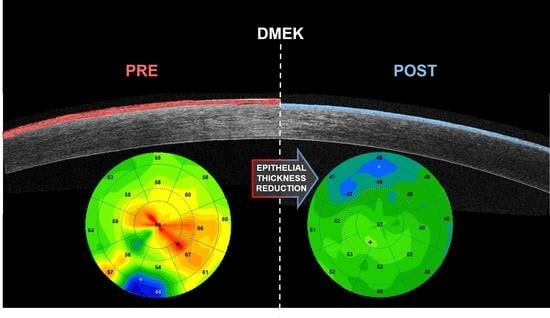
Descemet Membrane Endothelial Keratoplasty (DMEK) Reduces the Corneal Epithelial Thickness in Fuchs’ Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Procedure
2.2. Corneal Imaging
2.3. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elhalis, H.; Azizi, B.; Jurkunas, U.V. Fuchs Endothelial Corneal Dystrophy. Ocul. Surf. 2010, 8, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Krachmer, J.H.; Purcell, J.J.; Young, C.W.; Bucher, K.D. Corneal endothelial dystrophy. A study of 64 families. Arch. Ophthalmol. 1978, 96, 2036–2039. [Google Scholar] [CrossRef] [PubMed]
- Vithana, E.N.; Morgan, P.E.; Ramprasad, V.; Tan, D.T.; Yong, V.H.; Venkataraman, D.; Venkatraman, A.; Yam, G.H.; Nagasamy, S.; Law, R.W.; et al. SLC4A11 mutations in Fuchs endothelial corneal dystrophy. Hum. Mol. Genet. 2008, 17, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Aiello, F.; Gallo Afflitto, G.; Ceccarelli, F.; Cesareo, M.; Nucci, C. Global Prevalence of Fuchs Endothelial Corneal Dystrophy (FECD) in Adult Population: A Systematic Review and Meta-Analysis. J. Ophthalmol. 2022, 2022, 3091695. [Google Scholar] [CrossRef]
- Singh, A.; Zarei-Ghanavati, M.; Avadhanam, V.; Liu, C. Systematic review and meta-analysis of clinical outcomes of Descemet membrane endothelial keratoplasty versus descemet stripping endothelial keratoplasty/Descemet stripping automated endothelial keratoplasty. Cornea 2017, 36, 1437–1443. [Google Scholar] [CrossRef]
- Woo, J.H.; Ang, M.; Htoon, H.M.; Tan, D. Descemet membrane endothelial keratoplasty versus Descemet stripping automated endothelial keratoplasty and penetrating keratoplasty. Am. J. Ophthalmol. 2019, 207, 288–303. [Google Scholar] [CrossRef]
- Ong, H.S.; Ang, M.; Mehta, J. Evolution of Therapies for the Corneal Endothelium: Past, Present and Future Approaches. Br. J. Ophthalmol. 2021, 105, 454–467. [Google Scholar] [CrossRef]
- Marques, R.E.; Guerra, P.S.; Sousa, D.C.; Gonçalves, A.I.; Quintas, A.M.; Rodrigues, W. DMEK versus DSAEK for Fuchs’ Endothelial Dystrophy: A Meta-Analysis. Eur. J. Ophthalmol. 2019, 29, 15–22. [Google Scholar] [CrossRef]
