Mitral and Tricuspid Valve Disease in Athletes
Abstract
1. Introduction
2. Training-Induced Atrioventricular Valve Remodeling in Athletes
3. Multimodality Imaging Assessment of Mitral and Tricuspid Valve Disease in Athletes
3.1. Resting Echocardiography
3.2. Exercise Stress Echocardiography
3.3. Three-Dimensional Echocardiography
3.4. Myocardial Work
3.5. Cardiac Magnetic Resonance
3.6. Cardiac Computed Tomography
4. Atrioventricular Valve Disease in Athletes
4.1. Mitral Valve Prolapse
4.2. Mitral Valve Regurgitation
4.3. Mitral Valve Stenosis
4.4. Tricuspid Valve Regurgitation
4.5. Tricuspid Valve Stenosis
5. Eligibility for Sports
5.1. Mitral Valve Prolapse
5.2. Mitral Valve Regurgitation
5.3. Mitral Valve Stenosis
5.4. Tricuspid Valve Regurgitation
5.5. Tricuspid Valve Stenosis
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| AF | Atrial fibrillation |
| AFTR | Atrial functional tricuspid regurgitation |
| BP | Blood pressure |
| CAs | Competitive athletes |
| CCT | Cardiac computed tomography |
| CMR | Cardiac magnetic resonance |
| CO | Cardiac output |
| CV | Cardiovascular |
| DCM | Dilated cardiomyopathy |
| ECV | Extracellular volume |
| EICR | Exercise-induced cardiovascular remodeling |
| ESE | Exercise stress echocardiography |
| GCW | Global constructive work |
| GLS | Global longitudinal strain |
| GWE | Global work efficiency |
| GWI | Global work index |
| GWW | Global wasted work |
| HCM | Hypertrophic cardiomyopathy |
| HR | Heart rate |
| LA | Left atrium |
| LGE | Late gadolinium enhancement |
| LV | Left ventricle |
| LVEDD | Left ventricle end-diastolic diameter |
| LVEDV | Left ventricle end-diastolic volume |
| LVEF | Left ventricular ejection fraction |
| LVOT | Left ventricular outflow tract |
| MA | Mitral annulus |
| MAD | Mitral annular disjunction |
| MDTR | Mid-diastolic tricuspid regurgitation |
| MR | Mitral regurgitation |
| MS | Mitral stenosis |
| MV | Mitral valve |
| MVA | Mitral valve area |
| MVP | Mitral valve prolapse |
| MW | Myocardial work |
| PAP | Pulmonary artery pressure |
| PC | Phase-contrast |
| PISA | Proximal isovelocity surface area |
| PMs | Papillary muscles |
| PSL | Pressure-strain loop |
| RA | Right atrium |
| RV | Right ventricle |
| RVEIO | Right ventricle early inflow–outflow |
| RF | Regurgitant fraction |
| RVol | Regurgitant volume |
| RVOT | Right ventricular outflow tract |
| RWT | Relative wall thickness |
| SCD | Sudden cardiac death |
| SPAP | Systolic pulmonary artery pressure |
| SV | Stroke volume |
| TA | Tricuspid annulus |
| TR | Tricuspid regurgitation |
| TS | Tricuspid stenosis |
| TV | Tricuspid valve |
| TTE | Transthoracic echocardiography |
| VAs | Ventricular arrhythmias |
| VC | Vena contracta |
| VFTR | Ventricular functional tricuspid regurgitation |
| VHD | Valvular heart disease |
| VTI | Velocity time integral |
| 2DE | Two-dimensional echocardiography |
| 3DE | Three-dimensional echocardiography |
References
- Van Buuren, F.; Gati, S.; Sharma, S.; Papadakis, M.; Adami, P.E.; Niebauer, J.; Pelliccia, A.; Rudolph, V.; Börjesson, M.; Carre, F.; et al. Athletes with valvular heart disease and competitive sports: A position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology. Eur. J. Prev. Cardiol. 2021, 28, 1569–1578. [Google Scholar] [CrossRef]
- Petek, B.J.; Baggish, A.L. Valvular Heart Disease in Athletes. Curr. Treat. Options Cardiovasc. Med. 2021, 23, 69. [Google Scholar] [CrossRef]
- Gjerdalen, G.F.; Hisdal, J.; Solberg, E.E.; Andersen, T.E.; Radunovic, Z.; Steine, K. Atrial Size and Function in Athletes. Int. J. Sports Med. 2015, 36, 1170–1176. [Google Scholar] [CrossRef]
- Fábián, A.; Lakatos, B.K.; Tokodi, M.; Kiss, A.R.; Sydó, N.; Csulak, E.; Kispál, E.; Babity, M.; Szűcs, A.; Kiss, O.; et al. Geometrical remodeling of the mitral and tricuspid annuli in response to exercise training: A 3D echocardiographic study in elite athletes. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1774–H1785. [Google Scholar] [CrossRef]
- Gati, S.; Malhotra, A.; Sharma, S. Exercise recommendations in patients with valvular heart disease. Heart 2019, 105, 106–110. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease: The Task Force on sports cardiology and exercise in patients with cardiovascular disease of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Douglas, P.S.; Berman, G.O.; O’Toole, M.L.; Hiller, W.D.B.; Reichek, N. Prevalence of multivalvular regurgitation in athletes. Am. J. Cardiol. 1989, 64, 209–212. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.W.; Kim, J.H.; Shah, A.B.; Phelan, D.; Emery, M.S.; Wasfy, M.M.; Fernandez, A.B.; Bunch, T.J.; Dean, P.; Danielian, A.; et al. Exercise-Induced Cardiovascular Adaptations and Approach to Exercise and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1453–1470. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Caselli, S. Structural and functional adaptations in the athlete’s heart. In The ESC Textbook of Sports Cardiology, 1st ed.; Pellicia, A., Heidbuchel, H., Corrado, D., Börjesson, M., Sharma, S., Eds.; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Sharma, S.; Drezner, J.A.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Börjesson, M.; Salerno, J.C.; et al. International recommendations for electrocardiographic interpretation in athletes. Eur. Heart J. 2018, 39, 1466–1480. [Google Scholar] [CrossRef] [PubMed]
- Schmied, C.M.; Wilhelm, M. Athlete’s Heart: Basic Physiology and Adaptation to Exercise. In Textbook of Sports and Exercise Cardiology; Springer: Berlin/Heidelberg, Germany, 2020; pp. 29–51. [Google Scholar]
