiently to induce magnitudes of weight loss similar to that of standard dietary modification practices, and the diet did not appear to cause an unbalanced nutritional intake. |
| 10. Li C, et al., 2017 [] | RCT | T2D | T2D | ADF 1 week fasting | 7 | CTL = 64.4 ± 5.7 EG = 64.7 ± 7 | CTL—16 NS EG—16 NS | A 1-week fasting therapy is promising for the improvement of weight, waist circumference, SBP, DBP and HOMA-IR in T2D patients, when compared with typical T2D medication. CTL comprises T2D individuals that followed the principles of a Mediterranean diet. |
| 11. Catenacci VA et al., 2016 [] | RCT | - | Obesity | ADF 1 + 1 (NC) | 56 | EG = 39.6 ± 9.5 | EG—3 M/10 W | ADF is a safe and tolerable approach for weight loss, it improved weight, body composition, lipids and insulin sensitivity index at 8 weeks while not increasing the risk for weight regain up-to 24 weeks after completing the intervention. |
| 12. Hoddy KK, et al., 2014 [] | RCT | - | Obesity | ADF 1 + 1 (NC) | 70 | ADF-L: 45 ± 3 ADF-D: 45 ± 3 ADF-SM: 46 ± 2 | ADF-L: 3 M/17 W ADF-D: 4 M/15 W ADF-SM: 2 M/18 W | This study demonstrated there is flexibility in the timing of the fast day meal during ADF. People with obesity may feed at dinner or as small meals throughout the day, and experience similar weight loss, body composition and cardiovascular benefits as the traditional lunch time approach. |
| 13.Varady KA et al., 2009 [] | CRS | - | Obesity | ADF 1 + 1 (NC) | 56 | EG = 46 ± 2.4 | EG—4 M/12 W | ADF is a viable diet option to help obese individuals lose weight and decrease coronary artery disease risk. |
| 1. Kotarsky CJ, et al., 2021 [] | RCT | Overweight or Obesity | Overweight or Obesity | TRF 16 + 8 | 56 | CTL = 44 ± 2 EG = 45 ± 3 | CTL—9 M/1 W EG—9 M/2 W | IF associated with exercise training is a short-term dietary strategy for reducing fat mass and increasing lean mass in overweight and obese adults. CTL comprises overweight or obese individuals that maintained their usual diet. |
| 2. de Oliveira Maranhao Pureza IR, et al., 2021 [] | RCT | Overweight or Obesity | Overweight or Obesity | TRF 12 + 12 | 365 | 19–44 | EG –31 W | IF may help in the long-term management of obesity. |
| 3. Kunduraci YE, et al., 2020 [] | RCT | MetS | MetS | TRF 16 + 8 | 84 | EG = 47.44 ± 2.17 | EG—16 M/16 W | Weight, BMI, total cholesterol, LDL, triglycerides, fasting glucose, systolic and diastolic blood pressure, insulin resistance and fasting insulin were significantly improved after IF. |
| 4. Cienfuegos S, et al., 2020 [] | RCT | - | Obesity | TRF 20 + 4 18 + 6 | 56 | 4 h_TRF: 49 ± 2 6 h_TRF: 46 ± 3 | 4 h_TRF: 2 M/14 W 6 h_TRF: 1 M/18 W | 4- and 6-h TRF regimens lead to similar weight loss over the 2 months in peolple with obesity while also decreasing insulin resistance and oxidative stress. CTL comprises obese individuals that maintained their usual diet. |
| 5. Jones R, et al., 2020 [] | CCS | Healthy | Healthy | TRF 16 + 8 | 14 | CTL = 23 ± 1 EG = 23 ± 1 | CTL—8 M EG—8 M | Weight showed significant improvement after IF. CTL comprises healthy individuals following a dietary plan provided with all food and beverages that matched the macronutrient composition (45% CHO, 35% fat and 20% protein). |
| 6. Zhao L, et al., 2022 [] | CRS | Overweight | TRF 14 + 10 | 56 | 63 ± 4 | 15 M | This study demostrated that TRF had a net effect of reducing glycemia and dampening energy-consuming pathways in adipose tissue. | |
| 7. Parr EB, et al., 2020 [] | CRS | T2D | T2D | TRF 15 + 9 | 28 | 50.2 ± 8.9 | 9 M/10 W | Fasting insulin showed significant improvements after IF. |
| 8. Gabel K, et al., 2020 [] | CRS | - | Obesity | TRF 16 + 8 | 84 | - | 14 M | This study suggest that the mild weight loss (2%) induced by time restricted eating did not significantly alter the diversity or overall composition of the gut microbiome. |
| 9. Wilkinson MJ, et al., 2020 [] | CRS | - | MetS | TRF 14 + 10 | 84 | 59 ± 11.4 | 15 M/6 W | TRF mproved cardiometabolic health of patients with metabolic syndrome receiving standard medical care including high rates of statin and anti-hypertensive use. |
| 10. Anton SD, et al., 2019 [] | CRS | - | Overweight | TRF 16 + 8 | 21 | 77.1 | 4 M/6 W | TRF is an acceptable and feasible eating pattern for overweight, older adults to follow while also promoting short-term weight loss. |
| 11. Kesztyüs D, et al., 2019 [] | CRS | - | MetS | TRF 16 + 8 | 90 | 49.1± 12,4 | 9 M/31 W | TRE may help to reduce abdominal obesity and hence prevent cardio-metabolic diseases. |
| 12. Arnason FB, et al., 2017 [] | CRS | - | T2D | TRF 18–20 h fasting per day | 14 | 53.8 ± 9.11 | 1 M/9 W | Weight and BMI were improved after IF. |
| 13. Schroder JD, et al., 2021 [] | NRCT | Obesity | Obesity | TRF 16 + 8 | 90 | CTL = 42.3 ± 3.5 EG = 36.6 ± 1.6 | CTL—12 W EG—20 W | Weight, waist circumference, BMI, SBP and DBP were improved after IF. CTL comprises obese individuals that maintained their usual diet. |
| 1. Zouhal H, et al., 2020 [] | RCT | - | Obesity | RF Ramadan | 30 | CTL = 23.8 ± 3.8 EG = 24 ± 3.4 | CTL—14 M EG—14 M | Ramadan fasting improves systemic inflammation biomarkers in males with obesity. CTL comprises obese individuals that did not fast during Ramadan. |
| 2. Zouhal H, et al., 2020 [] | RCT | - | Obesity | RF Ramadan | 30 | CTL = 23.8 ± 3.7 EG = 24.5 ± 3.8 | CTL—15 M EG—15 M | IF during Ramadan is an effective strategy to modify appetite-regulating hormones, leading to improved body composition indices and reduced obesity. |
| 3. Abdullah K, et al., 2020 [] | CS | Healthy | Healthy or T2D | RF Ramadan | 30 | CTL = 34.61 ± 4.31 EG = 34.35 ± 3.83 EG = 50.17 ± 12.95 | CTL—31 M EG—37 M EG—30 M | This study compares the effect of IF in patients with T2D, their first-degree relatives and healthy individuals. Leptin, adiponectin, leptin:adiponectin ratio, HOMA-beta and HbA1c were significantly improved in all groups. Fasting blood glucose and growth hormone levels were improved in control and first-degree relatives. C-peptide, HOMA-IR, HOMA-S and insulin levels were improved in T2D patients and first-degree relatives. CTL comprises healthy control with fasting blood glucose <100 mg/dL. |
| 4. Yeoh ECK, et al., 2015 [] | CS | T2D | RF Ramadan | 30 | 57 ± 11 | 15 M/14 W | Ramadan fasting can be practiced safely with prior patient education and medication adjustment with modest benefits on metabolic profile and body composition, particularly among females. | |
| 5. Feizollahzadeh S, et al., 2014 [] | CS | - | Healthy with Overweight | RF Ramadan | 30 | 47.88 | 70 M | Weight and BMI were improved after IF although total cholesterol, triglycerides and fasting glucose levels were also increased. |
| 6. Karatoprak C, et al., 2013 [] | CS | T2D | RF Ramadan | 30 | 57.4 ± 10.1 | 19 M/57 W | Results showed no negative effects of extended fasting on glucose regulation in diabetic patients using certain medications. | |
| 7. McNeil J, et al., 2014 [] | CCS | Healthy | Obesity | RF Ramadan | 14 | 27 ± 4.5 | 10 M | Data demostrated significant increased of glucose, total cholesterol and LDL-C levels during Ramadan fast in normal-weight and obese men. Dietary restraint scores were also greater during Ramadan. Lastly, changes in anthropometric parameters were related to changes in metabolic profiles, dietary restraint and disinhibition eating behavior trait scores. |
| 8. Kovil R, et al., 2020 [] | CRS | - | T2D | RF Ramadan | 30 | 21–80 ** | 25 M/25 W | This study did not show any significant changes in the parameters evaluated after IF. |
| 9. Faris E, et al., 2019 [] | CRS | - | Overweight or Obesity | RF Ramadan | 30 | 36.2 ± 12.5 | 35 M/22 W | Weight, BMI, total cholesterol, triglycerides and SBP were improved after IF although HDL was also decreased. |
| 10. Madkour MI, et al., 2019 [] | CRS | - | Healthy or Obesity | RF Ramadan | 30 | CTL = 29.8 ± 14 EG = 35.72 ± 12.35 | CTL—6 EG—34 M/22 W | Fasting glucose, insulin, insulin resistance expressed in homeostatic model assessment (HOMA-IR) remained unchanged throughout the study, while significant (p < 0.05) decreases in total cholesterol, triglycerides and HDL cholesterol were observed. |
| 11. Abdessadek M, et al., 2019 [] | CRS | - | T2D | RF Ramadan | 30 | - | 57 M/93 W | This study showed a significant decrease in glycemic parameters (glycated haemoglobin and fasting blood glucose), and also significant variations in lipid profile before and after Ramadan, respectively. Furthermore, it also demostrated that in well-controlled T2D patients under antidiabetic drugs, the risk of hypoglycaemia is very low and patients may fast safely in Ramadan. |
| 12. Prasetya G, et al., 2018 [] | CRS | - | Healthy | RF Ramadan | 29 | 24.3 ± 3.7 | 27 M | Weight, waist circumference, BMI, insulin resistance and fasting insulin were improved after IF although HDL levels were also decreased. |
| 13. Kamble S, et al., 2018 [] | CRS | - | Healthy | RF Ramadan | 39 | 20–35 ** | 30 (NS) | No changes after IF. |
| 14. Sezen Y, et al., 2016 [] | CRS | - | Obesity | RF Ramadan | 30 | 37 ± 7 | 70 M | Ramadan fasting was beneficial for body composition, but had no effect on arterial stiffness and resting heart rate. |
| 15. Gnanou JV, et al., 2015 [] | CRS | - | Healthy | RF Ramadan | 39 | 19–23 ** | 20 M | Weight, BMI, fasting glucose, fasting insulin and insulin resistance were improved after IF. |
| 16. Sahin SB, et al., 2013 [] | CRS | - | T2D | RF Ramadan | 30 | 56.93 ± 9.57 | 40 M/82 W | Fasting during Ramadan did not worsen the glycemic control of T2D patients. |
| 17. Shariatpanahi MV, et al., 2012 [] | CRS | - | MetS | RF Ramadan | 30 | 40.14 ± 10.8 | 65 M | Change in the number and timing of meals and portioning of the daily energy consumption decreases inflammatory markers in MetS. |
| 18. Salehi M and Neghab M, 2007 [] | CRS | - | Obesity | RF Ramadan | 29 | 23.4 ± 1.3 | 28 M | Consumption of a medium calorie balanced diet in conjunction with sufficient fluid intake during Ramadan and fasting may significantly decrease serum levels of glucose, cholesterol, as well as weight and BMI. |
| 19. Khaled MB, et al., 2006 [] | CRS | - | Obesity | RF Ramadan | 29 | 23.4 ± 1.3 | 60 W | Beneficial effect of Ramadan fasting on glucose homeostasis, and an unbalanced profile on lipids. |
| 20. Khatib FA and Shafagoj YA, 2004 [] | CRS | - | T2D/Obesity | RF Ramadan | 29 | 51 ± 10 | 44 M | Non-insulin dependent T2D patients displayed a trend towards better glycemic control following Ramadan fasting. |
| 21. Bener A, et al., 2018 [] | CSS | T2D | RF Ramadan | 30 | 55.39 ± 15.3 | 593 M/653 W | Ramadan fasting has positive effects on T2D patients by decreasing blood pressure, blood glucose and HbA1C levels and BMI. It also improved sleep duration and physical activity. | |
| TOTAL | 1551 M/1491 W/ 68 NS |
RCT—Randomized controlled trial; CS—Cohort study; CRS—Case report series; NRCT—non-randomized controlled trial; CSS—Cross-sectional study; MetS—Metabolic syndrome; T2D—diabetes mellitus type 2; C—consecutive; NC—Non-consecutive; CTL—Control; EG—Experimental Group; NS—Not specified; ADF—alternate-day eating; CR—caloric restriction; TRF—Time-restrict eating; IF70—Intermittent fasting 70%; IF100—Intermittent fasting 100%; M—Men; W—Women; HOMA-IR—insulin resistance; BMI—Body mass index; SBP—Systolic blood pressure; DBP—Diastolic blood pressure; * mean (range); ** range.