- Pavlovic, I.; Shajari, M.; Herrmann, E.; Schmack, I.; Lencova, A.; Kohnen, T. Meta-Analysis of Postoperative Outcome Parameters Comparing Descemet Membrane Endothelial Keratoplasty Versus Descemet Stripping Automated Endothelial Keratoplasty. Cornea 2017, 36, 1445. [Google Scholar] [CrossRef]
- Weisenthal, R.W.; Yin, H.Y.; Jarstad, A.R.; Wang, D.; Verdier, D.D. Long-Term Outcomes in Fellow Eyes Comparing DSAEK and DMEK for Treatment of Fuchs Corneal Dystrophy. Am. J. Ophthalmol. 2022, 233, 216–226. [Google Scholar] [CrossRef]
- Ang, M.; Baskaran, M.; Werkmeister, R.M.; Chua, J.; Schmidl, D.; Aranha Dos Santos, V.; Garhöfer, G.; Mehta, J.S.; Schmetterer, L. Anterior segment optical coherence tomography. Prog. Retin. Eye. Res 2018, 66, 132–156. [Google Scholar] [CrossRef]
- Venkateswaran, N.; Galor, A.; Wang, J.; Karp, C.L. Optical Coherence Tomography for Ocular Surface and Corneal Diseases: A Review. Eye. Vis. 2018, 5, 13. [Google Scholar] [CrossRef]
- Arnalich-Montiel, F.; Ortiz-Toquero, S.; Auladell, C.; Couceiro, A. Accuracy of Corneal Thickness by Swept-Source Optical Coherence Tomography and Scheimpflug Camera in Virgin and Treated Fuchs Endothelial Dystrophy. Cornea 2018, 37, 727. [Google Scholar] [CrossRef]
- Fisenko, N.V.; Trufanov, S.V.; Demura, T.A. Morphological features of the cornea in eyes with pseudophakic bullous keratopathy and Fuchs endothelial corneal dystrophy. Vestn. Oftalmol. 2022, 138, 31–37. [Google Scholar] [CrossRef]
- Augustin, V.A.; Köppe, M.K.; Son, H.-S.; Meis, J.; Yildirim, T.M.; Khoramnia, R.; Auffarth, G.U. Scheimpflug Versus Optical Coherence Tomography to Detect Subclinical Corneal Edema in Fuchs Endothelial Corneal Dystrophy. Cornea 2022, 41, 1378. [Google Scholar] [CrossRef]
- Arnalich-Montiel, F.; Mingo-Botín, D.; Diaz-Montealegre, A. Keratometric, Pachymetric, and Surface Elevation Characterization of Corneas with Fuchs Endothelial Corneal Dystrophy Treated With DMEK. Cornea 2019, 38, 535. [Google Scholar] [CrossRef]