- Schmied, C.; Sharma, S. Mitral Valve Prolapse in Relation to Sport. In ESC Textbook of Sports Cardiology; Pelliccia, A., Heidbuchel, H., Corrado, D., Börjesson, M., Sanjay, S., Eds.; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Huonker, M.; Halle, M.; Keul, J. Structural and functional adaptations of the cardiovascular system by training. Int. J. Sport. Med. 1996, 17, S164–S172. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, R.; Schmied, C.; Niederseer, D.; Guazzi, M. Cardiopulmonary Exercise Test Parameters in Athletic Population: A Review. J. Clin. Med. 2021, 10, 5073. [Google Scholar] [CrossRef] [PubMed]
- Segreti, A.; Picarelli, F.; Di Gioia, G.; Coletti, F.; Crispino, S.P.; Fanale, V.; Fossati, C.; Antonelli Incalzi, R.; Pigozzi, F.; Grigioni, F. Athlete’s heart or heart disease in the athlete? Evaluation by cardiopulmonary exercise testing. J. Sports Med. Phys. Fitness 2023. online ahead of print. [Google Scholar] [CrossRef]
- Muraru, D.; Addetia, K.; Guta, A.C.; Ochoa-Jimenez, R.C.; Genovese, D.; Veronesi, F.; Basso, C.; Iliceto, S.; Badano, L.P.; Lang, R.M. Right atrial volume is a major determinant of tricuspid annulus area in functional tricuspid regurgitation: A three-dimensional echocardiographic study. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Silvestry, F.E. Mechanistic insights into mitral regurgitation due to atrial fibrillation: Atrial functional mitral regurgitation. Trends Cardiovasc. Med. 2016, 26, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Itabashi, Y.; Mihara, H.; Berdejo, J.; Kobayashi, S.; Siegel, R.J.; Shiota, T. Functional Tricuspid Regurgitation Caused by Chronic Atrial Fibrillation: A Real-Time 3-Dimensional Transesophageal Echocardiography Study. Circ. Cardiovasc. Imaging 2017, 10, e004897. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, R.F.; Burt, F.X.; Panidis, I.P.; Bove, A.A. Echocardiographic predictors of mitral regurgitation in high school and collegiate competitive athletes. Am. J. Cardiol. 2013, 112, 1652–1656. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Caselli, S.; Sharma, S.; Basso, C.; Bax, J.J.; Corrado, D.; D’Andrea, A.; D’Ascenzi, F.; Di Paolo, F.M.; Edvardsen, T.; et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) Joint Position Statement: Recommendations for the Indication and Interpretation of Cardiovascular Imaging in the Evaluation of the Athlete’s Heart. Eur. Heart J. 2018, 39, 1949–1969. [Google Scholar]
- D’andrea, A.; Sperlongano, S.; Russo, V.; D’ascenzi, F.; Benfari, G.; Renon, F.; Palermi, S.; Ilardi, F.; Giallauria, F.; Limongelli, G.; et al. The Role of Multimodality Imaging in Athlete’s Heart Diagnosis: Current Status and Future Directions. J. Clin. Med. 2021, 10, 5126. [Google Scholar] [CrossRef]
- Maron, M.S.; Olivotto, I.; Harrigan, C.; Appelbaum, E.; Gibson, C.M.; Lesser, J.R.; Haas, T.S.; Udelson, J.E.; Manning, W.J.; Maron, B.J. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation 2011, 124, 40–47. [Google Scholar] [CrossRef]
- Baggish, A.L.; Battle, R.W.; Beaver, T.A.; Border, W.L.; Douglas, P.S.; Kramer, C.M.; Martinez, M.W.; Mercandetti, J.H.; Phelan, D.; Singh, T.K.; et al. Recommendations on the Use of Multimodality Cardiovascular Imaging in Young Adult Competitive Athletes: A Report from the American Society of Echocardiography in Collaboration with the Society of Cardiovascular Computed Tomography and the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2020, 33, 523–549. [Google Scholar]
- Palmisano, A.; Darvizeh, F.; Cundari, G.; Rovere, G.; Ferrandino, G.; Nicoletti, V.; Cilia, F.; De Vizio, S.; Palumbo, R.; Esposito, A.; et al. Advanced cardiac imaging in athlete’s heart: Unravelling the grey zone between physiologic adaptation and pathology. Radiol. Med. 2021, 126, 1518–1531. [Google Scholar] [CrossRef] [PubMed]
- Caselli, S.; D’Ascenzi, F. Echocardiogram: Morphological and Functional Evaluation Including New Echocardiographic Techniques. In The ESC Textbook of Sports Cardiology; Pelliccia, A., Heidbuchel, H., Corrado, D., Börjesson, M., Sanjay, S., Eds.; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev. Esp. Cardiol. 2022, 75, 524. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Dhutia, H.; Finocchiaro, G.; Gatis, S.; Beasley, I.; Clift, P.; Cowie, C.; Kenny, A.; Mayet, J.; Oxborough, D.; et al. Outcomes of Cardiac Screening in Adolescent Soccer Players. N. Engl. J. Med. 2018, 379, 524–534. [Google Scholar] [CrossRef]
- Grigioni, F.; Russo, A.; Pasquale, F.; Biagini, E.; Barberini, F.; Ferlito, M.; Leone, O.; Rapezzi, C. Clinical Use of Doppler Echocardiography in Organic Mitral Regurgitation: From Diagnosis to Patients’ Management. J. Cardiovasc. Ultrasound. 2015, 23, 121–133. [Google Scholar] [CrossRef]
- Morganroth, J.; Maron, B.J. The Athlete’s Heart Syndrome: A New Perspective. Ann. N.Y. Acad. Sci. 1977, 301, 931–941. [Google Scholar] [CrossRef]
- Wasfy, M.M.; Weiner, R.B.; Wang, F.; Berkstresser, B.; Lewis, G.D.; DeLuca, J.R.; Hutter, A.M.; Picard, M.H.; Baggish, A.L. Endurance Exercise-Induced Cardiac Remodeling: Not All Sports Are Created Equal. J. Am. Soc. Echocardiogr. 2015, 28, 1434–1440. [Google Scholar] [CrossRef]
- Pelliccia, A.; Culasso, F.; Di Paolo, F.M.; Maron, B.J. Physiologic left ventricular cavity dilatation in elite athletes. Ann. Intern. Med. 1999, 130, 23–31. [Google Scholar] [CrossRef]
- D’Andrea, A.; Radmilovic, J.; Carbone, A.; Mandoli, G.E.; Santoro, C.; Evola, V.; Bandera, F.; D’Ascenzi, F.; Bossone, E.; Galderisi, M.; et al. Speckle tracking evaluation in endurance athletes: The “optimal” myocardial work. Int. J. Cardiovasc. Imaging 2020, 36, 1679–1688. [Google Scholar] [CrossRef]
- D’Andrea, A.; Carbone, A.; Radmilovic, J.; Russo, V.; Fabiani, D.; Di Maio, M.; Ilardi, F.; Giallauria, F.; Caputo, A.; Cirillo, T.; et al. Myocardial Work Efficiency in Physiologic Left Ventricular Hypertrophy of Power Athletes. J. Cardiovasc. Echogr. 2022, 32, 154–159. [Google Scholar]
- Iskandar, A.; Mujtaba, M.T.; Thompson, P.D. Left Atrium Size in Elite Athletes. JACC Cardiovasc. Imaging 2015, 8, 753–762. [Google Scholar] [CrossRef]