Of the 64 studies identified as relevant for this systematic review, the overall methodological quality of 45 was rated as “good” (70.3%), 17 studies as “moderate” (26.5%) and 2 as “weak” (3.1%). Overall, the quality of studies was good, and Table S2 summarizes the proportion of studies that met each criterion. Independently of the study type, the studies that presented good quality met criteria including, per example, the clarity of the study objective, the study randomization, the implications of the studies for practice, among others. The criteria that most of the studies failed to meet was the identification of confounding factors, as well as its interference in the study design, the groups’ randomization and the lack of demographic data (Table S2).
3.4. Meta-Analysis
The meta-analysis results of the effects of IF on the regulation of metabolic homeostasis in health and disease (T2D, MetS and obesity) were based on data from 47 studies selected considering the completeness of the outcomes measured in four main categories: adiposity (weight, BMI, waist circumference), lipid homeostasis (HDL-c, LDL-c, total cholesterol, triglycerides), insulin homeostasis (fasting glucose, fasting insulin, HOMA-IR) and blood pressure (SBP and DBP). According to IF protocols, these metabolic parameters were evaluated independently in 13 ADF, 13 TRF and 21 RF studies.
3.5. Characterization of Participants Included in Each IF Protocol
Changes in body weight, BMI, waist circumference, glucose and total cholesterol of the participants included in this meta-analysis are reported in Table 2. Overall, weight loss, BMI, waist circumference and total cholesterol levels were similar among participants included in each of the IF regimes. Nevertheless, we noticed that participants from RF studies presented higher glucose levels in comparison with the participants that integrate the ADF and TRF studies. This difference could be related to the fact that most of the RF studies were performed with T2D individuals, that by itself present higher glucose levels.

Table 2.
Characteristics of the participants that integrate the studies included in each intermittent fasting (IF) protocols. Mean (SD) values are shown.
3.6. Effects of IF on Regulation of Metabolic Homeostasis
3.6.1. Adiposity
Concerning the adiposity measurements, the analyzed outcomes were weight, BMI and waist circumference. In general, all the tested IF protocols (ADF, TRF and RF) showed significant positive results for the analyzed outcomes, when comparing pre- and post-fasting timepoints (Table 3, Table 4 and Table 5; Figure 2, Figure 3 and Figure 4).
For ADF protocols, a total of 13 studies were included and data showed a significant reduction in the body weight (k = 12, 5.54 (95% CI [4.21, 6.86]), p < 0.00001), BMI (k = 9, 2.50 (95% CI [1.78, 3.21]), p < 0.00001) and waist circumference (k = 7, 5.80 (95% CI [3.87, 7.72]), p < 0.00001), with high heterogeneity between studies (Q = 26.92, p = 0.008, I2 = 55.00%; Q = 18.59, p = 0.05, I2 = 57.00% and Q = 31.19, p < 0.00001, I2 = 78.00%, respectively) (Table 3; Figure 2).
A deeper analysis throughout stratification of the different metabolic conditions, people with obesity, T2D and MetS individuals, revealed that in relation to weight, ADF interventions presented statistical positive effects only for people with obesity (k = 8, 5.82 (95% CI [4.29, 7.36]), p < 0.00001), with high heterogeneity between studies (Q = 26.68, p = 0.0008, I2 = 70.00%) (Table 3; Figure 2).
Concerning BMI, analysis showed that obese and MetS individuals benefit with the ADF intervention (k = 6, 2.83 (95% CI [2.03, 3.64]), p < 0.00001; k = 1, 1.70 (95% CI [0.18, 3.22]), p = 0.03, respectively). Heterogeneity was high for the people with obesity (Q = 13.08, p = 0.02, I2 = 62.00%) (Table 3; Figure 2).
From the seven studies used to estimate the impact of ADF protocols in waist circumference, data showed that ADF decreases abdominal circumference of obese and MetS individuals (k = 3, 6.67 (95% CI [4.24, 9.10]), p < 0.00001; k = 2, 3.52 (95% CI [−0.06, 7.10]), p = 0.05). Studies with obese people displayed high heterogeneity (Q = 28.64, p < 0.00001, I2 = 90.00%), while low heterogeneity was observed for studies performed with MetS individuals (Q = 0.15, p = 0.70, I2 = 0.00%) (Table 3; Figure 2). It is worth noting that with the ADF studies no data related with healthy group were found.
Regarding the analysis of TRF protocol, the 13 assessed studies demonstrated a significant reduction in the body weight (k = 13, 3.05 (95% CI [0.87, 5.23]), p = 0.006), BMI (k = 9, 1.48 (95% CI [0.39, 2.57]), p = 0.008) and waist circumference (k = 8, 3.93 (95% CI [2.64, 5.21]), p < 0.00001), with high heterogeneity between studies for weight and BMI (Q = 46.60, p < 0.00001, I2 = 72.00% and Q = 45.32, p < 0.00001, I2 = 82.00%, respectively) and low heterogeneity for waist circumference studies (Q = 0.98, p = 1.00, I2 = 0.00%) (Table 4; Figure 3).
Specific analysis of each outcome showed that TRF significantly decreases the weight of obese (k = 7, 2.78 (95% CI [1.50, 4.06]), p < 0.00001) and MetS individuals (k = 3, 7.14 (95% CI [3.71, 10.57]), p < 0.00001). Heterogeneity was low in the obesity and MetS groups (Q = 1.39, p = 0.99, I2 = 0.00% and Q = 2.46, p = 0.29, I2 = 19.00%, respectively) (Table 4; Figure 3).
Studies demonstrated that TRF protocols significantly improved BMI in obese (k = 5, 1.01 (95% CI [0.56, 1.46]), p < 0.00001) and MetS (k = 3, 2.01 (95% CI [0.24, 3.78]), p = 0.03) individuals. Heterogeneity was low in obesity group (Q = 2.97, p = 0.56, I2 = 0.00%) and moderate for the MetS group (Q = 4.92, p = 0.09, I2 = 59.00%) (Table 4; Figure 3).
TRF protocols promoted a statistically significant reduction in waist circumference in individuals with obesity (k = 5, 3.89 (95% CI [2.54, 5.23]), p < 0.00001) and MetS (k = 2, 5.01 (95% CI [0.12, 9.90]), p = 0.04). Low heterogeneity was observed between the studies analyzed for the people with obesity (Q = 0.57, p = 0.97, I2 = 0.00%) or MetS (Q = 0.03, p = 0.87, I2 = 0.00%) (Table 4; Figure 3).

Table 3.
Analysis of the impact of alternate-day fasting (ADF) protocols on different outcomes in healthy individuals and/or individuals with metabolic related disorders as type 2 diabetes mellitus (T2D), metabolic syndrome (MetS) or obesity.
Table 3.
Analysis of the impact of alternate-day fasting (ADF) protocols on different outcomes in healthy individuals and/or individuals with metabolic related disorders as type 2 diabetes mellitus (T2D), metabolic syndrome (MetS) or obesity.
| Moderators | k 1 | Point Estimate | CI Lower | CI Upper | p-Value | Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|---|
| Q-Value | p-Value | I-Squared | |||||||
| Adiposity | Weight (kg) | ||||||||
| All studies | 12 | 5.54 | 4.21 | 6.86 | <0.00001 | 26.92 | 0.008 | 55.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 8 | 5.82 | 4.29 | 7.36 | <0.00001 | 26.68 | 0.0008 | 70.00 | |
| T2D | 2 | 3.67 | −3.71 | 11.04 | 0.33 | 0.00 | 0.99 | 0.00 | |
| MetS | 2 | 4.00 | −0.23 | 8.24 | 0.06 | 0.02 | 0.88 | 0.00 | |
| BMI (kg/m2) | |||||||||
| All studies | 9 | 2.50 | 1.78 | 3.21 | <0.00001 | 18.59 | <0.02 | 57.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 6 | 2.83 | 2.03 | 3.64 | <0.00001 | 13.08 | 0.02 | 62.00 | |
| T2D | 2 | 1.09 | −0.61 | 2.79 | 0.21 | 0.04 | 0.84 | 0.00 | |
| MetS | 1 | 1.70 | 0.18 | 3.22 | 0.03 | - | - | - | |
| Waist circumference (cm) | |||||||||
| All studies | 7 | 5.80 | 3.87 | 7.72 | <0.00001 | 31.19 | <0.00001 | 78.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 3 | 6.67 | 4.24 | 9.10 | <0.00001 | 28.64 | <0.00001 | 90.00 | |
| T2D | 2 | 4.12 | −1.09 | 9.33 | 0.12 | 0.04 | 0.84 | 0.00 | |
| MetS | 2 | 3.52 | −0.06 | 7.10 | 0.05 | 0.15 | 0.70 | 0.00 | |
| Lipid homeostasis | HDL-c (mg/dL) | ||||||||
| All studies | 11 | 1.49 | 0.72 | 2.26 | 0.0002 | 803.57 | <0.00001 | 98.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 8 | 1.59 | 0.79 | 2.39 | <0.00001 | 796.57 | <0.00001 | 99.00 | |
| T2D | 2 | −0.51 | −4.25 | 3.24 | 0.79 | 0.84 | 0.36 | 0.00 | |
| MetS | 1 | 1.00 | −2.92 | 4.92 | 0.62 | - | - | - | |
| LDL-c (mg/dL) | |||||||||
| All studies | 12 | 7.06 | 3.19 | 10.93 | 0.0004 | 22,243.31 | <0.00001 | 100.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 8 | 7.67 | 3.51 | 11.83 | 0.0003 | 22,238.46 | <0.00001 | 100.00 | |
| T2D | 2 | 3.49 | −12.02 | 19.00 | 0.66 | 0.01 | 0.94 | 0.00 | |
| MetS | 2 | 3.10 | −7.45 | 13.65 | 0.58 | 0.39 | 0.53 | 0.00 | |
| Total cholesterol (mg/dL) | |||||||||
| All studies | 12 | 13.16 | 3 | 17.16 | <0.00001 | 22,070.51 | <0.00001 | 100.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 8 | 13.87 | 9.62 | 18.12 | <0.00001 | 22,062.59 | <0.00001 | 100.00 | |
| T2D | 2 | 8.84 | −7.79 | 25.48 | 0.30 | 0.77 | 0.38 | 0.00 | |
| MetS | 2 | 7.39 | −5.11 | 19.90 | 0.25 | 0.52 | 0.47 | 0.00 | |
| Triglycerides (mg/dL) | |||||||||
| All studies | 10 | 26.17 | −0.62 | 55.45 | 0.06 | 1134.75 | <0.00001 | 99.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 7 | 25.38 | −4.68 | 55.45 | 0.10 | 1128.37 | <0.00001 | 99.00 | |
| T2D | 2 | 11.20 | −15.84 | 38.24 | 0.42 | 0.23 | 0.63 | 0.00 | |
| MetS | 1 | 52.00 | 9.41 | 94.59 | 0.02 | - | - | - | |
| Insulin homeostasis | Fasting glucose (mg/dL) | ||||||||
| All studies | 10 | 4.33 | −1.14 | 9.79 | 0.12 | 99,283 | <0.00001 | 99.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 7 | 1.14 | −1.89 | 4.17 | 0.46 | 157.83 | <0.00001 | 96.00 | |
| T2D | 2 | 19.17 | 14.63 | 23.72 | <0.00001 | 1.15 | 0.28 | 13.00 | |
| MetS | 1 | 5.00 | 0.71 | 9.29 | 0.02 | - | - | - | |
| Fasting insulin (mU/L) | |||||||||
| All studies | 8 | 2.55 | −0.88 | 5.98 | 0.15 | 330.18 | <0.00001 | 98.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 5 | 2.36 | −1.76 | 6.48 | 0.26 | 329.38 | <0.00001 | 98.00 | |
| T2D | 1 | 3.70 | −5.31 | 12.71 | 0.42 | - | - | - | |
| MetS | 2 | 2.54 | −0.08 | 5.15 | 0.06 | 0.06 | 0.80 | 0.00 | |
| HOMA-IR | |||||||||
| All studies | 6 | 0.90 | −0.14 | 1.95 | 0.09 | 329.27 | <0.00001 | 98.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 4 | 0.88 | −0.33 | 2.10 | 0.16 | 328.54 | <0.00001 | 99.00 | |
| T2D | 1 | 1.50 | −1.63 | 4.63 | 0.53 | - | - | - | |
| MetS | 1 | 0.73 | −0.01 | 1.47 | <0.00001 | - | - | - | |
| Blood pressure | SBP (mmHg) | ||||||||
| All studies | 8 | 6.08 | 4.08 | 8.08 | <0.00001 | 24.27 | 0.004 | 63.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 4 | 5.49 | 3.41 | 7.57 | <0.00001 | 17.68 | 0.003 | 72.00 | |
| T2D | 2 | 8.21 | −1.38 | 17.80 | 0.09 | 1.83 | 0.18 | 45.00 | |
| MetS | 2 | 10.46 | 2.08 | 18.83 | 0.01 | 1.58 | 0.21 | 37.00 | |
| DBP (mmHg) | |||||||||
| All studies | 8 | 3.52 | 2.55 | 4.49 | <0.00001 | 17.14 | 0.05 | 48.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 4 | 3.23 | 2.32 | 4.13 | <0.00001 | 10.19 | 0.07 | 51.00 | |
| T2D | 2 | 5.79 | −0.08 | 11.65 | 0.05 | 1.39 | 0.24 | 28.00 | |
| MetS | 2 | 5.76 | 0.30 | 11.22 | 0.04 | 2.09 | 0.15 | 52.00 | |
1 k—number of studies; MetS—Metabolic syndrome; T2D—diabetes mellitus type 2; HOMA—insulin resistance; BMI—Body mass index; SBP—Systolic Blood Pressure; DBP—Diastolic Blood Pressure.