- Brockmann, T.; Pilger, D.; Brockmann, C.; Maier, A.-K.B.; Bertelmann, E.; Torun, N. Predictive Factors for Clinical Outcomes after Primary Descemet’s Membrane Endothelial Keratoplasty for Fuchs’ Endothelial Dystrophy. Curr. Eye. Res. 2019, 44, 147–153. [Google Scholar] [CrossRef]
- Machalińska, A.; Kuligowska, A.; Kaleta, K.; Kuśmierz-Wojtasik, M.; Safranow, K. Changes in Corneal Parameters after DMEK Surgery: A Swept-Source Imaging Analysis at 12-Month Follow-Up Time. J. Ophthalmol. 2021, 2021, 3055722. [Google Scholar] [CrossRef]
- Okumura, N.; Padmanaban, V.; Balaji, J.J.; Srinivasan, B.; Hanada, N.; Komori, Y.; Yoshii, K.; Srinivas, S.P.; Koizumi, N.; Padmanabhan, P. Clinical, Morphological, and Optical Correlates of Visual Function in Patients with Fuchs Endothelial Corneal Dystrophy. Cornea 2022, 41, 171. [Google Scholar] [CrossRef]
- Podskochy, A. Protective role of corneal epithelium against ultraviolet radiation damage. Acta. Ophthalmol. Scand 2004, 82, 714–717. [Google Scholar] [CrossRef]
- Eckard, A.; Stave, J.; Guthoff, R.F. In vivo investigations of the corneal epithelium with the confocal Rostock Laser Scanning Microscope (RLSM). Cornea 2006, 25, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, D.Z.; Archer, T.J.; Gobbe, M.; Silverman, R.H.; Coleman, D.J. Epithelial Thickness in the Normal Cornea: Three-Dimensional Display with Very High Frequency Ultrasound. J. Refract. Surg. 2008, 24, 571–581. [Google Scholar] [PubMed]
- Rocha, K.M.; Straziota, C.P.; Stulting, R.D.; Randleman, J.B. Spectral-Domain OCT Analysis of Regional Epithelial Thickness Profiles in Keratoconus, Postoperative Corneal Ectasia, and Normal Eyes. J. Refrac. Surg. 2013, 29, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Sella, R.; Zangwill, L.M.; Weinreb, R.N.; Afshari, N.A. Repeatability and Reproducibility of Corneal Epithelial Thickness Mapping With Spectral-Domain Optical Coherence Tomography in Normal and Diseased Cornea Eyes. Am. J. Ophthalmol. 2019, 197, 88–97. [Google Scholar] [CrossRef]