- Pelliccia, A.; Maron, B.J.; Di Paolo, F.M.; Biffi, A.; Quattrini, F.M.; Pisicchio, C.; Roselli, A.; Caselli, S.; Culasso, F. Prevalence and clinical significance of left atrial remodeling in competitive athletes. J. Am. Coll. Cardiol. 2005, 46, 690–696. [Google Scholar] [CrossRef]
- Haykowsky, M.J.; Samuel, T.J.; Nelson, M.D.; La Gerche, A. Athlete’s Heart: Is the Morganroth Hypothesis Obsolete? Heart Lung Circ. 2018, 27, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- La Gerche, A.; Rakhit, D.J.; Claessen, G. Exercise and the right ventricle: A potential Achilles’ heel. Cardiovasc. Res. 2017, 113, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Riegler, L.; Golia, E.; Cocchia, R.; Scarafile, R.; Salerno, G.; Pezzullo, E.; Nunziata, L.; Citro, R.; Cuomo, S.; et al. Range of right heart measurements in top-level athletes: The training impact. Int. J. Cardiol. 2013, 164, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Pelliccia, A.; Natali, B.M.; Zacà, V.; Cameli, M.; Alvino, F.; Malandrino, A.; Palmitesta, P.; Zorzi, A.; Corrado, D.; et al. Morphological and functional adaptation of left and right atria induced by training in highly trained female athletes. Circ. Cardiovasc. Imaging 2014, 7, 222–229. [Google Scholar] [CrossRef]
- McClean, G.; George, K.; Lord, R.; Utomi, V.; Jones, N.; Somauroo, J.; Fletcher, S.; Oxborough, D. Chronic adaptation of atrial structure and function in elite male athletes. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 417–422. [Google Scholar] [CrossRef]
- Grossmann, G.; Giesler, M.; Stein, M.; Kochs, M.; Höher, M.; Hombach, V. Quantification of mitral and tricuspid regurgitation by the proximal flow convergence method using two-dimensional colour Doppler and colour Doppler M-mode: Influence of the mechanism of regurgitation. Int. J. Cardiol. 1998, 66, 299–307. [Google Scholar] [CrossRef]
- Delling, F.N.; Vasan, R.S. Epidemiology and pathophysiology of mitral valve prolapse: New insights into disease progression, genetics, and molecular basis. Circulation 2014, 129, 2158–2170. [Google Scholar] [CrossRef]
- Fukuda, S.; Song, J.K.; Mahara, K.; Kuwaki, H.; Jang, J.Y.; Takeuchi, M.; Sun, B.J.; Kim, Y.J.; Miyamoto, T.; Oginosawa, Y.; et al. Basal Left Ventricular Dilatation and Reduced Contraction in Patients with Mitral Valve Prolapse Can Be Secondary to Annular Dilatation: Preoperative and Postoperative Speckle-Tracking Echocardiographic Study on Left Ventricle and Mitral Valve Annulus Interaction. Circ. Cardiovasc. Imaging 2016, 9, e005113. [Google Scholar]
- Lee, A.P.-W.; Hsiung, M.C.; Salgo, I.S.; Fang, F.; Xie, J.-M.; Zhang, Y.-C.; Lin, Q.-S.; Looi, J.-L.; Wan, S.; Wong, R.H.L.; et al. Quantitative analysis of mitral valve morphology in mitral valve prolapse with real-time 3-dimensional echocardiography: Importance of annular saddle shape in the pathogenesis of mitral regurgitation. Circulation 2013, 127, 832–841. [Google Scholar] [CrossRef]
- Lauretta, L.; Casalino, G.; Amzulescu, M.; David-Cojocariu, A.; Unger, P. How to improve tissue Doppler imaging sensitivity to detect the Pickelhaube sign. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 746. [Google Scholar] [CrossRef]
- Muthukumar, L.; Rahman, F.; Jan, M.F.; Shaikh, A.; Kalvin, L.; Dhala, A.; Jahangir, A.; Tajik, A.J. The Pickelhaube Sign: Novel Echocardiographic Risk Marker for Malignant Mitral Valve Prolapse Syndrome. JACC Cardiovasc. Imaging 2017, 10, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Capoulade, R.; Piriou, N.; Serfaty, J.-M.; Le Tourneau, T. Multimodality imaging assessment of mitral valve anatomy in planning for mitral valve repair in secondary mitral regurgitation. J. Thorac. Dis. 2017, 9, S640–S660. [Google Scholar] [CrossRef] [PubMed]
- Grayburn, P.A.; Thomas, J.D. Basic Principles of the Echocardiographic Evaluation of Mitral Regurgitation. JACC Cardiovasc. Imaging 2021, 14, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Grayburn, P.A.; Sannino, A.; Packer, M. Proportionate and Disproportionate Functional Mitral Regurgitation: A New Conceptual Framework That Reconciles the Results of the MITRA-FR and COAPT Trials. JACC Cardiovasc. Imaging 2019, 12, 353–362. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef]
- Obadia, J.F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef]
- Hahn, R.T.; Thomas, J.D.; Khalique, O.K.; Cavalcante, J.L.; Praz, F.; Zoghbi, W.A. Imaging Assessment of Tricuspid Regurgitation Severity. JACC Cardiovasc. Imaging 2019, 12, 469–490. [Google Scholar] [CrossRef]
- Hahn, R.T.; Zamorano, J.L. The need for a new tricuspid regurgitation grading scheme. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1342–1343. [Google Scholar] [CrossRef]
- Izgi, I.A.; Acar, E.; Kilicgedik, A.; Guler, A.; Cakmak, E.O.; Demirel, M.; Izci, S.; Yilmaz, M.F.; Inanir, M.; Kirma, C. A new and simple method for clarifying the severity of tricuspid regurgitation. Echocardiography 2017, 34, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.-W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef] [PubMed]
- Millar, L.M.; Fanton, Z.; Finocchiaro, G.; Sanchez-Fernandez, G.; Dhutia, H.; Malhotra, A.; Merghani, A.; Papadakis, M.; Behr, E.R.; Bunce, N.; et al. Differentiation between athlete’s heart and dilated cardiomyopathy in athletic individuals. Heart 2020, 106, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Booher, A.M.; Bach, D.S. Exercise hemodynamics in valvular heart disease. Curr. Cardiol. Rep. 2011, 13, 226–233. [Google Scholar] [CrossRef]
- Bonow, R.O.; Nishimura, R.A.; Thompson, P.D.; Udelson, J.E.; American Heart Association Electrocardiography and Arrhythmias Committee of Council on Clinical Cardiology; Council on Cardiovascular Disease in Young; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology and American College of Cardiology. Eligibility and Disqualification Recommendations for Competitive Athletes with Cardiovascular Abnormalities: Task Force 5: Valvular Heart Disease: A Scientific Statement from the American Heart Association and American College of Cardiology. J. Am. Coll. Cardiol. 2015, 66, 2385–2392. [Google Scholar] [CrossRef]