Regarding RF interventions, results revealed less impact on the adiposity outcomes when compared with ADF or TRF protocols. The RF studies revealed positive effects on reduction in weight in healthy (k = 5, 2.44 (95% CI [0.74, 4.14]), p = 0.005) and obese individuals (k = 6, 3.01 (95% CI [0.92, 5.10]), p = 0.005). Low heterogeneity was observed between the studies analyzed in each group (Table 5; Figure 4).
RF improved BMI in healthy (k = 5, 0.70 (95% CI [0.32, 1.09]), p = 0.0004), obese (k = 7, 0.84 (95% CI [0.43, 1.26]), p < 0.00001) and T2D (k = 8, 1.26 (95% CI [0.98, 1.54]), p < 0.00001) individuals. Heterogeneity was low in the healthy, obesity and T2D groups (Q = 2.46, p = 0.65, I2 = 0.00%; Q = 2.57, p = 0.86, I2 = 0.00% and Q = 7.23, p = 0.70, I2 = 0.00%, respectively (Table 5; Figure 4).
3.6.2. Lipid Homeostasis
To study the impact of the different types of IF on lipid homeostasis, the outcomes analyzed were HDL-c, LDL-c, total cholesterol and triglyceride. ADF, TRF and RF showed distinct impact on the analyzed lipid homeostasis outcomes (Table 3, Table 4 and Table 5; Figure 5, Figure 6 and Figure 7). Starting with the ADF protocols, data collected revealed that this intervention promotes statistically significant positive effects on HDL-c (k = 11, 1.49 (95% CI [0.72, 2.26]), p = 0.0002), LDL-c (k = 12, 7.06 (95% CI [3.19, 10.93]), p = 0.0004) and total cholesterol (k = 12, 13.18 (95% CI [9.16, 17.16]), p < 0.00001) levels. Heterogeneity between studies, related with each outcome, was high (Table 3; Figure 5).
Regarding HDL-c, LDL-c and total cholesterol parameters, only people with obesity presented statistically significant positive effects (k = 8, 1.59 (95% CI [0.79, 2.39]), p < 0.00001; (k = 8, 7.67 (95% CI [3.51, 11.83], p = 0.0003 and k = 8, 13.87 (95% CI [9.62, 18.12, p < 0.00001, respectively) (Table 3). High heterogeneity was observed in studies involving obese group (Q = 796.57, p < 0.00001, I2 = 99.00%; Q = 22,238.46, p < 0.00001, I2 = 100.00% and Q = 22,062.59, p < 0.00001, I2 = 100.00%, respectively) (Table 3; Figure 5).
Finally, ADF protocols also significantly decreased triglycerides levels in MetS participants (k = 1, 52.00 (95% CI [9.41, 94.59]), p = 0.02) (Table 3; Figure 5).

Figure 2.
Forest plot of the data from random effects meta-analysis shown as mean difference with 95% confidence intervals on adiposity outcomes, weight, body mass index (BMI) and waist circumference, for the studies that presented data concerning these parameters. Data presented are related with alternate-day fasting (ADF) and the metabolic conditions: healthy or disease condition, obesity, T2D or MetS. For each study, the square represents the mean difference between baseline and fasting conditions, with the horizontal line intersecting it as the lower and upper limits of the 95% confidence interval [,,,,,,,,,,,].

Figure 3.
Forest plot of the data from random effects meta-analysis shown as mean difference with 95% confidence intervals on adiposity outcomes, weight, body mass index (BMI) and waist circumference, for the studies that presented data concerning these parameters. Data presented are related with time-restricted fasting (TRF) and the metabolic conditions: healthy or disease condition, obesity, T2D or MetS. For each study, the square represents the mean difference between baseline and fasting conditions, with the horizontal line intersecting it as the lower and upper limits of the 95% confidence interval [,,,,,,,,,,,,].

Figure 4.
Forest plot of the data from random effects meta-analysis shown as mean difference with 95% confidence intervals on adiposity outcomes, weight, body mass index (BMI) and waist circumference, for the studies that presented data concerning these parameters. Data presented are related with and religious fasting (RF) and the metabolic conditions: healthy or disease condition, obesity, T2D or MetS. For each study, the square represents the mean difference between baseline and fasting conditions, with the horizontal line intersecting it as the lower and upper limits of the 95% confidence interval [,,,,,,,,,,,,,,,,,,,,].

Table 4.
Analysis of the impact of Time-Restricted Fasting (TRF) protocols on different outcomes in healthy individuals and/or individuals with metabolic related disorders as type 2 diabetes mellitus (T2D), metabolic syndrome (MetS) or obesity.
Table 4.
Analysis of the impact of Time-Restricted Fasting (TRF) protocols on different outcomes in healthy individuals and/or individuals with metabolic related disorders as type 2 diabetes mellitus (T2D), metabolic syndrome (MetS) or obesity.
| Moderators | k 1 | Point Estimate | CI Lower | CI Upper | p-Value | Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|---|
| Q-Value | p-Value | I-Squared | |||||||
| Adiposity | Weight (kg) | ||||||||
| All studies | 13 | 3.05 | 0.87 | 5.23 | 0.006 | 46.60 | <0.00001 | 72.00 | |
| Healthy | 1 | 1.04 | −1.89 | 3.97 | 0.49 | - | - | - | |
| Obesity | 7 | 2.78 | 1.50 | 4.06 | <0.00001 | 1.37 | 0.99 | 0.00 | |
| T2D | 2 | 0.89 | −6.55 | 8.34 | 0.81 | 0.00 | 0.95 | 0.00 | |
| MetS | 3 | 7.14 | 3.71 | 10.57 | <0.00001 | 2.46 | 0.29 | 19.00 | |
| BMI (kg/m2) | |||||||||
| All studies | 9 | 1.48 | 0.39 | 2.57 | 0.008 | 45.32 | <0.0001 | 82.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 5 | 1.01 | 0.56 | 1.46 | <0.00001 | 2.97 | 0.56 | 0.00 | |
| T2D | 1 | 3.50 | −3.69 | 10.69 | 0.34 | - | - | - | |
| MetS | 3 | 2.01 | 0.24 | 3.78 | 0.03 | 4.92 | 0.09 | 59.00 | |
| Waist circumference (cm) | |||||||||
| All studies | 8 | 3.93 | 2.64 | 5.21 | <0.00001 | 0.98 | 1.00 | 0.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 5 | 3.89 | 2.54 | 5.23 | <0.00001 | 0.57 | 0.97 | 0.00 | |
| T2D | 1 | 1.80 | −7.93 | 11.53 | 0.72 | - | - | - | |
| MetS | 2 | 5.01 | 0.12 | 9.90 | 0.04 | 0.03 | 0.87 | 0.00 | |
| Lipid homeostasis | HDL-c (mg/dL) | ||||||||
| All studies | 8 | 0.00 | −0.60 | 0.61 | 0.99 | 8.96 | 0.44 | 0.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 5 | 0.43 | −0.46 | 1.31 | 0.34 | 5.08 | 0.41 | 2.00 | |
| T2D | 1 | 0.90 | −3.36 | 5.16 | 0.72 | - | - | - | |
| MetS | 3 | −0.44 | −1.29 | 0.42 | 0.32 | 1.82 | 0.40 | 0.00 | |
| LDL-c (mg/dL) | |||||||||
| All studies | 7 | 4.48 | −7.60 | 16.56 | 0.47 | 200.73 | <0.00001 | 97.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 3 | −1.47 | −7.32 | 4.38 | 0.62 | 15.32 | 0.002 | 80.00 | |
| T2D | 1 | 3.87 | −17.06 | 24.80 | 0.72 | - | - | - | |
| MetS | 3 | 14.16 | −7.92 | 36.28 | 0.21 | 12.49 | 0.002 | 84.00 | |
| Total cholesterol (mg/dL) | |||||||||
| All studies | 8 | 8.14 | −8.37 | 24.65 | 0.33 | 154.28 | <0.00001 | 95.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 4 | −4.21 | −10.36 | 1.94 | 0.18 | 4.75 | 0.19 | 37.00 | |
| T2D | 1 | 3.80 | −17.13 | 24.73 | 0.72 | - | - | - | |
| MetS | 3 | 28.80 | 25.26 | 32.34 | <0.00001 | 1.98 | 0.37 | 0.00 | |
| Triglycerides (mg/dL) | |||||||||
| All studies | 9 | 8.93 | −1.58 | 19.45 | 0.10 | 80.70 | <0.00001 | 89.00 | |
| Healthy | 1 | 7.08 | −2.08 | 16.24 | 0.13 | - | - | - | |
| Obesity | 4 | 3.41 | −2.80 | 9.63 | 0.28 | 10.66 | 0.03 | 62.00 | |
| T2D | 1 | 0.00 | −42.34 | 42.34 | 1.00 | - | - | - | |
| MetS | 3 | 20.61 | −12.67 | 53.90 | 0.22 | 6.59 | 0.04 | 70.00 | |
| Insulin homeostasis | Fasting glucose (mg/dL) | ||||||||
| All studies | 10 | 5.89 | 2.52 | 9.26 | 0.0006 | 338.62 | <0.00001 | 97.00 | |
| Healthy | 1 | 0.54 | −1.33 | 2.41 | 0.57 | - | - | - | |
| Obesity | 5 | 4.25 | 0.32 | 8.18 | 0.03 | 102.00 | <0.00001 | 95.00 | |
| T2D | 2 | 7.22 | 3.74 | 10.71 | <0.00001 | 13.45 | 0.0002 | 93.00 | |
| MetS | 2 | 13.75 | 7.99 | 19.50 | <0.00001 | 1.73 | 0.19 | 42.00 | |
| Fasting insulin (mU/L) | |||||||||
| All studies | 9 | 2.34 | 0.17 | 4.51 | 0.003 | 24.85 | 0.0002 | 68.00 | |
| Healthy | 1 | 1.58 | −8.05 | 11.21 | 0.75 | - | - | - | |
| Obesity | 5 | 1.19 | 0.14 | 2.23 | 0.03 | 18.09 | 0.0001 | 78.00 | |
| T2D | 1 | −2.70 | −15.93 | 10.53 | 0.69 | - | - | - | |
| MetS | 2 | 2.27 | 1.04 | 3.51 | 0.0003 | 0.15 | 0.70 | 0.00 | |
| HOMA-IR | |||||||||
| All studies | 5 | 0.75 | 0.35 | 1.15 | 0.0002 | 15.54 | 0.0008 | 68.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 2 | 0.57 | 0.21 | 0.93 | 0.002 | 4.14 | 0.13 | 52.00 | |
| T2D | 1 | 0.40 | −1.98 | 2.78 | 0.74 | - | - | - | |
| MetS | 2 | 1.29 | 0.99 | 1.60 | <0.00001 | 0.03 | 0.86 | 0.00 | |
| Blood pressure | SBP (mmHg) | ||||||||
| All studies | 11 | 3.99 | 1.64 | 6.34 | 0.0009 | 57.42 | <0.00001 | 81.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 7 | 3.18 | 0.29 | 6.07 | 0.03 | 38.68 | <0.00001 | 82.00 | |
| T2D | 2 | 4.77 | −1.11 | 10.64 | 0.11 | 0.05 | 0.83 | 0.00 | |
| MetS | 2 | 7.27 | 5.91 | 8.63 | <0.00001 | 0.35 | 0.55 | 0.00 | |
| DBP (mmHg) | |||||||||
| All studies | 11 | 2.86 | 1.78 | 3.94 | <0.00001 | 38.22 | <0.00001 | 71.00 | |
| Healthy | 0 | - | - | - | - | - | - | - | |
| Obesity | 7 | 2.36 | 1.47 | 3.24 | <0.00001 | 11.78 | 0.11 | 41.00 | |
| T2D | 2 | 3.80 | 0.66 | 6.94 | 0.02 | 0.24 | 0.62 | 0.00 | |
| MetS | 2 | 4.77 | 4.15 | 5.38 | <0.00001 | 0.29 | 0.59 | 0.00 | |
1 k—number of studies; MetS—Metabolic syndrome; T2D—diabetes mellitus type 2; HOMA—insulin resistance; BMI—Body mass index; SBP—Systolic Blood Pressure; DBP—Diastolic Blood Pressure.