- Ma, X.J.; Wang, L.; Koch, D.D. Repeatability of corneal epithelial thickness measurements using Fourier-domain optical coherence tomography in normal and post-LASIK eyes. Cornea 2013, 32, 1544–1548. [Google Scholar] [CrossRef]
- Eleiwa, T.; Elsawy, A.; Tolba, M.; Feuer, W.; Yoo, S.; Shousha, M.A. Diagnostic Performance of 3-Dimensional Thickness of the Endothelium–Descemet Complex in Fuchs’ Endothelial Cell Corneal Dystrophy. Ophthalmology 2020, 127, 874–887. [Google Scholar] [CrossRef]
- Agha, B.; Ahmad, N.; Dawson, D.G.; Kohnen, T.; Schmack, I. Refractive Outcome and Tomographic Changes after Descemet Membrane Endothelial Keratoplasty in Pseudophakic Eyes with Fuchs’ Endothelial Dystrophy. Int. Ophthalmol. 2021, 41, 2897–2904. [Google Scholar] [CrossRef]
- Machalińska, A.; Kuligowska, A.; Kowalska, B.; Safranow, K. Comparative Analysis of Corneal Parameters in Swept-Source Imaging between DMEK and UT-DSAEK Eyes. J. Clin. Med. 2021, 10, 5119. [Google Scholar] [CrossRef]
- Price, F.W.J.; Price, M.O. Combined Cataract/DSEK/DMEK: Changing Expectations. Asia-Pac. J. Ophthalmol. 2017, 6, 388. [Google Scholar]
- Terry, M.A.; Shamie, N.; Chen, E.S.; Phillips, P.M.; Shah, A.K.; Hoar, K.L.; Friend, D.J. Endothelial Keratoplasty for Fuchs’ Dystrophy with Cataract: Complications and Clinical Results with the New Triple Procedure. Ophthalmology 2009, 116, 631–639. [Google Scholar] [CrossRef]
- Röck, T.; Bartz-Schmidt, K.U.; Röck, D.; Yoeruek, E. Refractive changes after Descemet membrane endothelial keratoplasty. Ophthalmol 2014, 111, 649–653. [Google Scholar] [CrossRef]
- Laaser, K.; Bachmann, B.O.; Horn, F.K.; Cursiefen, C.; Kruse, F.E. Descemet Membrane Endothelial Keratoplasty Combined with Phacoemulsification and Intraocular Lens Implantation: Advanced Triple Procedure. Am. J. Ophthalmol. 2012, 154, 47–55. [Google Scholar] [CrossRef]
- Dimasi, D.P.; Burdon, K.P.; Craig, J.E. The genetics of central corneal thickness. Br. J. Ophthalmol. 2010, 94, 971–976. [Google Scholar] [CrossRef]