- Leung, D.Y.; Griffin, B.P.; Stewart, W.J.; Cosgrove, D.M.; Thomas, J.D.; Marwick, T.H. Left ventricular function after valve repair for chronic mitral regurgitation: Predictive value of preoperative assessment of contractile reserve by exercise echocardiography. J. Am. Coll. Cardiol. 1996, 28, 1198–1205. [Google Scholar] [CrossRef]
- Magne, J.; Mahjoub, H.; Dulgheru, R.; Pibarot, P.; Pierard, L.A.; Lancellotti, P. Left ventricular contractile reserve in asymptomatic primary mitral regurgitation. Eur. Heart J. 2014, 35, 1608–1616. [Google Scholar] [CrossRef]
- Lancellotti, P.; Fattouch, K.; La Canna, G. Therapeutic decision-making for patients with fluctuating mitral regurgitation. Nat. Rev. Cardiol. 2015, 12, 212–219. [Google Scholar] [CrossRef]
- Magne, J.; Lancellotti, P.; Piérard, L.A. Exercise-induced changes in degenerative mitral regurgitation. J. Am. Coll. Cardiol. 2010, 56, 300–309. [Google Scholar] [CrossRef]
- Giga, V.; Ostojic, M.; Vujisic-Tesic, B.; Djordjevic-Dikic, A.; Stepanovic, J.; Beleslin, B.; Petrovic, M.; Nedeljkovic, M.; Nedeljkovic, I.; Milic, N. Exercise-induced changes in mitral regurgitation in patients with prior myocardial infarction and left ventricular dysfunction: Relation to mitral deformation and left ventricular function and shape. Eur. Heart J. 2005, 26, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Oxborough, D.; Augustine, D.X.; Bedair, R.; Harkness, A.; Rana, B.; Robinson, S.; Badano, L.P.; Education Committee of the British Society of Echocardiography. Echocardiographic assessment of the tricuspid and pulmonary valves: A practical guideline from the British Society of Echocardiography. Echo. Res. Pract. 2020, 7, G95–G122. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, S.; Walker, S.; Loudon, B.L.; Gollop, N.D.; Wilson, A.M.; Lowery, C.; Frenneaux, M.P. Assessment of pulmonary artery pressure by echocardiography—A comprehensive review. Int. J. Cardiol. Heart Vasc. 2016, 12, 45–51. [Google Scholar] [CrossRef]
- Zamorano, J.; Cordeiro, P.; Sugeng, L.; de Isla, L.P.; Weinert, L.; Macaya, C.; Rodríguez, E.; Lang, R.M. Real-time three-dimensional echocardiography for rheumatic mitral valve stenosis evaluation: An accurate and novel approach. J. Am. Coll. Cardiol. 2004, 43, 2091–2096. [Google Scholar] [CrossRef] [PubMed]
- Muraru, D.; Badano, L.P.; Sarais, C.; Soldà, E.; Iliceto, S. Evaluation of tricuspid valve morphology and function by transthoracic three-dimensional echocardiography. Curr. Cardiol. Rep. 2011, 13, 242–249. [Google Scholar] [CrossRef]
- Mantegazza, V.; Gripari, P.; Tamborini, G.; Muratori, M.; Fusini, L.; Ali, S.G.; Garlaschè, A.; Pepi, M. 3D echocardiography in mitral valve prolapse. Front. Cardiovasc. Med. 2022, 9, 1050476. [Google Scholar] [CrossRef]
- Antoine, C.; Mantovani, F.; Benfari, G.; Mankad, S.V.; Maalouf, J.F.; Michelena, H.I.; Enriquez-Sarano, M. Pathophysiology of Degenerative Mitral Regurgitation: New 3-Dimensional Imaging Insights. Circ. Cardiovasc. Imaging 2018, 11, e005971. [Google Scholar] [CrossRef]
- Lee, A.P.; Jin, C.N.; Fan, Y.; Wong, R.H.L.; Underwood, M.J.; Wan, S. Functional Implication of Mitral Annular Disjunction in Mitral Valve Prolapse: A Quantitative Dynamic 3D Echocardiographic Study. JACC Cardiovasc. Imaging 2017, 10, 1424–1433. [Google Scholar] [CrossRef]
- Badano, L.P.; Miglioranza, M.H.; Mihăilă, S.; Peluso, D.; Xhaxho, J.; Marra, M.P.; Cucchini, U.; Soriani, N.; Iliceto, S.; Muraru, D. Left Atrial Volumes and Function by Three-Dimensional Echocardiography: Reference Values, Accuracy, Reproducibility, and Comparison with Two-Dimensional Echocardiographic Measurements. Circ. Cardiovasc. Imaging 2016, 9, e004229. [Google Scholar] [CrossRef]
- Addetia, K.; Muraru, D.; Badano, L.P.; Lang, R.M. New Directions in Right Ventricular Assessment Using 3-Dimensional Echocardiography. JAMA Cardiol. 2019, 4, 936–944. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Liu, S.; Datta, S.; Rajagopalan, S.; Ryan, T.; Igo, S.R.; Jackson, M.S.; Little, S.H.; De Michelis, N.; Vannan, M.A. Quantification of chronic functional mitral regurgitation by automated 3-dimensional peak and integrated proximal isovelocity surface area and stroke volume techniques using real-time 3-dimensional volume color Doppler echocardiography: In vitro and clinical validation. Circ. Cardiovasc. Imaging 2013, 6, 125–133. [Google Scholar] [PubMed]
- Hahn, R.T.; Badano, L.P.; Bartko, P.E.; Muraru, D.; Maisano, F.; Zamorano, J.L.; Donal, E. Tricuspid regurgitation: Recent advances in understanding pathophysiology, severity grading and outcome. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 913–929. [Google Scholar] [CrossRef] [PubMed]
- Abawi, D.; Rinaldi, T.; Faragli, A.; Pieske, B.; Morris, D.A.; Kelle, S.; Tschöpe, C.; Zito, C.; Alogna, A. The non-invasive assessment of myocardial work by pressure-strain analysis: Clinical applications. Heart Fail. Rev. 2022, 27, 1261–1279. [Google Scholar] [CrossRef] [PubMed]
- Tokodi, M.; Oláh, A.; Fábián, A.; Lakatos, B.K.; Hizoh, I.; Ruppert, M.; Sayour, A.A.; Barta, B.A.; Kiss, O.; Sydó, N.; et al. Novel insights into the athlete’s heart: Is myocardial work the new champion of systolic function? Eur. Heart J. Cardiovasc. Imaging 2022, 23, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Ilardi, F.; D’Andrea, A.; D’Ascenzi, F.; Bandera, F.; Benfari, G.; Esposito, R.; Malagoli, A.; Mandoli, G.E.; Santoro, C.; Russo, V.; et al. Myocardial Work by Echocardiography: Principles and Applications in Clinical Practice. J. Clin. Med. 2021, 10, 4521. [Google Scholar] [CrossRef]
- Yingchoncharoen, T.; Agarwal, S.; Popovic, Z.B.; Marwick, T.H. Normal ranges of left ventricular strain: A meta-analysis. J. Am. Soc. Echocardiogr. 2013, 26, 185–191. [Google Scholar] [CrossRef]