For TRF protocols, the analyzed data revealed distinct results among the lipid homeostasis outcomes. TRF interventions showed null effects for HDL-c (k = 8, 0.00 (95% CI [−0.60, 0.61]), p = 0.99), and a positive trend to reduce the LDL-c (k = 7, 4.48 (95% CI [−7.60, 16.56]), p = 0.47), total cholesterol (k = 8, 8.14 (95% CI [−8.37, 24.65]), p = 0.33) and triglycerides (k = 9, 8.93 (95% CI [−1.58, 19.45]), p = 0.10) levels (Table 3; Figure 3). With exception between the HDL-c studies, the heterogeneity between the studies compiling the data for LDL-c, total cholesterol and triglycerides was high (Table 4; Figure 6).
Individual analysis revealed the only MetS individuals displayed statistically significant decreased levels of total cholesterol after TRF intervention (k = 3, 28.80 (95% CI [25.26, 32.34, p < 0.00001). Low heterogeneity was observed between the studies for the MetS group (Q = 1.98, p = 0.37, I2 = 0.00%) (Table 4; Figure 6).
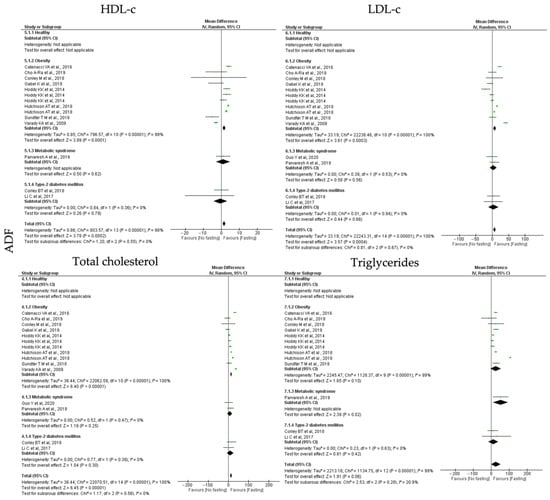
Figure 5.
Forest plot of the data from random effects meta-analysis shown as mean difference with 95% confidence intervals on lipid homeostasis outcomes, HDL-c, LDL-c, total cholesterol, triglycerides, for the studies that presented data concerning these parameters. Data are presented according to alternate-day fasting (ADF) and the metabolic conditions: healthy or disease condition, obesity, T2D or MetS. For each study, the square represents the mean difference between baseline and fasting conditions, with the horizontal line intersecting it as the lower and upper limits of the 95% confidence interval [,,,,,,,,,,,].
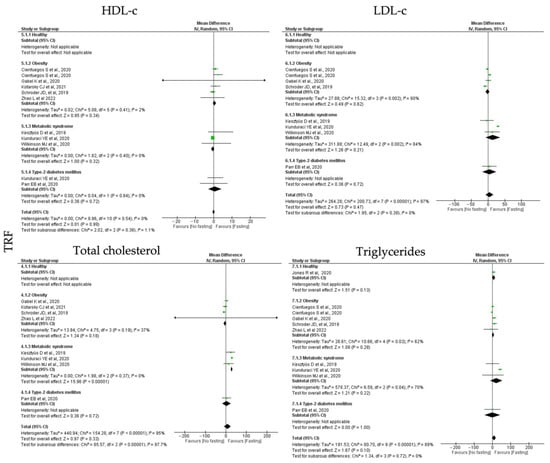
Figure 6.
Forest plot of the data from random effects meta-analysis shown as mean difference with 95% confidence intervals on lipid homeostasis outcomes, HDL-c, LDL-c, total cholesterol, triglycerides, for the studies that presented data concerning these parameters. Data are presented according to time-restricted fasting (TRF) and the metabolic conditions: healthy or disease condition, obesity, T2D or MetS. For each study, the square represents the mean difference between baseline and fasting conditions, with the horizontal line intersecting it as the lower and upper limits of the 95% confidence interval [,,,,,,,,].
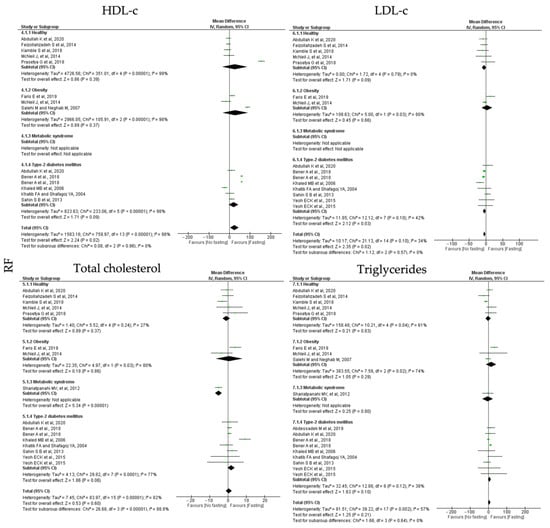
Figure 7.
Forest plot of the data from random effects meta-analysis shown as mean difference with 95% confidence intervals on lipid homeostasis outcomes, HDL-c, LDL-c, total cholesterol, triglycerides, for the studies that presented data concerning these parameters. Data are presented according to religious fasting (RF) and the metabolic conditions: healthy or disease condition, obesity, T2D or MetS. For each study, the square represents the mean difference between baseline and fasting conditions, with the horizontal line intersecting it as the lower and upper limits of the 95% confidence interval [,,,,,,,,,,,,].

Table 5.
Analysis of the impact of religious protocols, namely Ramadan, on different outcomes in healthy individuals and/or individuals with metabolic related disorders as type 2 diabetes mellitus (T2D), metabolic syndrome (MetS) or obesity.
Table 5.
Analysis of the impact of religious protocols, namely Ramadan, on different outcomes in healthy individuals and/or individuals with metabolic related disorders as type 2 diabetes mellitus (T2D), metabolic syndrome (MetS) or obesity.
| Moderators | k 1 | Point Estimate | CI Lower | CI Upper | p-Value | Heterogeneity | |||
|---|---|---|---|---|---|---|---|---|---|
| Q-Value | p-Value | I-Squared | |||||||
| Adiposity | Weight (kg) | ||||||||
| All studies | 14 | 2.22 | 1.07 | 3.38 | 0.0002 | 8.11 | 0.80 | 0.00 | |
| Healthy | 5 | 2.44 | 0.74 | 4.14 | 0.005 | 3.84 | 0.43 | 0.00 | |
| Obesity | 6 | 3.01 | 0.92 | 5.10 | 0.005 | 1.95 | 0.75 | 0.00 | |
| T2D | 4 | 0.78 | −1.60 | 3.15 | 0.52 | 0.29 | 0.99 | 0.00 | |
| MetS | 0 | - | - | - | - | - | - | ||
| BMI (kg/m2) | |||||||||
| All studies | 19 | 1.01 | 0.81 | 1.21 | <0.00001 | 18.42 | 0.73 | 0.00 | |
| Healthy | 5 | 0.70 | 0.32 | 1.09 | 0.0004 | 2.46 | 0.65 | 0.00 | |
| Obesity | 7 | 0.84 | 0.43 | 1.26 | <0.00001 | 2.57 | 0.86 | 0.00 | |
| T2D | 8 | 1.26 | 0.98 | 1.54 | <0.00001 | 7.23 | 0.70 | 0.00 | |
| MetS | 1 | 0.75 | −0.67 | 2.17 | <0.00001 | - | - | - | |
| Waist circumference (cm) | |||||||||
| All studies | 6 | 1.66 | 0.18 | 3.15 | 0.03 | 2.09 | 0.95 | 0.00 | |
| Healthy | 3 | 1.32 | −1.35 | 3.99 | 0.33 | 1.04 | 0.59 | 0.00 | |
| Obesity | 2 | 1.30 | −2.84 | 5.44 | 0.54 | 0.09 | 0.77 | 0.00 | |
| T2D | 2 | 0.76 | −2.54 | 4.07 | 0.65 | 0.03 | 0.87 | 0.00 | |
| MetS | 1 | 2.61 | 0.12 | 5.10 | 0.04 | - | - | - | |
| Lipid homeostasis | HDL-c (mg/dL) | ||||||||
| All studies | 12 | 0.44 | −1.21 | 2.10 | 0.60 | 83.97 | <0.00001 | 82.00 | |
| Healthy | 5 | −0.91 | −2.90 | 1.08 | 0.37 | 5.52 | 0.24 | 27.00 | |
| Obesity | 2 | 0.67 | −6.61 | 7.96 | 0.86 | 4.97 | 0.03 | 80.00 | |
| T2D | 6 | 1.77 | −0.10 | 3.64 | 0.06 | 29.82 | <0.00001 | 77.00 | |
| MetS | 1 | −5.13 | −7.01 | 3.25 | <0.00001 | - | - | - | |
| LDL-c (mg/dL) | |||||||||
| All studies | 11 | −3.81 | −6.99 | −0.63 | 0.02 | 21.13 | 0.10 | 34.00 | |
| Healthy | 5 | −5.68 | −12.21 | 0.85 | 0.09 | 1.72 | 0.79 | 0.00 | |
| Obesity | 2 | 3.64 | −12.36 | 19.64 | 0.66 | 5.00 | 0.03 | 80.00 | |
| T2D | 6 | −4.55 | −8.75 | −0.35 | 0.03 | 12.12 | 0.10 | 42.00 | |
| MetS | 0 | - | - | - | - | - | - | - | |
| Total cholesterol (mg/dL) | |||||||||
| All studies | 11 | 24.29 | 3.05 | 45.53 | 0.02 | 759.97 | <0.00001 | 98.00 | |
| Healthy | 5 | 26.54 | −34.18 | 87.26 | 0.39 | 351.01 | <0.00001 | 99.00 | |
| Obesity | 3 | 28.36 | −33.99 | 90.70 | 0.37 | 105.91 | <0.00001 | 98.00 | |
| T2D | 5 | 20.58 | −3.03 | 44.19 | 0.09 | 233.06 | <0.00001 | 98.00 | |
| MetS | 0 | - | - | - | |||||
| Triglycerides (mg/dL) | |||||||||
| All studies | 14 | 4.31 | −2.44 | 11.07 | 0.21 | 39.22 | 0.002 | 57.00 | |
| Healthy | 5 | −1.61 | −16.33 | 13.11 | 0.83 | 10.21 | 0.04 | 61.00 | |
| Obesity | 3 | 14.96 | −12.98 | 42.89 | 0.29 | 7.59 | 0.02 | 74.00 | |
| T2D | 7 | 6.06 | −1.24 | 13.35 | 0.10 | 12.88 | 0.12 | 38.00 | |
| MetS | 1 | −3.78 | −33.78 | 26.22 | 0.80 | - | - | - | |
| Insulin homeostasis | Fasting glucose (mg/dL) | ||||||||
| All studies | 13 | 13.75 | 2.47 | 25.02 | 0.02 | 1360.6 | <0.00001 | 99.00 | |
| Healthy | 3 | −3.65 | −8.24 | 0.95 | 0.12 | 7.85 | 0.02 | 75.00 | |
| Obesity | 4 | 1.06 | −5.23 | 7.34 | 0.74 | 7.71 | 0.05 | 61.00 | |
| T2D | 6 | 29.90 | 21.29 | 38.50 | <0.00001 | 64.87 | <0.00001 | 91.00 | |
| MetS | 1 | 20.24 | 12.38 | 28.10 | <0.00001 | - | - | - | |
| Fasting insulin (mU/L) | |||||||||
| All studies | 7 | −1.06 | −3.17 | 1.06 | 0.33 | 39.87 | <0.00001 | 80.00 | |
| Healthy | 4 | 0.05 | −1.24 | 1.34 | 0.94 | 3.64 | 0.30 | 18.00 | |
| Obesity | 3 | −3.20 | −7.14 | 0.74 | 0.11 | 5.18 | 0.07 | 61.00 | |
| T2D | 2 | −1.07 | −8.28 | 6.13 | 0.77 | 5.52 | 0.02 | 82.00 | |
| MetS | 0 | - | - | - | - | - | - | - | |
| HOMA-IR | |||||||||
| All studies | 6 | 0.32 | −0.91 | 1.54 | 0.64 | 448.84 | <0.00001 | 98.00 | |
| Healthy | 4 | −0.05 | −0.26 | 0.16 | 0.63 | 3.61 | 0.31 | 17.00 | |
| Obesity | 3 | 1.49 | −1.68 | 4.67 | 0.36 | 202.77 | <0.00001 | 99.00 | |
| T2D | 1 | −0.68 | −1.51 | 0.15 | 0.11 | - | - | - | |
| MetS | 0 | - | - | - | - | - | - | - | |
| Blood pressure | SBP (mmHg) | ||||||||
| All studies | 7 | 1.54 | −1.29 | 4.37 | 0.29 | 79.27 | <0.00001 | 89.00 | |
| Healthy | 1 | −0.22 | −1.92 | 1.48 | 0.80 | - | - | - | |
| Obesity | 3 | 2.00 | −1.19 | 5.19 | 0.22 | 4.14 | 0.13 | 52.00 | |
| T2D | 4 | 1.84 | −3.11 | 6.78 | 0.47 | 71.50 | <0.00001 | 93.00 | |
| MetS | 0 | - | - | - | - | - | - | - | |
| DBP (mmHg) | |||||||||
| All studies | 7 | 0.51 | −0.38 | 1.40 | 0.27 | 16.33 | 0.06 | 45.00 | |
| Healthy | 1 | −0.67 | −2.42 | 1.08 | 0.45 | - | - | - | |
| Obesity | 3 | 0.92 | −1.12 | 2.96 | 0.38 | 0.59 | 0.74 | 0.00 | |
| T2D | 4 | 0.65 | −0.58 | 1.88 | 0.30 | 13.59 | 0.02 | 63.00 | |
| MetS | 0 | - | - | - | - | - | - | - | |
1 k—number of studies; MetS—Metabolic syndrome; T2D—diabetes mellitus type 2; HOMA—insulin resistance; BMI—Body mass index; SBP—Systolic Blood Pressure; DBP—Diastolic Blood Pressure.