- Diener, R.; Treder, M.; Lauermann, J.L.; Eter, N.; Alnawaiseh, M. Optimizing intraocular lens power calculation using adjusted conventional keratometry for cataract surgery combined with Descemet membrane endothelial keratoplasty. Graefes. Arch. Clin. Exp. Ophthalmol. 2022, 260, 3087–3093. [Google Scholar] [CrossRef]
- Ham, L.; Dapena, I.; Moutsouris, K.; Balachandran, C.; Frank, L.E.; van Dijk, K.; Melles, G.R. Refractive change and stability after Descemet membrane endothelial keratoplasty. Effect of corneal dehydration-induced hyperopic shift on intraocular lens power calculation. J. Cataract. Refract. Surg. 2011, 37, 1455–1464. [Google Scholar] [CrossRef]
- Hayashi, T.; Kobayashi, A.; Takahashi, H.; Oyakawa, I.; Kato, N.; Yamaguchi, T. Optical characteristics after Descemet membrane endothelial keratoplasty: 1-year results. PLoS ONE 2020, 15, e0240458. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Yap, T.E.; Archer, T.J.; Gobbe, M.; Silverman, R.H. Comparison of Corneal Epithelial Thickness Measurement between Fourier-Domain OCT and Very High-Frequency Digital Ultrasound. J. Refract. Surg. 2015, 31, 438–445. [Google Scholar] [CrossRef]

| FECD Cohort | Control Cohort | ||
|---|---|---|---|
| Preoperative | Postoperative | ||
| n (patients) | 38 | 35 | |
| n (eyes) | 38 | 35 | |
| age (years) | 67.24 (59.79; 74.70) | 67.92 (60.14; 75.22) | 70.31 (62.93; 77.02) |
| gender (m:f) | 19:19 | 16:19 | |
| eye (r:l) | 16:22 | 18:17 | |
| visual acuity [logMAR] | 0.50 (0.70; 0.40) | 0.20 (0.30; 0.10) | 0.10 (0.20; 0.00) |
| spherical equivalent [D] | 0.00 (−1.38; 0.94) | −0.35 (−1.12; 0.34) | −0.25 (−1.18; 0.45) |
| Localization | Corneal Thickness Median (25% Quartile; 75% Quartile) | p Values | |||||
|---|---|---|---|---|---|---|---|
| Pre-Operative | Post-Operative | Controls | Pre- vs. Post-Operative | Pre-Operative vs. Controls | Post-Operative vs. Controls | ||
| Total | Central | 617.70 (564.21; 672.34) | 527.68 (484.75; 555.53) | 532.31 (501.71; 571.28) | <0.01 | <0.01 | 0.28 |