- Voigt, J.U.; Cvijic, M. 2- and 3-Dimensional Myocardial Strain in Cardiac Health and Disease. JACC Cardiovasc. Imaging 2019, 12, 1849–1863. [Google Scholar] [CrossRef]
- Borzì, D.D.; Saladino, S.; Losi, V.; Faro, D.C.; Monte, I.P. Strain and Myocardial Work Index during Echo Exercise to Evaluate Myocardial Function in Athletes. J. Cardiovasc. Echogr. 2022, 32, 82–88. [Google Scholar]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure–strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef]
- Basso, C.; Marra, M.P.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Van Wijngaarden, S.E.; Abou, R.; Hiemstra, Y.L.; Marsan, N.A.; Bax, J.J.; Delgado, V. Regional Left Ventricular Myocardial Mechanics in Degenerative Myxomatous Mitral Valve Disease: A Comparison Between Fibroelastic Deficiency and Barlow’s Disease. JACC Cardiovasc. Imaging 2018, 11, 1362–1364. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, R.; Marchetta, S.; Dulgheru, R.; Ilardi, F.; Sugimoto, T.; Robinet, S.; Cimino, S.; Go, Y.Y.; Bernard, A.; Kacharava, G.; et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, S.; Gulhar, R.; Lim, L.; Bibby, D.; Fang, Q.; Nah, G.; Abraham, T.P.; Schiller, N.B.; Delling, F.N. Left ventricular mechanical dispersion predicts arrhythmic risk in mitral valve prolapse. Heart 2019, 105, 1063–1069. [Google Scholar] [CrossRef]
- Yedidya, I.; Lustosa, R.P.; Fortuni, F.; van der Bijl, P.; Namazi, F.; Vo, N.M.; Meucci, M.C.; Marsan, N.A.; Bax, J.J.; Delgado, V. Prognostic Implications of Left Ventricular Myocardial Work Indices in Patients with Secondary Mitral Regurgitation. Circ. Cardiovasc. Imaging 2021, 14, e012142. [Google Scholar] [CrossRef] [PubMed]
- Butcher, S.C.; Fortuni, F.; Montero-Cabezas, J.M.; Abou, R.; El Mahdiui, M.; Van Der Bijl, P.; Van Der Velde, E.T.; Marsan, N.A.; Bax, J.J.; Delgado, V. Right ventricular myocardial work: Proof-of-concept for non-invasive assessment of right ventricular function. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, X.; Huang, K.; Gao, Q.; Tian, Y.; Lin, B.; Tang, Y.; Chen, X.; Su, M. Correlations among noninvasive right ventricular myocardial work indices and the main parameters of systolic and diastolic functions. J. Clin. Ultrasound 2022, 50, 873–884. [Google Scholar] [CrossRef]
- Claessen, G.; La Gerche, A. Cardiac Magnetic Resonance Imaging. In The ESC Textbook of Sports Cardiology; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Mantegazza, V.; Volpato, V.; Gripari, P.; Ali, S.G.; Fusini, L.; Italiano, G.; Muratori, M.; Pontone, G.; Tamborini, G.; Pepi, M. Multimodality imaging assessment of mitral annular disjunction in mitral valve prolapse. Heart 2021, 107, 25–32. [Google Scholar] [CrossRef]
- Marra, M.P.; Basso, C.; De Lazzari, M.; Rizzo, S.; Cipriani, A.; Giorgi, B.; Lacognata, C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Morphofunctional Abnormalities of Mitral Annulus and Arrhythmic Mitral Valve Prolapse. Circ. Cardiovasc. Imaging 2016, 9, e005030. [Google Scholar]
- Daza, A.R.; Chokshi, A.; Pardo, P.; Maneiro, N.; Contreras, A.G.; Larrañaga-Moreira, J.M.; Ibañez, B.; Fuster, V.; Friera, L.F.; Solís, J.; et al. Mitral valve prolapse morphofunctional features by cardiovascular magnetic resonance: More than just a valvular disease. J. Cardiovasc. Magn. Reson. 2021, 23, 107. [Google Scholar] [CrossRef]
- El-Tallawi, K.C.; Messika-Zeitoun, D.; Zoghbi, W.A. Assessment of the severity of native mitral valve regurgitation. Prog. Cardiovasc. Dis. 2017, 60, 322–333. [Google Scholar] [CrossRef]
- Kitkungvan, D.; Nabi, F.; Kim, R.J.; Bonow, R.O.; Khan, A.; Xu, J.; Little, S.H.; Quinones, M.A.; Lawrie, G.M.; Zoghbi, W.A.; et al. Myocardial Fibrosis in Patients with Primary Mitral Regurgitation with and without Prolapse. J. Am. Coll. Cardiol. 2018, 72, 823–834. [Google Scholar] [CrossRef]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update: Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. J. Cardiovasc. Magn. Reson. 2020, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Pavon, A.G.; Arangalage, D.; Pascale, P.; Hugelshofer, S.; Rutz, T.; Porretta, A.P.; Le Bloa, M.; Muller, O.; Pruvot, E.; Schwitter, J.; et al. Myocardial extracellular volume by T1 mapping: A new marker of arrhythmia in mitral valve prolapse. J. Cardiovasc. Magn. Reson. 2021, 23, 102. [Google Scholar] [CrossRef] [PubMed]
- Gulsin, G.S.; Singh, A.; McCann, G.P. Cardiovascular magnetic resonance in the evaluation of heart valve disease. BMC Med. Imaging 2017, 17, 67. [Google Scholar] [CrossRef]
- Mathew, R.C.; Löffler, A.I.; Salerno, M. Role of Cardiac Magnetic Resonance Imaging in Valvular Heart Disease: Diagnosis, Assessment, and Management. Curr. Cardiol. Rep. 2018, 20, 119. [Google Scholar] [CrossRef] [PubMed]
- Cawley, P.J.; Maki, J.H.; Otto, C.M. Cardiovascular magnetic resonance imaging for valvular heart disease: Technique and validation. Circulation 2009, 119, 468–478. [Google Scholar] [CrossRef]
- Myerson, S.G. Heart valve disease: Investigation by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2012, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Swift, A.J.; Zhong, L.; Carlhäll, C.-J.; Ebbers, T.; Westenberg, J.; Hope, M.D.; Bucciarelli-Ducci, C.; Bax, J.J.; Myerson, S.G. Assessment of mitral valve regurgitation by cardiovascular magnetic resonance imaging. Nat. Rev. Cardiol. 2020, 17, 298–312. [Google Scholar] [CrossRef]
- Mehta, N.K.; Kim, J.; Siden, J.Y.; Rodriguez-Diego, S.; Alakbarli, J.; Di Franco, A.; Weinsaft, J.W. Utility of cardiac magnetic resonance for evaluation of mitral regurgitation prior to mitral valve surgery. J. Thorac. Dis. 2017, 9, S246–S256. [Google Scholar] [CrossRef]