RF protocols promoted a non-significant increase in HDL-c (k = 12, 0.44 (95% CI [−1.21, 2.10]), p = 0.60) and triglycerides levels (k = 14, 4.31 (95% CI [−2.44, 11.07]), p = 0.21); a significant reduction in total cholesterol (k = 11, 24.29 (95% CI [3.05, 45.53]), p = 0.02) and a negative significant increase in LDL-c levels (k = 11, −3.81 (95% CI [−6.99, −0.63]), p = 0.02) (Table 5). Heterogeneity between the studies for LDL-c and total cholesterol was high (Table 4) and it was moderate between the studies presenting data for LDL-c and triglycerides levels (Table 5; Figure 7).
MetS individuals are negatively affected by RF intervention, with a statistically significance decrease in HDL-c levels (k = 1, −5.13 (95% CI [−7.01, 3.25]), p < 0.00001) (Table 5). Whereas, for T2D individuals, RT protocols increased LDL-c levels, (k = 6, −4.55 (95% CI [−8.75, −0.35], p = 0.03), with moderate heterogeneity between the studies (Q = 12.12, p = 0.10, I2 = 42.00) (Table 5; Figure 7).
3.6.3. Insulin Homeostasis
Regarding insulin homeostasis, the parameters analyzed were fasting glucose, fasting insulin and HOMA-IR levels. In relation to the impact of ADF interventions on the outcomes of insulin homeostasis, global data analysis revealed that ADF protocols showed a non-significant reduction in fasting glucose, fasting insulin and HOMA-IR levels (k = 10, 4.33 (95% CI [−1.14, 9.79]), p = 0.12; k = 8, 2.55 (95% CI [−0.88, 5.98]), p = 0.15 and k = 6, 0.90 (95% CI [−0.14, 1.95]), p = 0.09, respectively). High heterogeneity was detected between the studies (Table 3; Figure 8).
Group stratification revealed that ADF protocols promoted a significant reduction in fasting glucose levels in T2D and MetS participants (k = 2, 19.17 (95% CI [14.68, 23.72]), p < 0.00001 and k = 1, 5.00 (95% CI [0.71, 9.29]), p = 0.02, respectively). Studies comprising T2D participants have low heterogeneity (Q = 1.15, p = 0.28, I2 = 13.00%) (Table 3; Figure 4). Furthermore, HOMA-IR levels were statistically significant reduced in MetS individuals after ADF protocols (k = 1, 0.73 (95% CI [−0.01, 1.47, p < 0.00001) (Table 3; Figure 8).
For the TRF protocols, data revealed a significant reduction in fasting glucose, fasting insulin and HOMA-IR levels (k = 10, 5.89 (95% CI [2.52, 9.26]), p = 0.0006; k = 9, 2.34 (95% CI [0.17, 4.51]), p = 0.003 and k = 5, 0.75 (95% CI [0.35, 1.15]), p = 0.0002, respectively). High heterogeneity was observed between the studies (Table 4; Figure 9).
Fasting glucose levels were statistically significant reduced by TRF protocols in obese, T2D and MetS individuals (k = 5, 4.25 (95% CI [0.32, 8.18]), p = 0.03; k = 2, 7.22 (95% CI [3.74, 10.71]), p < 0.00001 and k = 2, 13.75 (95% CI [7.99, 19.50]), p < 0.00001, respectively). Heterogeneity between the studies was high for obese and T2D individuals (Q = 102.00, p < 0.00001, I2 = 95.00% and Q = 13.45, p = 0.0002, I2 = 93.00%, respectively), and moderate for MetS individuals (Q = 1.73, p = 0.19, I2 = 42.00%) (Table 4; Figure 9).
Concerning the fasting insulin levels, they displayed a statistically significant reduction in obese and MetS individuals (k = 5, 1.19 (95% CI [0.14, 2.23]), p = 0.03 and k = 2, 2.27 (95% CI [1.04, 3.51]), p = 0.0003, respectively). Low heterogeneity was observed between the studies for the MetS group (Q = 0.15, p = 0.70, I2 = 0.00%), while moderate heterogeneity was detected for the obesity group (Q = 18.09, p = 0.0001, I2 = 78.00%) (Table 4; Figure 9).
Finally, TRF protocols promoted a statistically significant reduction in HOMA-IR levels in obese and MetS individuals (k = 2, 0.57 (95% CI [0.21, 0.93]), p = 0.002 and k = 2, 1.29 (95% CI [0.99, 1.60]), p < 0.00001, respectively). Heterogeneity was moderate in studies with obesity (Q = 4.14, p = 0.13, I2 = 52.00%) and low for the studies with MetS participants (Q = 0.03, p = 0.86, I2 = 0.00%) groups (Table 4; Figure 9).
In relation to the RF protocols, data showed that these interventions promote a statistically significant reduction in fasting glucose (k = 13, 13.75 (95% CI [2.47, 25.02]), p = 0.02), a non-significant alteration of HOMA-IR levels (k = 6, 0.32 (95% CI [−0.91, 1.54]), p = 0.64) and a slight increase in fasting insulin (k = 7, −1.06 (95% CI [−3.17, 1.06]), p = 0.33) (Table 4). Heterogeneity between the studies was high (Table 5; Figure 10).
The stratification by groups showed that RF protocols only promoted a significant reduction in fasting glucose levels in T2D and MetS individuals (k = 6, 29.90 (95% CI [21.29, 38.50]), p < 0.00001 and k = 1, 20.24 (95% CI [12.38, 28.10]), p < 0.00001, respectively). T2D group showed high heterogeneity between the selected studies (Q = 64.87, p < 0.00001, I2 = 91.00%) (Table 5; Figure 10).

Figure 8.
Forest plot of the data from random effects meta-analysis shown as mean difference with 95% confidence intervals on insulin homeostasis outcomes, fasting glucose, fasting insulin, insulin resistance (HOMA-IR), for the studies that presented data concerning these parameters. Data are presented according to alternate-day fasting (ADF) and the metabolic conditions: healthy or disease condition, obesity, T2D or MetS. For each study, the square represents the mean difference between baseline and fasting conditions, with the horizontal line intersecting it as the lower and upper limits of the 95% confidence interval [,,,,,,,,,,].
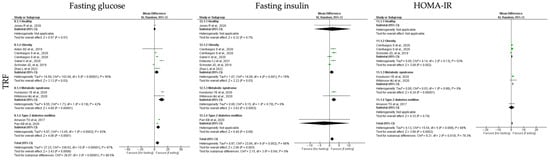
Figure 9.
Forest plot of the data from random effects meta-analysis shown as mean difference with 95% confidence intervals on insulin homeostasis outcomes, fasting glucose, fasting insulin, insulin resistance (HOMA-IR), for the studies that presented data concerning these parameters. Data are presented according to time-restricted fasting (TRF) and the metabolic conditions: healthy or disease condition, obesity, T2D or MetS. For each study, the square represents the mean difference between baseline and fasting conditions, with the horizontal line intersecting it as the lower and upper limits of the 95% confidence interval [,,,,,,,,,].

Figure 10.
Forest plot of the data from random effects meta-analysis shown as mean difference with 95% confidence intervals on insulin homeostasis outcomes, fasting glucose, fasting insulin, insulin resistance (HOMA-IR), for the studies that presented data concerning these parameters. Data are presented according to religious fasting (RF) and the metabolic conditions: healthy or disease condition, obesity, T2D or MetS. For each study, the square represents the mean difference between baseline and fasting conditions, with the horizontal line intersecting it as the lower and upper limits of the 95% confidence interval [,,,,,,,,,,,,,].
3.6.4. Blood Pressure
To study the impact of different types of IF protocols on blood pressure, the outcomes systolic blood pressure (SBP) and diastolic blood pressure (DBP) were analyzed. Relatively to blood pressure outcomes, ADF, TRF and RF showed positive impact (Table 3, Table 4 and Table 5 and Figure 11, Figure 12 and Figure 13).
ADF protocols promoted a statistically significant reduction in SBP (k = 8, 6.08 (95% CI [4.08, 8.08]), p < 0.00001) and DBP (k = 8, 3.52 (95% CI [2.55, 4.49]), p < 0.00001) (Table 3). For each outcome, the heterogeneity between studies was moderate (Table 3; Figure 11).
SBP was statistically significant reduced in obese and MetS individuals (k = 4, 5.49 (95% CI [3.41, 7.57]), p < 0.00001 and k = 2, 10.46 (95% CI [2.08, 18.83]), p = 0.01, respectively), after ADF protocols. Heterogeneity between the studies for obesity and MetS groups was moderate (Q = 17.68, p = 0.003, I2 = 72.00% and Q = 1.58, p = 0.21, I2 = 37.00%, respectively). In relation to DBP, a statistically significant reduction was observed for obese, T2D and MetS individuals (k = 4, 3.23 (95% CI [2.32, 4.13]), p < 0.00001, k = 2, 5.79 (95% CI [−0.08, 11.65]), p = 0.05 and k = 2, 5.76 (95% CI [0.30, 11.22]), p = 0.04, respectively), with moderate heterogeneity between studies (Q = 10.19, p = 0.07, I2 = 51.00%, Q = 1.39, p = 0.24, I2 = 28.00% and Q = 2.09, p = 0.15, I2 = 52.00%, respectively) (Table 3; Figure 11).
TRF protocols also promoted a statistically significant positive reduction in both SBP and DBP (k = 11, 3.99 (95% CI [1.64, 6.34]), p = 0.0009 and k = 11, 2.86 (95% CI [1.47, 3.24]), p < 0.00001, respectively), with high and moderate studies heterogeneity, respectively (Q = 57.42, p < 0.00001, I2 = 81.00% and Q = 38.22, p < 0.00001, I2 = 71.00%, respectively) (Table 4; Figure 12).
A statistically significant reduction in SBP was observed in people with obesity and MetS individuals (k = 7, 3.18 (95% CI [0.29, 6.07]), p = 0.03 and k = 2, 7.27 (95% CI [5.91, 8.63]), p < 0.00001, respectively), after TRF protocols. Heterogeneity between the studies was high for the obesity group (Q = 38.68, p < 0.00001, I2 = 82.00%) and low for MetS groups (Q = 0.35, p = 0.55, I2 = 0.00%). Concerning DBP, a statistically significant reduction was observed in obese, T2D and MetS individuals (k = 4, 3.23 (95% CI [2.32, 4.13]), p < 0.00001, k = 2, 5.79 (95% CI [−0.08, 11.65]), p = 0.05 and k = 2, 5.76 (95% CI [0.30, 11.22]), p = 0.04, respectively), after TRF protocols. Heterogeneity was moderate for obese group and low for T2D and MetS groups (Q = 11.78, p = 0.11, I2 = 41.00%, Q = 0.24, p = 0.62, I2= 0.00% and Q = 0.29, p = 0.59, I2 = 0.00%, respectively) (Table 4; Figure 12).
Finally, RF protocols only promoted no significant impact of SBP and DBP (k = 7, 1.54 (95% CI [−1.29, 4.37]), p = 0.29 and k = 7, 0.51 (95% CI [−0.38, 1.40]), p = 0.27, respectively), with high and moderate heterogeneity between studies, respectively (Q = 79.27, p < 0.00001, I2 = 89.00% and Q = 16.33, p = 0.06, I2 = 45.00, respectively) (Table 5; Figure 13).
This meta-analysis demonstrates high heterogeneity in the results, particularly in studies of individuals with obesity and MetS (Table 3, Table 4 and Table 5). Therefore, a sensitive analysis was performed by two different strategies. In the first, the sensitive analysis integrated all the studies (Table S3), and in the second analysis the impact of IF, without the RF studies was evaluated (Table S4). The sensitivity analysis did not decrease heterogeneity neither when all studies are integrated nor when RF studies were removed. Therefore, data suggest that heterogeneity is most probably related with differences in study design, populations, interventions or outcomes across the included studies.
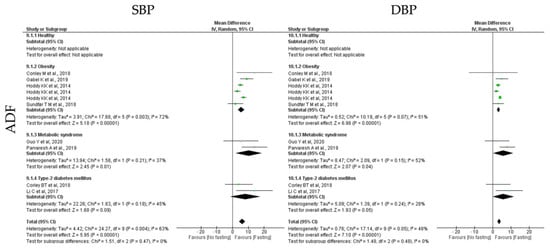
Figure 11.
Forest plot of the data from random effects meta-analysis shown as mean difference with 95% confidence intervals on blood pressure outcomes systolic blood pressure (SBP) and diastolic blood pressure (DBP), for the studies that presented data concerning these parameters. Data are presented according to alternate-day fasting (ADF) and the metabolic conditions: healthy or disease condition, obesity, T2D or MetS. For each study, the square represents the mean difference between baseline and fasting conditions, with the horizontal line intersecting it as the lower and upper limits of the 95% confidence interval [,,,,,,,].