| Paracentral | 608.28 (576.53; 677.58) | 551.22 (506.39; 576.94) | 549.53 (519.94; 590.16) | <0.01 | <0.01 | 0.51 | |
| Mid-peripheral | 616.48 (592.23; 676.66) | 579.65 (535.20; 607.68) | 577.36 (543.73; 614.87) | <0.01 | <0.01 | 0.71 | |
| Stroma | Central | 563.46 (510.17; 617.01) | 478.83 (430.75; 499.64) | 466.86 (439.32; 507.78) | <0.01 | <0.01 | 0.75 |
| Paracentral | 554.71 (520.82; 622.22) | 502.91 (455.57; 526.95) | 481.18 (460.00; 526.18) | <0.01 | <0.01 | 0.96 | |
| Mid-peripheral | 562.38 (495.85; 620.82) | 532.41 (483.37; 557.18) | 505.23 (483.10; 552.22) | <0.01 | <0.01 | 0.68 | |
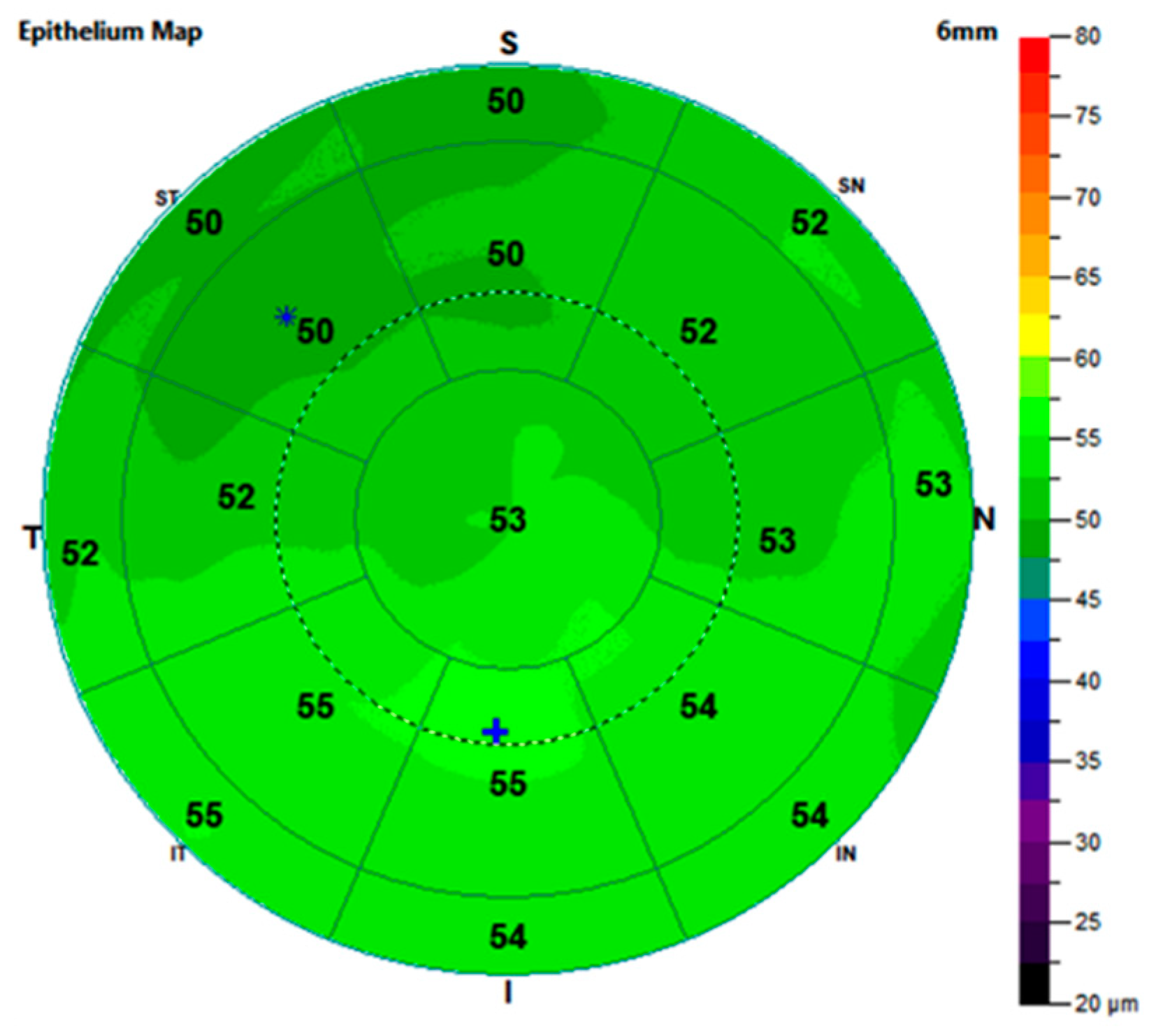
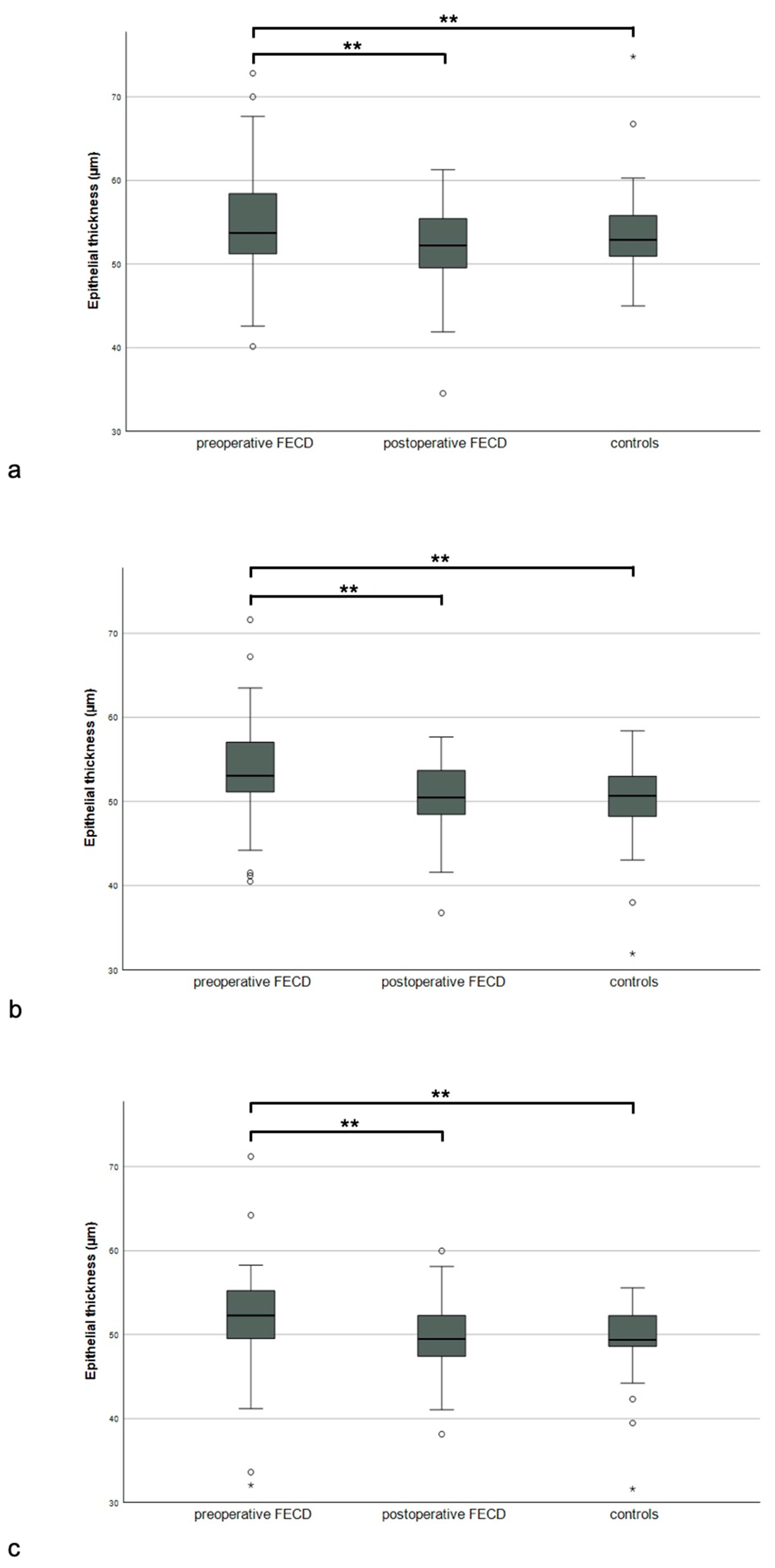
| Epithelium | Central | 54.42 (51.79; 60.20) | 52.10 (48.68; 55.41) | 52.60 (50.67; 54.59) | <0.01 | 0.01 | 0.51 |
| Paracentral | 53.67 (51.12; 57.85) | 50.25 (47.34; 53.62) | 50.26 (48.20; 52.67) | <0.01 | <0.01 | 0.87 | |
| Mid-peripheral | 52.55 (49.55; 55.87) | 48.95 (46.68; 52.23) | 49.25 (48.58; 51.97) | <0.01 | 0.02 | 0.54 | |
| Localization | Epithelial Thickness Median (25% Quartile; 75% Quartile) | p-Value | ||||
|---|---|---|---|---|---|---|
| Preoperative | Postoperative | Controls | Pre- vs. Postoperative | Post-Operative vs. Controls | ||
| paracentral | temporal | 53.07 (49.46; 57.32) | 50.17 (47.05; 53.50) | 49.67 (47.55; 52.43) | 0.01 | 0.96 |
| superior-temporal | 52.00 (48.61; 56.19) | 49.02 (45.52; 51.37) | 48.90 (47.22; 50.79) | <0.01 | 0.90 | |
| superior | 51.59 (49.55; 55.70) | 48.00 (45.94; 51.63) | 48.12 (46.83; 50.90) | <0.01 | 0.90 | |
| superior-nasal | 52.70 (49.70; 56.74) | 49.96 (46.78; 52.84) | 49.16 (47.50; 51.84) | 0.01 | 0.67 | |
| nasal | 53.49 (50.50; 57.57) | 49.84 (47.92; 53.95) | 50.57 (48.18; 53.92) | 0.02 | 0.83 | |
| inferior-nasal | 55.13 (52.46; 58.63) | 52.00 (48.17; 54.91) | 51.48 (49.87; 54.04) | <0.01 | 0.76 | |
| inferior | 55.07 (53.09; 58.84) | 53.15 (49.20; 55.91) | 51.50 (49.80; 55.09) | <0.01 | 0.68 | |