- Krieger, E.; Lee, J.; Branch, K.; Hamilton-Craig, C. Quantitation of mitral regurgitation with cardiac magnetic resonance imaging: A systematic review. Heart 2016, 102, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.; Nielsen-Kudsk, J.E.; Noordegraaf, A.V.; de Man, F.S. Right Ventricular Fibrosis. Circulation 2019, 139, 269–285. [Google Scholar] [CrossRef]
- Wang, T.K.M.; Unai, S.; Xu, B. Contemporary review in the multi-modality imaging evaluation and management of tricuspid regurgitation. Cardiovasc. Diagn. Ther. 2021, 11, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; La Canna, G.; Pepi, M.; Dulgheru, R.; Dweck, M.; Delgado, V.; Garbi, M.; Vannan, M.A.; et al. Multi-modality imaging assessment of native valvular regurgitation: An EACVI and ESC council of valvular heart disease position paper. Eur. Heart J. Cardiovasc. Imaging 2022, 23, e171–e232. [Google Scholar] [CrossRef]
- Ko, S.M.; Hwang, S.H.; Lee, H.J. Role of Cardiac Computed Tomography in the Diagnosis of Left Ventricular Myocardial Diseases. J. Cardiovasc. Imaging 2019, 27, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Eleid, M.F.; Foley, T.A.; Said, S.M.; Pislaru, S.V.; Rihal, C.S. Severe Mitral Annular Calcification: Multimodality Imaging for Therapeutic Strategies and Interventions. JACC Cardiovasc. Imaging. 2016, 9, 1318–1337. [Google Scholar] [CrossRef]
- Nazari, M.; Khajouei, A.S.; Esfahani, M.A.; Moradi, M. Comparison of the accuracy of cardiac computed tomography angiography and transthoracic echocardiography in the diagnosis of mitral valve prolapse. Adv. Biomed. Res. 2015, 4, 221. [Google Scholar] [CrossRef]
- Toh, H.; Mori, S.; Izawa, Y.; Fujita, H.; Miwa, K.; Suzuki, M.; Takahashi, Y.; Toba, T.; Watanabe, Y.; Kono, A.K.; et al. Prevalence and extent of mitral annular disjunction in structurally normal hearts: Comprehensive 3D analysis using cardiac computed tomography. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 614–622. [Google Scholar] [CrossRef]
- Naoum, C.; Blanke, P.; Cavalcante, J.L.; Leipsic, J. Cardiac Computed Tomography and Magnetic Resonance Imaging in the Evaluation of Mitral and Tricuspid Valve Disease: Implications for Transcatheter Interventions. Circ. Cardiovasc. Imaging 2017, 10, e005331. [Google Scholar] [CrossRef]
- Carbone, A.; D’Andrea, A.; Scognamiglio, G.; Scarafile, R.; Tocci, G.; Sperlongano, S.; Martone, F.; Radmilovic, J.; D’Amato, M.; Liccardo, B.; et al. Mitral Prolapse: An Old Mysterious Entity—The Incremental Role of Multimodality Imaging in Sports Eligibility. J. Cardiovasc. Echogr. 2018, 28, 207–217. [Google Scholar]
- Barlow, J.B.; Bosman, C.K.; Pocock, W.A.; Marchand, P. Late systolic murmurs and non-ejection (“mid-late”) systolic clicks. An analysis of 90 patients. Br. Heart J. 1968, 30, 203–218. [Google Scholar] [CrossRef]
- Aluru, J.S.; Barsouk, A.; Saginala, K.; Rawla, P.; Barsouk, A. Valvular Heart Disease Epidemiology. Med. Sci. 2022, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Jeresaty, R.M. Mitral valve prolapse: Definition and implications in athletes. J. Am. Coll. Cardiol. 1986, 7, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Hancock, E.W.; Cohn, K. The syndrome associated with midsystolic click and late systolic murmur. Am. J. Med. 1966, 41, 183–196. [Google Scholar] [CrossRef]
- Basso, C.; Rizzo, S.; Thiene, G. Cardiovascular Causes of Sudden Death in Athletes. In The ESC Textbook of Sports Cardiology; Pelliccia, A., Heidbuchel, H., Corrado, D., Börjesson, M., Sanjay, S., Eds.; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Sloman, G.; Wong, M.; Walker, J. Arrhythmias on exercise in patients with abnormalities of the posterior leaflet of the mitral valve. Am. Heart J. 1972, 83, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Cavarretta, E.; Peruzzi, M.; Versaci, F.; Frati, G.; Sciarra, L. How to manage an athlete with mitral valve prolapse. Eur. J. Prev. Cardiol. 2020, 28, 1110–1117. [Google Scholar] [CrossRef]
- Basso, C.; Iliceto, S.; Thiene, G.; Marra, M.P. Mitral Valve Prolapse, Ventricular Arrhythmias, and Sudden Death. Circulation 2019, 140, 952–964. [Google Scholar] [CrossRef]
- Alenazy, A.; Eltayeb, A.; Alotaibi, M.K.; Anwar, M.K.; Mulafikh, N.; Aladmawi, M.; Vriz, O. Diagnosis of Mitral Valve Prolapse: Much More than Simple Prolapse. Multimodality Approach to Risk Stratification and Therapeutic Management. J. Clin. Med. 2022, 11, 455. [Google Scholar] [CrossRef] [PubMed]
- Carmo, P.; Andrade, M.J.; Aguiar, C.; Rodrigues, R.; Gouveia, R.; Silva, A.J. Mitral annular disjunction in myxomatous mitral valve disease: A relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc. Ultrasound 2010, 8, 53. [Google Scholar] [CrossRef]
- Essayagh, B.; Sabbag, A.; Antoine, C.; Benfari, G.; Yang, L.-T.; Maalouf, J.; Asirvatham, S.; Michelena, H.; Enriquez-Sarano, M. Presentation and Outcome of Arrhythmic Mitral Valve Prolapse. J. Am. Coll. Cardiol. 2020, 76, 637–649. [Google Scholar] [CrossRef]
- Caselli, S.; Mango, F.; Clark, J.; Pandian, N.G.; Corrado, D.; Autore, C.; Pelliccia, A. Prevalence and Clinical Outcome of Athletes with Mitral Valve Prolapse. Circulation 2018, 137, 2080–2082. [Google Scholar] [CrossRef]
- Levine, R.A.; Schwammenthal, E. Ischemic mitral regurgitation on the threshold of a solution: From paradoxes to unifying concepts. Circulation 2005, 112, 745–758. [Google Scholar] [CrossRef]
- Silbiger, J.J. Novel pathogenetic mechanisms and structural adaptations in ischemic mitral regurgitation. J. Am. Soc. Echocardiogr. 2013, 26, 1107–1117. [Google Scholar] [CrossRef]
- Deferm, S.; Bertrand, P.B.; Verbrugge, F.H.; Verhaert, D.; Rega, F.; Thomas, J.D.; Vandervoort, P.M. Atrial Functional Mitral Regurgitation: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Silbiger, J.J. Does left atrial enlargement contribute to mitral leaflet tethering in patients with functional mitral regurgitation? Proposed role of atriogenic leaflet tethering. Echocardiography 2014, 31, 1310–1311. [Google Scholar] [CrossRef] [PubMed]