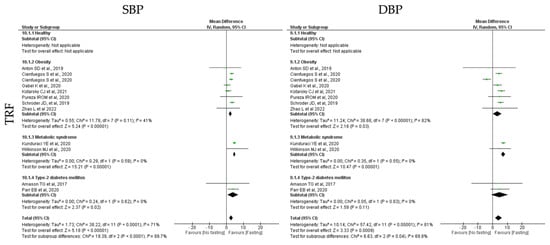
Figure 12.
Forest plot of the data from random effects meta-analysis shown as mean difference with 95% confidence intervals on blood pressure outcomes systolic blood pressure (SBP) and diastolic blood pressure (DBP), for the studies that presented data concerning these parameters. Data are presented according to time-restricted fasting (TRF) and the metabolic conditions: healthy or disease condition, obesity, T2D or MetS. For each study, the square represents the mean difference between baseline and fasting conditions, with the horizontal line intersecting it as the lower and upper limits of the 95% confidence interval [,,,,,,,,,,].
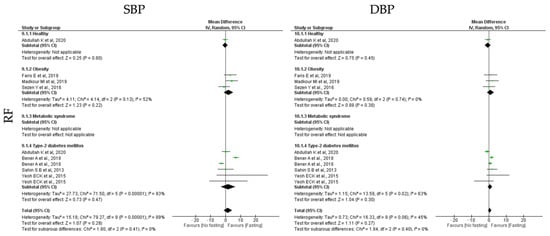
Figure 13.
Forest plot of the data from random effects meta-analysis shown as mean difference with 95% confidence intervals on blood pressure outcomes systolic blood pressure (SBP) and diastolic blood pressure (DBP), for the studies that presented data concerning these parameters. Data are presented according to religious fasting (RF) and the metabolic conditions: healthy or disease condition, obesity, T2D or MetS. For each study, the square represents the mean difference between baseline and fasting conditions, with the horizontal line intersecting it as the lower and upper limits of the 95% confidence interval [,,,,,,].
4. Discussion
The main purpose of this systematic review and meta-analysis was to summarize scientific evidence on the impact of IF protocols on metabolic-related outcomes, both on healthy and metabolic-related disease conditions, namely obesity, T2D and MetS. To our knowledge, this is the first meta-analysis that presents comprehensive results on the effects of different IF protocols, Alternate-Day Fasting (ADF), Time-Restricted Fasting (TRF) and Religious Fasting (RF), on specific parameters both in health and in metabolic-related disorders. Despite the amount of data already available and the fact that many studies point to beneficial effects of IF protocols on several metabolic parameters, data are conflicting. While reasons are still elusive, we hypothesized that different basal metabolic conditions such as those herein analyzed, as well as the multitude of IF protocols could contribute for distinct results and conclusions.
Herein, we have considered not only the effectiveness of different IF protocols but also how they impact in individuals with different metabolic status. For our analysis we selected three main common metabolic disorders: obesity, T2D and MetS. Regarding each of the metabolic parameters, for the adiposity outcomes, it was possible to observe that the different IF approaches resulted in the reduction in weight, BMI and waist circumference. These data suggest that independently of the IF intervention protocol, and most probably the reasons behind these observations are most likely related with the inability of individuals to fully compensate, during non-fasting periods, the calorie deficit associated with IF protocols [,,]. Furthermore, fasting periods might also diminish the hunger that these individuals usually feel [,,,,]. For the remaining outcomes, namely lipid, insulin and blood pressure homeostasis, ADF was the type of IF that showed the most beneficial effects in comparison with TRF and RF. The ADF major effectiveness might be related with higher fasting time of this intervention and a greater overall caloric restriction. These alterations can elicit an augmented autophagy, improved insulin sensitivity and reduce inflammation, possibly by modulating the gut microbiota and the release of inflammatory cytokines [,,]. Furthermore, the increment of fasting periods contributes to a decreased in energy intake [], to a depletion of liver glycogen and a metabolic switch from lipid/cholesterol synthesis and fat storage to the utilization of fat as a substrate []. Therefore, IF protocols’ effectiveness might be depend on different factors such as the duration and frequency of fasting periods, the amount and type of food consumed during feeding periods, and individual variation in genetic and metabolic factors [,,].
Although RF have a similar fasting time, when compared to TRF, this last appears to present more pronounced beneficial effects. The differences between TRF and RF are most probably associated with the alterations promoted by RF in the circadian rhythm. It has been postulated that TRF, due to the limited eating windows, allow for the synchronization of the circadian system, consequently optimizing the metabolic function []. Indeed, it was already described that both TRF and ADF have beneficial effects aligned with the circadian rhythm, which have consequently benefits on glucose regulation, beta cell responsiveness, body composition and weight, reduction in oxidative stress and metabolic switch [,,,]. RF disturbs the circadian system, which, as previously described, can predispose to several dysfunctions, such as the impairment of glucose tolerance, reduction in sensitivity to insulin and increased arterial blood pressure [,,,].
Our analysis shows that a significant weight loss, reduction in BMI and waist circumference is particularly observed in obese and MetS individuals independently of IF approach. MetS constitutes a group with a small number of studies that did not provide enough information to allow a categorization of the participants in either people with obesity or T2D. However, one has to keep in mind that MetS is a combination of several metabolic disfunctions, including obesity, (one of the metabolic disorders herein analyzed) insulin resistance, hypertriglyceridemia, hypercholesterolemia, hypertension and reduced high-density lipoprotein (HDL)-cholesterol concentrations []. Therefore, the promoted metabolic shift and the reduction in fat mass, induced by the IF protocols, could underlie the beneficial effects observed in the lipid profile of obese and MetS individuals, in regards to the increment of HDL-c and the reduction in the total cholesterol and triglycerides, as already reported in the literature (reviewed in []). T2D individuals benefit by the implementation of IF protocols through the reduction in fasting glucose and insulin. T2D is triggered by a combination of two essential factors, a defect in insulin secretion and/or the inability of insulin-sensitive tissues to respond appropriately to insulin []. It is proposed that these insulin defects are linked to increased adiposity and subsequent chronic inflammation, leading to the development of insulin resistance in tissues []. Therefore, we hypothesized that reduction in caloric intake and the consequent metabolic changes, implied by IF, underly the establishment of a better insulin homeostasis. Furthermore, it is also described that IF protocols might incite a prolonged decrease in insulin production and increased levels of AMPK, which can result in the improvement of insulin sensitivity and glucose homeostasis []. In relation to healthy individuals, data herein presented have no power to infer the role of IF in the modulation of the metabolic parameters analyzed, under healthy circumstances. Cohorts of healthy individuals are reduced and essentially subjected to RF, which presented the worst IF results in health promotion.
This work faced several limitations including great heterogeneity between studies, lack of blinding and variable quality of result reporting. The high heterogeneity observed in the global analysis can be explained by the strategy of grouping participants with different metabolic status, the diversity of IF interventions, variation in population age, gender ratio and geographical localization. In addition, individual weight loss and behavior change, such as increased physical activity or improved dietary habits, can be confounding factors when assessing the benefits of IF. Weight loss, a common outcome of IF, can, independently of IF intervention, lead to improvements in metabolic markers, such as insulin sensitivity and lipid profiles, making it difficult to determine which factor is responsible for the enhancement of metabolic health [,]. Therefore, it is important to control for these factors when designing and conducting studies on the effects of IF. However, the observed heterogeneity, by other side, can be seen as a major find, suggesting that IF should be personalized. Other aspect is that the literature search ranged from 2000 to June 2022. Although the concept of IF and its variations have been around for centuries, and various cultures and religions have practiced fasting for spiritual, health or cultural reasons, the scientific understanding and interest in IF gained more attention and recognition in the scientific community in the 21st century. By the year 2000, the term IF was widely used in the scientific literature to describe a variety of different fasting protocols, including ADF, TRF and RF. Furthermore, it is only in the last few decades that IF protocols have become more standardized. Therefore, for a more consistent classification, we decided to select studies published after the year 2000, keeping in mind that this is one of the study limitations.
Another point to consider in this study is that the commonly used measures of IF, such as changes in body weight, blood glucose levels or lipid profiles, may not be perfect proxies to estimate the metabolic effects of IF. Indeed, these measures can be easily influenced by other factors, for example body weight, changes in water weight, muscle mass and measurements under fasting or feeding conditions, rather than just changes in fat mass [,]. Importantly, there are other measures that may provide more accurate insights into the cellular mechanisms underlying the metabolic IF benefits. Examples include changes in biomarkers of cellular stress, oxidative damage or even autophagy [,,]. Nevertheless, the measurement of these biomarkers is not well defined in the clinical context, neither included in the hospitals’ routine analysis. Therefore, new approaches must be applied in order to overcome this drawback.
Despite the described limitations, one of the main findings of this study is that IF interventions have beneficial effects for most of individuals included in the studies, and independently of the IF protocol used. IF has been associated with improvements in weight loss, control of blood sugar and blood pressure and cholesterol levels [,,,,,]. However, not all individuals may benefit from IF, since it is described that some individuals may experience negative side effects such as hunger, fatigue and irritability [,]. IF benefits are mostly dependent of mechanisms associated with a metabolic shift towards the predominant use of fatty acids as fuel for energy [,]. However, IF benefits are far more complex and not only restricted to the positive effects of fatty acids usage for energy generation. IF interventions involve alternated periods of fasting and feeding, which correspond to different metabolic homeostasis status. In the fasting time, cells adopt a stress-resistance mode through reduction in insulin signaling and overall protein synthesis [,,,,,]. This stress-resistance mode is associated with activation of signaling pathways, which improve mitochondrial function, stress resistance and antioxidant defenses, and also increase autophagy to remove damaged molecules and recycle their components [,,]. In contrast, during the feeding, glucose levels and protein synthesis increase while ketone bodies levels drop, allowing cellular growth and repair [,,,,]. Therefore, the maintenance of IF regimens lead to long-term adaptations, most likely through a hormesis effect that improve cellular homeostasis and increase disease resistance [,,,,,,].
5. Conclusions
Overall, we verified that the implementation of the different types of IF protocols has distinct effects. According to data herein presented, ADF and TRF protocols have major beneficial effects in the improvement of dysregulated metabolic conditions. In addition, IF protocols have a major beneficial impact for obese and MetS individuals, through the improvement of adiposity, lipid homeostasis and blood pressure. For T2D individuals, the IF beneficial effects were limited, but associated with their major metabolic dysfunctions. These individuals carefully require consideration of IF protocols, proper medication adjustment and self-monitoring of blood glucose levels to improve results (Graphical abstract).
One of the caveats of this work is related with the high heterogeneity seen, particularly in studies of individuals with obesity and MetS, which can be explained by diversity of IF interventions, as well as, the metabolic status of the individuals, strengthen the notion that IF should be tailored to the individual. Therefore, our data clearly show that IF impacts metabolic homeostasis differently depending on the individual’s basal metabolic status.
In a forward-looking perspective, there is still much research to be carried out in this area, and more studies are needed to fully understand the potential benefits and risks of IF for different populations and under different conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12113699/s1, Table S1: Overview of the main analysis of studies included in qualitative synthesis, Table S2: Assessment of studies quality, Table S3: Analysis of the impact of intermittent fasting on different outcomes and Table S4: Analysis of the impact of intermittent fasting, without the fasting Ramadan studies, on different outcomes.