| inferior-temporal | 54.24 (51.16; 58.31) | 52.65 (48.07; 55.79) | 51.32 (48.44; 54.24) | 0.04 | 0.39 | |
| mid-peripheral | temporal | 50.96 (49.43; 54.04) | 49.41 (46.11; 52.18) | 49.70 (47.75; 51.86) | 0.01 | 0.72 |
| superior-temporal | 49.92 (46.97; 53.81) | 47.35 (43.54; 50.05) | 48.12 (44.94; 49.74) | <0.01 | 0.87 | |
| superior | 50.38 (47.55; 53.19) | 46.34 (43.00; 49.33) | 46.22 (43.90; 49.34) | <0.01 | 0.83 | |
| superior-nasal | 52.19 (49.34; 55.64) | 48.89 (44.79; 51.68) | 46.79 (45.31; 52.64) | <0.01 | 0.62 | |
| nasal | 54.02 (49.79; 56.22) | 50.43 (46.58; 53.38) | 50.37 (48.14; 52.63) | 0.05 | 0.67 | |
| inferior-nasal | 54.19 (50.64; 56.98) | 51.39 (47.80; 54.70) | 51.57 (49.96; 53.48) | 0.01 | 0.69 | |
| inferior | 54.68 (49.55; 57.55) | 52.03 (48.16; 54.32) | 51.57 (49.16; 53.40) | 0.02 | 0.98 | |
| inferior-temporal | 52.55 (49.81; 56.26) | 51.78 (47.95; 54.37) | 52.76 (50.77; 53.83) | 0.14 | 0.22 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Storp, J.J.; Lahme, L.; Al-Nawaiseh, S.; Eter, N.; Alnawaiseh, M. Descemet Membrane Endothelial Keratoplasty (DMEK) Reduces the Corneal Epithelial Thickness in Fuchs’ Patients. J. Clin. Med. 2023, 12, 3573. https://doi.org/10.3390/jcm12103573
Storp JJ, Lahme L, Al-Nawaiseh S, Eter N, Alnawaiseh M. Descemet Membrane Endothelial Keratoplasty (DMEK) Reduces the Corneal Epithelial Thickness in Fuchs’ Patients. Journal of Clinical Medicine. 2023; 12(10):3573. https://doi.org/10.3390/jcm12103573
Chicago/Turabian StyleStorp, Jens Julian, Larissa Lahme, Sami Al-Nawaiseh, Nicole Eter, and Maged Alnawaiseh. 2023. "Descemet Membrane Endothelial Keratoplasty (DMEK) Reduces the Corneal Epithelial Thickness in Fuchs’ Patients" Journal of Clinical Medicine 12, no. 10: 3573. https://doi.org/10.3390/jcm12103573
APA StyleStorp, J. J., Lahme, L., Al-Nawaiseh, S., Eter, N., & Alnawaiseh, M. (2023). Descemet Membrane Endothelial Keratoplasty (DMEK) Reduces the Corneal Epithelial Thickness in Fuchs’ Patients. Journal of Clinical Medicine, 12(10), 3573. https://doi.org/10.3390/jcm12103573