- Gaasch, W.H.; Meyer, T.E. Left ventricular response to mitral regurgitation: Implications for management. Circulation 2008, 118, 2298–2303. [Google Scholar] [CrossRef] [PubMed]
- Lesniak-SobelgaEwa, A.; Wicher-Muniak, E.; Kostkiewicz, M.; Olszowska, M.; Musiałek, P.; Klimeczek, P.; Banyś, P.; Pasowicz, M.; Tracz, W.; Podolec, P. Relationship between mitral leaflets angles, left ventricular geometry and mitral deformation indices in patients with ischemic mitral regurgitation: Imaging by echocardiography and cardiac magnetic resonance. Int. J. Cardiovasc. Imaging 2012, 28, 59–67. [Google Scholar] [CrossRef]
- Yoshida, K.; Obase, K. Assessment of mitral valve complex by three-dimensional echocardiography: Therapeutic strategy for functional mitral regurgitation. J. Cardiovasc. Ultrasound 2012, 20, 69–76. [Google Scholar] [CrossRef]
- Schiros, C.G.; Ahmed, M.I.; Sanagala, T.; Zha, W.; McGiffin, D.C.; Bamman, M.M.; Gupta, H.; Lloyd, S.G.; Denney, T.S., Jr.; Dell’Italia, L.J. Importance of three-dimensional geometric analysis in the assessment of the athlete’s heart. Am. J. Cardiol. 2013, 111, 1067–1072. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, 2440–2492. [Google Scholar] [CrossRef]
- Fernandez, A.B.; Thompson, P.D. Exercise Participation for Patients with Valvular Heart Disease: A Review of the Current Guidelines. Curr. Cardiol. Rep. 2021, 23, 49. [Google Scholar] [CrossRef]
- Chandrashekhar, Y.; Westaby, S.; Narula, J. Mitral stenosis. Lancet 2009, 374, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, K.; Izumo, M.; Umemoto, T.; Suzuki, K.; Kitanaka, Y.; Oi, K.; Mizuno, T.; Harada, T.; Ashikaga, T.; Miyairi, T.; et al. Geometry of Tricuspid Valve Apparatus in Patients with Mitral Regurgitation due to Fibroelastic Deficiency versus Barlow Disease: A Real-Time Three-dimensional Transesophageal Echocardiography Study. J. Am. Soc. Echocardiogr. 2020, 33, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- La Gerche, A.; Burns, A.T.; Mooney, D.J.; Inder, W.J.; Taylor, A.J.; Bogaert, J.; MacIsaac, A.I.; Heidbüchel, H.; Prior, D.L. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur. Heart J. 2012, 33, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Spinner, E.M.; Shannon, P.; Buice, D.; Jimenez, J.H.; Veledar, E.; del Nido, P.J.; Adams, D.H.; Yoganathan, A.P. In vitro characterization of the mechanisms responsible for functional tricuspid regurgitation. Circulation 2011, 124, 920–929. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Perhonen, M.; Howden, E.; Peshock, R.; Zhang, R.; Adams-Huet, B.; Haykowsky, M.J.; Levine, B.D. Cardiac remodeling in response to 1 year of intensive endurance training. Circulation 2014, 130, 2152–2161. [Google Scholar] [CrossRef]
- Schindler, M.J.; Schoenfeld, J.; Halle, M.; Scherr, J. Mid-diastolic tricuspid regurgitation: A novel echocardiographic marker for an athlete’s heart? Eur. Heart J. Cardiovasc. Imaging 2020, 21, 820. [Google Scholar] [CrossRef]
- Nemoto, N.; Lesser, J.R.; Pedersen, W.R.; Sorajja, P.; Spinner, E.; Garberich, R.F.; Vock, D.M.; Schwartz, R.S. Pathogenic structural heart changes in early tricuspid regurgitation. J. Thorac. Cardiovasc. Surg. 2015, 150, 323–330. [Google Scholar] [CrossRef]
- Dietz, M.F.; Prihadi, E.A.; van der Bijl, P.; Goedemans, L.; Mertens, B.J.; Gursoy, E.; van Genderen, O.S.; Marsan, N.A.; Delgado, V.; Bax, J.J. Prognostic Implications of Right Ventricular Remodeling and Function in Patients with Significant Secondary Tricuspid Regurgitation. Circulation 2019, 140, 836–845. [Google Scholar] [CrossRef]
- Prihadi, E.A.; van der Bijl, P.; Dietz, M.; Abou, R.; Vollema, E.M.; Marsan, N.A.; Delgado, V.; Bax, J.J. Prognostic Implications of Right Ventricular Free Wall Longitudinal Strain in Patients with Significant Functional Tricuspid Regurgitation. Circ. Cardiovasc. Imaging 2019, 12, e008666. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.; Pellikka, P.A.; Quiñones, M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur. J. Echocardiogr. 2009, 10, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Biffi, A.; Delise, P.; Zeppilli, P.; Giada, F.; Pelliccia, A.; Penco, M.; Casasco, M.; Colonna, P.; D’Andrea, A.; D’Andrea, L.; et al. Italian cardiological guidelines for sports eligibility in athletes with heart disease: Part 1. J. Cardiovasc. Med. 2013, 14, 477–499. [Google Scholar] [CrossRef]
- Delise, P.; Mos, L.; Sciarra, L.; Basso, C.; Biffi, A.; Cecchi, F.; Colivicchi, F.; Corrado, D.; D’Andrea, A.; Di Cesare, E.; et al. Italian Cardiological Guidelines (COCIS) for Competitive Sport Eligibility in athletes with heart disease: Update 2020. J. Cardiovasc. Med. 2021, 22, 874–891. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T. Finding concordance in discord: The value of discordant invasive and echocardiographic pulmonary artery pressure measurements with severe tricuspid regurgitation. Eur. Heart J. 2020, 41, 2796–2798. [Google Scholar] [CrossRef] [PubMed]
| 3D Echo | Stress Echo | MW | CMR | CCT | |
|---|---|---|---|---|---|
| MVP | Identification of leaflet billowing, prolapse, and flail; characterization of MAD. | Evaluation of sPAP increase during exercise. | Insight into myocardial contraction. | Fibrosis detection. | Characterization of mitral valve apparatus anatomy. |
| MR | Valve apparatus morphology and quantification of regurgitation. | Evaluation of hemodynamic consequences and arrhythmias during exercise. | Capacity to reflect “true” EF. | Tissue characterization for insight into LV remodeling. | Assessment of MV geometry; cardiac chambers measurement. |
| MS | Valve apparatus morphology and assessment of MVA. | Symptoms, sPAP, and MV dynamic gradients evaluation during exercise. | Information about LV impairment. | Evaluation of MVA. | Assessment of leaflet and annular calcification; cardiac chambers measurement. |