Author Contributions
Conceptualization, B.S.-M. and P.L.; methodology, B.S.-M., A.I.S., M.D. and F.P.-R.; software, B.S.-M. and F.P.-R.; validation, B.S.-M., F.P.-R. and P.L.; writing—original draft preparation, A.I.S., M.D, F.P.-R., B.S.-M. and P.L.; writing—review and editing, B.S.-M. and P.L.; supervision, B.S.-M. and P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been funded by National funds, through the Foundation for Science and Technology (FCT)—project UIDB/50026/2020 and UIDP/50026/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rynders, C.A.; Thomas, E.A.; Zaman, A.; Pan, Z.; Catenacci, V.A.; Melanson, E.L. Effectiveness of Intermittent Fasting and Time-Restricted Feeding Compared to Continuous Energy Restriction for Weight Loss. Nutrients 2019, 11, 2442. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Sears, D.D. Metabolic Effects of Intermittent Fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef] [PubMed]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Antoni, R.; Johnston, K.L.; Collins, A.L.; Robertson, M.D. Effects of intermittent fasting on glucose and lipid metabolism. Proc. Nutr. Soc. 2017, 76, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Antoni, R.; Johnston, K.L.; Collins, A.L.; Robertson, M.D. Intermittent v. continuous energy restriction: Differential effects on postprandial glucose and lipid metabolism following matched weight loss in overweight/obese participants. Br. J. Nutr. 2018, 119, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Clifton, P.M.; Keogh, J.B. The effect of intermittent compared with continuous energy restriction on glycaemic control in patients with type 2 diabetes: 24-month follow-up of a randomised noninferiority trial. Diabetes Res. Clin. Pract. 2019, 151, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kunduraci, Y.E.; Ozbek, H. Does the Energy Restriction Intermittent Fasting Diet Alleviate Metabolic Syndrome Biomarkers? A Randomized Controlled Trial. Nutrients 2020, 12, 3213. [Google Scholar] [CrossRef]
- Pinto, A.M.; Bordoli, C.; Buckner, L.P.; Kim, C.; Kaplan, P.C.; Del Arenal, I.M.; Jeffcock, E.J.; Hall, W.L. Intermittent energy restriction is comparable to continuous energy restriction for cardiometabolic health in adults with central obesity: A randomized controlled trial; the Met-IER study. Clin. Nutr. 2020, 39, 1753–1763. [Google Scholar] [CrossRef]
- Corley, B.T.; Carroll, R.W.; Hall, R.M.; Weatherall, M.; Parry-Strong, A.; Krebs, J.D. Intermittent fasting in Type 2 diabetes mellitus and the risk of hypoglycaemia: A randomized controlled trial. Diabet. Med. 2018, 35, 588–594. [Google Scholar] [CrossRef]
- Zubrzycki, A.; Cierpka-Kmiec, K.; Kmiec, Z.; Wronska, A. The role of low-calorie diets and intermittent fasting in the treatment of obesity and type-2 diabetes. J. Physiol. Pharmacol. 2018, 69, 663–683. [Google Scholar] [CrossRef]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Israel, H.; Richter, R.R. A guide to understanding meta-analysis. J. Orthop. Sport. Phys. Ther. 2011, 41, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Munn, Z. JBI Manual for Evidence Synthesis; JBI: Tokyo, Japan, 2020. [Google Scholar]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Abdessadek, M.; Khabbal, Y.; Magoul, R.; Marmouzi, I.; Ajdi, F. Follow-up of glycemic index before and after Ramadan fasting in type 2 diabetes patients under antidiabetic medications. Ann. Pharm. Fr. 2019, 77, 374–381. [Google Scholar] [CrossRef]
- Abdullah, K.; Al-Habori, M.; Al-Eryani, E. Ramadan Intermittent Fasting Affects Adipokines and Leptin/Adiponectin Ratio in Type 2 Diabetes Mellitus and Their First-Degree Relatives. BioMed. Res. Int. 2020, 2020, 1281792. [Google Scholar] [CrossRef]
- Anton, S.D.; Lee, S.A.; Donahoo, W.T.; McLaren, C.; Manini, T.; Leeuwenburgh, C.; Pahor, M. The Effects of Time Restricted Feeding on Overweight, Older Adults: A Pilot Study. Nutrients 2019, 11, 1500. [Google Scholar] [CrossRef]
- Arnason, T.G.; Bowen, M.W.; Mansell, K.D. Effects of intermittent fasting on health markers in those with type 2 diabetes: A pilot study. World J. Diabetes 2017, 8, 154–164. [Google Scholar] [CrossRef]
- Bener, A.; Al-Hamaq, A.O.; Ozturk, M.; Catan, F.; Haris, P.I.; Rajput, K.U.; Omer, A. Effect of ramadan fasting on glycemic control and other essential variables in diabetic patients. Ann. Afr. Med. 2018, 17, 196–202. [Google Scholar] [CrossRef]
- Catenacci, V.A.; Pan, Z.; Ostendorf, D.; Brannon, S.; Gozansky, W.S.; Mattson, M.P.; Martin, B.; MacLean, P.S.; Melanson, E.L.; Troy Donahoo, W. A randomized pilot study comparing zero-calorie alternate-day fasting to daily caloric restriction in adults with obesity. Obesity 2016, 24, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.R.; Moon, J.Y.; Kim, S.; An, K.Y.; Oh, M.; Jeon, J.Y.; Jung, D.H.; Choi, M.H.; Lee, J.W. Effects of alternate day fasting and exercise on cholesterol metabolism in overweight or obese adults: A pilot randomized controlled trial. Metab. Clin. Exp. 2019, 93, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4- and 6-h Time-Restricted Feeding on Weight and Cardiometabolic Health: A Randomized Controlled Trial in Adults with Obesity. Cell Metab. 2020, 32, 366–378. [Google Scholar] [CrossRef]
- Conley, M.; Le Fevre, L.; Haywood, C.; Proietto, J. Is two days of intermittent energy restriction per week a feasible weight loss approach in obese males? A randomised pilot study. Nutr. Diet. J. Dietit. Assoc. Aust. 2018, 75, 65–72. [Google Scholar] [CrossRef]
- de Oliveira Maranhao hr, I.R.; da Silva Junior, A.E.; Silva Praxedes, D.R.; Lessa Vasconcelos, L.G.; de Lima Macena, M.; Vieira de Melo, I.S.; de Menezes Toledo Florencio, T.M.; Bueno, N.B. Effects of time-restricted feeding on body weight, body composition and vital signs in low-income women with obesity: A 12-month randomized clinical trial. Clin. Nutr. 2021, 40, 759–766. [Google Scholar] [CrossRef]
- Faris, M.A.E.; Madkour, M.I.; Obaideen, A.K.; Dalah, E.Z.; Hasan, H.A.; Radwan, H.; Jahrami, H.A.; Hamdy, O.; Mohammad, M.G. Effect of Ramadan diurnal fasting on visceral adiposity and serum adipokines in overweight and obese individuals. Diabetes Res. Clin. Pract. 2019, 153, 166–175. [Google Scholar] [CrossRef]
- Feizollahzadeh, S.; Rasuli, J.; Kheirouri, S.; Alizadeh, M. Augmented plasma adiponectin after prolonged fasting during ramadan in men. Health Promot. Perspect. 2014, 4, 77–81. [Google Scholar] [CrossRef]
- Gabel, K.; Kroeger, C.M.; Trepanowski, J.F.; Hoddy, K.K.; Cienfuegos, S.; Kalam, F.; Varady, K.A. Differential Effects of Alternate-Day Fasting Versus Daily Calorie Restriction on Insulin Resistance. Obesity 2019, 27, 1443–1450. [Google Scholar] [CrossRef]
- Gabel, K.; Marcell, J.; Cares, K.; Kalam, F.; Cienfuegos, S.; Ezpeleta, M.; Varady, K.A. Effect of time restricted feeding on the gut microbiome in adults with obesity: A pilot study. Nutr. Health 2020, 26, 79–85. [Google Scholar] [CrossRef]
- Gnanou, J.V.; Caszo, B.A.; Khalil, K.M.; Abdullah, S.L.; Knight, V.F.; Bidin, M.Z. Effects of Ramadan fasting on glucose homeostasis and adiponectin levels in healthy adult males. J. Diabetes Metab. Disord. 2015, 14, 55. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, S.; Ye, Y.; Yin, S.; Fan, J.; Xia, M. Intermittent Fasting Improves Cardiometabolic Risk Factors and Alters Gut Microbiota in Metabolic Syndrome Patients. J. Clin. Endocrinol. Metab. 2021, 106, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Hoddy, K.K.; Kroeger, C.M.; Trepanowski, J.F.; Barnosky, A.; Bhutani, S.; Varady, K.A. Meal timing during alternate day fasting: Impact on body weight and cardiovascular disease risk in obese adults. Obesity 2014, 22, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.T.; Liu, B.; Wood, R.E.; Vincent, A.D.; Thompson, C.H.; O’Callaghan, N.J.; Wittert, G.A.; Heilbronn, L.K. Effects of Intermittent Versus Continuous Energy Intakes on Insulin Sensitivity and Metabolic Risk in Women with Overweight. Obesity 2019, 27, 50–58. [Google Scholar] [CrossRef]
- Jones, R.; Pabla, P.; Mallinson, J.; Nixon, A.; Taylor, T.; Bennett, A.; Tsintzas, K. Two weeks of early time-restricted feeding (eTRF) improves skeletal muscle insulin and anabolic sensitivity in healthy men. Am. J. Clin. Nutr. 2020, 112, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Kamble, S.; Hiremath, S. Insulin resistance and blood lipid levels during fasting. Natl. J. Physiol. Pharm. Pharmacol. 2018, 8, 1158–1161. [Google Scholar] [CrossRef]
- Karatoprak, C.; Yolbas, S.; Cakirca, M.; Cinar, A.; Zorlu, M.; Kiskac, M.; Cikrikcioglu, M.A.; Erkoc, R.; Tasan, E. The effects of long term fasting in Ramadan on glucose regulation in type 2 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2512–2516. [Google Scholar]
- Kesztyus, D.; Cermak, P.; Gulich, M.; Kesztyus, T. Adherence to Time-Restricted Feeding and Impact on Abdominal Obesity in Primary Care Patients: Results of a Pilot Study in a Pre-Post Design. Nutrients 2019, 11, 2854. [Google Scholar] [CrossRef]
- Khaled, B.M.; Bendahmane, M.; Belbraouet, S. Ramadan fasting induces modifications of certain serum components in obese women with type 2 diabetes. Saudi Med. J. 2006, 27, 23–26. [Google Scholar]
- Khatib, F.A.; Shafagoj, Y.A. Metabolic alterations as a result of Ramadan fasting in non-insulin-dependent diabetes mellitus patients in relation to food intake. Saudi Med. J. 2004, 25, 1858–1863. [Google Scholar]
- Kotarsky, C.J.; Johnson, N.R.; Mahoney, S.J.; Mitchell, S.L.; Schimek, R.L.; Stastny, S.N.; Hackney, K.J. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol. Rep. 2021, 9, e14868. [Google Scholar] [CrossRef]
- Kovil, R.; Shaikh, N. Study of Beneficial Impact on Specific Biomarkers in Type 2 Diabetes During Ramadan Fasting (Unintentional Intermittent Fasting). J. Assoc. Physicians India 2020, 68, 26–29. [Google Scholar] [PubMed]
- Li, C.; Sadraie, B.; Steckhan, N.; Kessler, C.; Stange, R.; Jeitler, M.; Michalsen, A. Effects of A One-week Fasting Therapy in Patients with Type-2 Diabetes Mellitus and Metabolic Syndrome—A Randomized Controlled Explorative Study. Exp. Clin. Endocrinol. Diabetes 2017, 125, 618–624. [Google Scholar] [CrossRef]
- Madkour, M.I.; El-Serafi, A.T.; Jahrami, H.A.; Sherif, N.M.; Hassan, R.E.; Awadallah, S.; Faris, M.A.E. Ramadan diurnal intermittent fasting modulates SOD2, TFAM, Nrf2, and sirtuins (SIRT1, SIRT3) gene expressions in subjects with overweight and obesity. Diabetes Res. Clin. Pract. 2019, 155, 107801. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.; Mamlouk, M.M.; Duval, K.; Schwartz, A.; Nardo Junior, N.; Doucet, E. Alterations in metabolic profile occur in normal-weight and obese men during the Ramadan fast despite no changes in anthropometry. J. Obes. 2014, 2014, 482547. [Google Scholar] [CrossRef] [PubMed]
- Parr, E.B.; Devlin, B.L.; Lim, K.H.C.; Moresi, L.N.Z.; Geils, C.; Brennan, L.; Hawley, J.A. Time-Restricted Eating as a Nutrition Strategy for Individuals with Type 2 Diabetes: A Feasibility Study. Nutrients 2020, 12, 3228. [Google Scholar] [CrossRef] [PubMed]
- Parvaresh, A.; Razavi, R.; Abbasi, B.; Yaghoobloo, K.; Hassanzadeh, A.; Mohammadifard, N.; Safavi, S.M.; Hadi, A.; Clark, C.C.T. Modified alternate-day fasting vs. calorie restriction in the treatment of patients with metabolic syndrome: A randomized clinical trial. Complement. Ther. Med. 2019, 47, 102187. [Google Scholar] [CrossRef] [PubMed]
- Prasetya, G.; Sapwarobol, S. Intermittent Fasting During Ramadan Improves Insulin Sensitivity and Anthropometric Parameters in Healthy Young Muslim Men. Am. J. Lifestyle Med. 2021, 15, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.B.; Ayaz, T.; Ozyurt, N.; Ilkkilic, K.; Kirvar, A.; Sezgin, H. The impact of fasting during Ramadan on the glycemic control of patients with type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2013, 121, 531–534. [Google Scholar] [CrossRef]
- Salehi, M.; Neghab, M. Effects of fasting and a medium calorie balanced diet during the holy month Ramadan on weight, BMI and some blood parameters of overweight males. Pak. J. Biol. Sci. 2007, 10, 968–971. [Google Scholar] [CrossRef]
- Schroder, J.D.; Falqueto, H.; Manica, A.; Zanini, D.; de Oliveira, T.; de Sa, C.A.; Cardoso, A.M.; Manfredi, L.H. Effects of time-restricted feeding in weight loss, metabolic syndrome and cardiovascular risk in obese women. J. Transl. Med. 2021, 19, 3. [Google Scholar] [CrossRef]
- Sezen, Y.; Altiparmak, I.H.; Erkus, M.E.; Kocarslan, A.; Kaya, Z.; Gunebakmaz, O.; Demirbag, R. Effects of Ramadan fasting on body composition and arterial stiffness. J. Pak. Med. Assoc. 2016, 66, 1522–1527. [Google Scholar] [PubMed]
- Shariatpanahi, M.V.; Shariatpanahi, Z.V.; Shahbazi, S.; Moshtaqi, M. Effect of fasting with two meals on BMI and inflammatory markers of metabolic syndrome. Pak. J. Biol. Sci. 2012, 15, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Sundfor, T.M.; Svendsen, M.; Tonstad, S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Bhutani, S.; Church, E.C.; Klempel, M.C. Short-term modified alternate-day fasting: A novel dietary strategy for weight loss and cardioprotection in obese adults. Am. J. Clin. Nutr. 2009, 90, 1138–1143. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metab. 2020, 31, 92–104.e105. [Google Scholar] [CrossRef]
- Yeoh, E.C.; Zainudin, S.B.; Loh, W.N.; Chua, C.L.; Fun, S.; Subramaniam, T.; Sum, C.F.; Lim, S.C. Fasting during Ramadan and Associated Changes in Glycaemia, Caloric Intake and Body Composition with Gender Differences in Singapore. Ann. Acad. Med. Singap. 2015, 44, 202–206. [Google Scholar] [CrossRef]
- Zhao, L.; Hutchison, A.T.; Liu, B.; Yates, C.L.; Teong, X.T.; Wittert, G.A.; Thompson, C.H.; Nguyen, L.; Au, J.; Manoogian, E.N.C.; et al. Time-restricted eating improves glycemic control and dampens energy-consuming pathways in human adipose tissue. Nutrition 2022, 96, 111583. [Google Scholar] [CrossRef]
- Zouhal, H.; Bagheri, R.; Ashtary-Larky, D.; Wong, A.; Triki, R.; Hackney, A.C.; Laher, I.; Abderrahman, A.B. Effects of Ramadan intermittent fasting on inflammatory and biochemical biomarkers in males with obesity. Physiol. Behav. 2020, 225, 113090. [Google Scholar] [CrossRef]
- Zouhal, H.; Bagheri, R.; Triki, R.; Saeidi, A.; Wong, A.; Hackney, A.C.; Laher, I.; Suzuki, K.; Ben Abderrahman, A. Effects of Ramadan Intermittent Fasting on Gut Hormones and Body Composition in Males with Obesity. Int. J. Environ. Res. Public Health 2020, 17, 5600. [Google Scholar] [CrossRef]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.; Bhutani, S.; Hoddy, K.K.; Rood, J.; Ravussin, E.; Varady, K.A. Effects of alternate-day fasting or daily calorie restriction on body composition, fat distribution, and circulating adipokines: Secondary analysis of a randomized controlled trial. Clin. Nutr. 2018, 37, 1871–1878. [Google Scholar] [CrossRef]
- Carter, S.; Clifton, P.M.; Keogh, J.B. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res. Clin. Pract. 2016, 122, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Furmli, S.; Elmasry, R.; Ramos, M.; Fung, J. Therapeutic use of intermittent fasting for people with type 2 diabetes as an alternative to insulin. BMJ Case Rep. 2018, 2018, bcr-2017. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e1213. [Google Scholar] [CrossRef] [PubMed]
- Shafras, M.S.; Monaragala, L.M.; Samarakkody, I.S.; Ahamed, R.A.; Nawarathna, L.N.; Sanas, M.S.; Antonypillai, C.A. Comparison of glycaemic control and anthropometric parameters before and after Ramadan fasting in a selected cohort of patients with type 2 diabetes mellitus in Sri Lanka. Ceylon Med. J. 2020, 65, 79–85. [Google Scholar] [CrossRef]
- Hassanein, M.; Abdelgadir, E.; Obaid, H.A.; Ahmed, F.S.; Alsharhan, M.; Thabit, S.; Haidar, M.; Elsayed, M.; Alawadi, F. Biometric and metabolic changes in patients with diabetes prior, during and after the holy month of Ramadan (ABCD Study). Diabetes Res. Clin. Pract. 2021, 173, 108678. [Google Scholar] [CrossRef] [PubMed]
- Harbuwono, D.S.; Kurniawan, F.; Sudarsono, N.C.; Tahapary, D.L. The impact of Ramadan fasting on glucose variability in type 2 diabetes mellitus patients on oral anti diabetic agents. PLoS ONE 2020, 15, e0234443. [Google Scholar] [CrossRef] [PubMed]
- Kalam, F.; Kroeger, C.M.; Trepanowski, J.F.; Gabel, K.; Song, J.H.; Cienfuegos, S.; Varady, K.A. Beverage intake during alternate-day fasting: Relationship to energy intake and body weight. Nutr. Health 2019, 25, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Kroeger, C.M.; Barnosky, A.; Klempel, M.C.; Bhutani, S.; Hoddy, K.K.; Gabel, K.; Freels, S.; Rigdon, J.; Rood, J.; et al. Effect of Alternate-Day Fasting on Weight Loss, Weight Maintenance, and Cardioprotection Among Metabolically Healthy Obese Adults: A Randomized Clinical Trial. JAMA Intern. Med. 2017, 177, 930–938. [Google Scholar] [CrossRef]
- Li, C.; Ostermann, T.; Hardt, M.; Ludtke, R.; Broecker-Preuss, M.; Dobos, G.; Michalsen, A. Metabolic and psychological response to 7-day fasting in obese patients with and without metabolic syndrome. Forsch Komplementmed 2013, 20, 413–420. [Google Scholar] [CrossRef]
- Vidmar, A.P.; Naguib, M.; Raymond, J.K.; Salvy, S.J.; Hegedus, E.; Wee, C.P.; Goran, M.I. Time-Limited Eating and Continuous Glucose Monitoring in Adolescents with Obesity: A Pilot Study. Nutrients 2021, 13, 3697. [Google Scholar] [CrossRef]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men With Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Aksungar, F.B.; Sarikaya, M.; Coskun, A.; Serteser, M.; Unsal, I. Comparison of Intermittent Fasting Versus Caloric Restriction in Obese Subjects: A Two Year Follow-Up. J. Nutr. Health Aging 2017, 21, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Kessler, C.S.; Stange, R.; Schlenkermann, M.; Jeitler, M.; Michalsen, A.; Selle, A.; Raucci, F.; Steckhan, N. A nonrandomized controlled clinical pilot trial on 8 wk of intermittent fasting (24 h/wk). Nutrition 2018, 46, 143–152.e142. [Google Scholar] [CrossRef] [PubMed]
- Mindikoglu, A.L.; Abdulsada, M.M.; Jain, A.; Choi, J.M.; Jalal, P.K.; Devaraj, S.; Mezzari, M.P.; Petrosino, J.F.; Opekun, A.R.; Jung, S.Y. Intermittent fasting from dawn to sunset for 30 consecutive days is associated with anticancer proteomic signature and upregulates key regulatory proteins of glucose and lipid metabolism, circadian clock, DNA repair, cytoskeleton remodeling, immune system and cognitive function in healthy subjects. J. Proteom. 2020, 217, 103645. [Google Scholar] [CrossRef]
- Johnstone, A.M.; Faber, P.; Gibney, E.R.; Elia, M.; Horgan, G.; Golden, B.E.; Stubbs, R.J. Effect of an acute fast on energy compensation and feeding behaviour in lean men and women. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2002, 26, 1623–1628. [Google Scholar] [CrossRef]
- Klempel, M.C.; Bhutani, S.; Fitzgibbon, M.; Freels, S.; Varady, K.A. Dietary and physical activity adaptations to alternate day modified fasting: Implications for optimal weight loss. Nutr. J. 2010, 9, 35. [Google Scholar] [CrossRef]
- Klempel, M.C.; Kroeger, C.M.; Varady, K.A. Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metab. Clin. Exp. 2013, 62, 137–143. [Google Scholar] [CrossRef]
- Hoddy, K.K.; Gibbons, C.; Kroeger, C.M.; Trepanowski, J.F.; Barnosky, A.; Bhutani, S.; Gabel, K.; Finlayson, G.; Varady, K.A. Changes in hunger and fullness in relation to gut peptides before and after 8 weeks of alternate day fasting. Clin. Nutr. 2016, 35, 1380–1385. [Google Scholar] [CrossRef]
- Horne, B.D.; Muhlestein, J.B.; Anderson, J.L. Health effects of intermittent fasting: Hormesis or harm? A systematic review. Am. J. Clin. Nutr. 2015, 102, 464–470. [Google Scholar] [CrossRef]
- Johnson, J.B.; Summer, W.; Cutler, R.G.; Martin, B.; Hyun, D.H.; Dixit, V.D.; Pearson, M.; Nassar, M.; Telljohann, R.; Maudsley, S.; et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic. Biol. Med. 2007, 42, 665–674. [Google Scholar] [CrossRef]
- Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Aggour, E.; Calvo, Y.; Trepanowski, J.F.; Hoddy, K.K.; Varady, K.A. Effect of exercising while fasting on eating behaviors and food intake. J. Int. Soc. Sport. Nutr. 2013, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Patterson, R.E.; Laughlin, G.A.; LaCroix, A.Z.; Hartman, S.J.; Natarajan, L.; Senger, C.M.; Martinez, M.E.; Villasenor, A.; Sears, D.D.; Marinac, C.R.; et al. Intermittent Fasting and Human Metabolic Health. J. Acad. Nutr. Diet. 2015, 115, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; McStay, M.; Gabel, K.; Varady, K.A. Time restricted eating for the prevention of type 2 diabetes. J. Physiol. 2022, 600, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Ratamess, N.A.; Faigenbaum, A.D.; Bush, J.A.; Beller, N.; Vargas, A.; Fardman, B.; Andriopoulos, T. Effect of Time-Restricted Feeding on Anthropometric, Metabolic, and Fitness Parameters: A Systematic Review. J. Am. Nutr. Assoc. 2022, 41, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; La Bounty, P.M. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr. Rev. 2015, 73, 661–674. [Google Scholar] [CrossRef]
- Mandal, S.; Simmons, N.; Awan, S.; Chamari, K.; Ahmed, I. Intermittent fasting: Eating by the clock for health and exercise performance. BMJ Open Sport Exerc. Med. 2022, 8, e001206. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.A. Intermittent fasting and time-restricted eating role in dietary interventions and precision nutrition. Front. Public Health 2022, 10, 1017254. [Google Scholar] [CrossRef]
- Vasim, I.; Majeed, C.N.; DeBoer, M.D. Intermittent Fasting and Metabolic Health. Nutrients 2022, 14, 631. [Google Scholar] [CrossRef]
- Santos, H.O.; Macedo, R.C.O. Impact of intermittent fasting on the lipid profile: Assessment associated with diet and weight loss. Clin. Nutr. ESPEN 2018, 24, 14–21. [Google Scholar] [CrossRef]
- Albosta, M.; Bakke, J. Intermittent fasting: Is there a role in the treatment of diabetes? A review of the literature and guide for primary care physicians. Clin. Diabetes Endocrinol. 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Halberg, N.; Henriksen, M.; Soderhamn, N.; Stallknecht, B.; Ploug, T.; Schjerling, P.; Dela, F. Effect of intermittent fasting and refeeding on insulin action in healthy men. J. Appl. Physiol. 2005, 99, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Ingersen, A.; Helset, H.R.; Calov, M.; Chabanova, E.; Harreskov, E.G.; Jensen, C.; Hansen, C.N.; Prats, C.; Helge, J.W.; Larsen, S.; et al. Metabolic effects of alternate-day fasting in males with obesity with or without type 2 diabetes. Front. Physiol. 2022, 13, 1061063. [Google Scholar] [CrossRef]
- Harvie, M.; Howell, A. Potential Benefits and Harms of Intermittent Energy Restriction and Intermittent Fasting Amongst Obese, Overweight and Normal Weight Subjects-A Narrative Review of Human and Animal Evidence. Behav. Sci. 2017, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Villani, V.; Buono, R.; Wei, M.; Kumar, S.; Yilmaz, O.H.; Cohen, P.; Sneddon, J.B.; Perin, L.; Longo, V.D. Fasting-Mimicking Diet Promotes Ngn3-Driven beta-Cell Regeneration to Reverse Diabetes. Cell 2017, 168, 775–788.e712. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Peri, C.; Cricri, D.; Coppi, L.; Caruso, D.; Mitro, N.; De Fabiani, E.; Crestani, M. Ketogenic Diet: A New Light Shining on Old but Gold Biochemistry. Nutrients 2019, 11, 2497. [Google Scholar] [CrossRef]
- Wang, W.; Wei, R.; Pan, Q.; Guo, L. Beneficial effect of time-restricted eating on blood pressure: A systematic meta-analysis and meta-regression analysis. Nutr. Metab. 2022, 19, 77. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, R. The Effect of Fasting on Human Metabolism and Psychological Health. Dis. Mrk. 2022, 2022, 5653739. [Google Scholar] [CrossRef]
- Mattson, M.P.; Allison, D.B.; Fontana, L.; Harvie, M.; Longo, V.D.; Malaisse, W.J.; Mosley, M.; Notterpek, L.; Ravussin, E.; Scheer, F.A.; et al. Meal frequency and timing in health and disease. Proc. Natl. Acad. Sci. USA 2014, 111, 16647–16653. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G., 3rd; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Wan, R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J. Nutr. Biochem. 2005, 16, 129–137. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).