| TR | RV sizing measurement with calculation of 3D RVEF. | Symptoms, sPAP, and TV dynamic gradients evaluation during exercise. | Identification of earlier modifications in RV contractility. | Tissue characterization for insight into RV remodeling. | Assessment of leaflet and annular calcification; cardiac chambers measurement. |
| TS |
| General Population | Athletes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Imaging Parameters | Mitral Valve | Tricuspid Valve | Mitral Valve | Tricuspid Valve | |||||
| MS | MR | TS | TR | MS | MR | TS | TR | ||
| TTE/ CRM | Origin | Primary rheumatic | Primary and secondary | Primary | Primary and secondary | Primary rheumatic | Mixed FR * | Primary | Mixed FR * |
| LV size | n. ** | n./↑/↑↑ | n. | n. | ↑ *** | ↑/↑↑ | ↑ | ↑ | |
| LVEF | n./↓ | ↓ **** | n. | n./↓ | n. | n. | n. | n. | |
| RV size | n./↑ | n./↑ | n./↑ | n./↑/↑↑ | ↑ | ↑ | ↑ | ↑/↑↑ | |
| RV function | n./↓ | n./↓ | n./↓ | n./↓ | n./↓ transitory | n./↓ transitory | n./↓ transitory | n./↓ transitory | |
| LA size | ↑↑ | n./↑/↑↑ | n. | n. | ↑↑↑ | ↑/↑↑ | ↑ | ↑ | |
| RA size | n. | n. | ↑↑ | n/↑/↑↑ | n./↑ | n./↑ | ↑↑↑ | ↑/↑↑ | |
| VC | ≥7 (≥8 mm) | >7 mm | =¶ | = | |||||
| PISA | >9 mm | >9 mm | = | = | |||||
| EROA | ≥40 (or 30 mm2) | ≥40 mm2 | ≥30 mm2 | = | |||||
| RVol | ≥60 mL (or 45 mL) | ≥45 mL | = | = | |||||
| RF | ≥50% | ≥50% | = | = | |||||
| Valve area | ≤1.5 cm2 | ≤1 cm2 | = | = | |||||
| PHT | ≥150 ms | ≥190 ms | = | = | |||||
| sPAP | >50 mmHg | n./↑ | >> 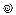 | ↑/↑↑ | |||||
| Mean gradient | >10 mmHg | ≥5 mmHg | = | = | |||||
| 3D Echo | VCa/qEROA # | ≥75 mm2 | = | ||||||
| Valve morphology/ leaflet motion | Commissural fusion | Tethering n./↑/↑↑ | Leaflets’ thickening | TA↑ | = | ↑Saddle-shaped, ↑ tenting | = | TA↑↑ | |
| Stress Echo | Mean gradient | ≥15 mmHg | = | ||||||
| sPAP | >60 mmHg | ≥60 mm Hg | 45 mmHg | >> | >> | 55–60 mmHg | |||
| GLS and MW Parameters | GLS↓↓ | ↓GWW | ↓GCW if HF | GLS n./↓ | n. GWW | GCW n./↑ | |||
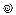 higher values; # 3D VC area or quantitative EROA 95–114 mm2 for massive TR and ≥115 mm2 for torrential TR; Abbreviations: CMR: cardiac magnetic resonance; EROA: effective regurgitant orifice area; FR: functional regurgitation; GLS: global longitudinal strain; GCW: global constructive work; GWW: global wasted work; HF: heart failure; LA: left atrium; LV: left ventricle; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; MS: mitral stenosis; MW: myocardial work; PHT: pressure half-time; PISA: proximal isovelocity surface area; RA: right atrium; RF: regurgitant fraction; RV: right ventricle; RVol: regurgitant volume; sPAP: systolic pulmonary artery pressure; TA: tricuspid annulus; TR: tricuspid regurgitation; TS: tricuspid stenosis; TTE: transthoracic echocardiography; VC: vena contracta.
higher values; # 3D VC area or quantitative EROA 95–114 mm2 for massive TR and ≥115 mm2 for torrential TR; Abbreviations: CMR: cardiac magnetic resonance; EROA: effective regurgitant orifice area; FR: functional regurgitation; GLS: global longitudinal strain; GCW: global constructive work; GWW: global wasted work; HF: heart failure; LA: left atrium; LV: left ventricle; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; MS: mitral stenosis; MW: myocardial work; PHT: pressure half-time; PISA: proximal isovelocity surface area; RA: right atrium; RF: regurgitant fraction; RV: right ventricle; RVol: regurgitant volume; sPAP: systolic pulmonary artery pressure; TA: tricuspid annulus; TR: tricuspid regurgitation; TS: tricuspid stenosis; TTE: transthoracic echocardiography; VC: vena contracta.| Valvular Heart Disease | Disease Severity | Recommendation |
|---|---|---|
| Mitral Valve Prolapse | Mild to moderate MR or no alert criteria 1 | No restriction. All levels of exercise. |
| Alert criteria 1 or severe MR | Restriction. Low-intensity sports if suitable. | |
| Mitral Regurgitation | Mild | No restriction. All levels of exercise. |
| Moderate | No restriction if suitable. 2 All levels of exercise. | |
| Severe | Restriction. Low-intensity sports if suitable. 2 | |
| Mitral Stenosis | Mild | No restriction if suitable. 3 All levels of exercise. |
| Moderate | Restriction Low-intensity sports if suitable. 3 | |
| Severe | Restriction Avoid all competitive sports. | |
| Tricuspid Regurgitation | Mild | No restriction if suitable. 4 All levels of exercise. |
| Moderate | No restriction if suitable. 4,5 All levels of exercise. | |
| Severe | Restriction. Low-intensity sports if suitable. 4 | |
| Tricuspid Stenosis | Mild | No restriction if suitable. 6 All levels of exercise. |
| Moderate | Restriction. Avoid all competitive sports. | |
| Severe | Restriction. Avoid all competitive sports. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segreti, A.; Celeski, M.; Monticelli, L.M.; Perillo, A.; Crispino, S.P.; Di Gioia, G.; Cammalleri, V.; Fossati, C.; Mega, S.; Papalia, R.; et al. Mitral and Tricuspid Valve Disease in Athletes. J. Clin. Med. 2023, 12, 3562. https://doi.org/10.3390/jcm12103562
Segreti A, Celeski M, Monticelli LM, Perillo A, Crispino SP, Di Gioia G, Cammalleri V, Fossati C, Mega S, Papalia R, et al. Mitral and Tricuspid Valve Disease in Athletes. Journal of Clinical Medicine. 2023; 12(10):3562. https://doi.org/10.3390/jcm12103562
Chicago/Turabian StyleSegreti, Andrea, Mihail Celeski, Luigi Maria Monticelli, Alfonso Perillo, Simone Pasquale Crispino, Giuseppe Di Gioia, Valeria Cammalleri, Chiara Fossati, Simona Mega, Rocco Papalia, and et al. 2023. "Mitral and Tricuspid Valve Disease in Athletes" Journal of Clinical Medicine 12, no. 10: 3562. https://doi.org/10.3390/jcm12103562
APA StyleSegreti, A., Celeski, M., Monticelli, L. M., Perillo, A., Crispino, S. P., Di Gioia, G., Cammalleri, V., Fossati, C., Mega, S., Papalia, R., Pigozzi, F., Ussia, G. P., & Grigioni, F. (2023). Mitral and Tricuspid Valve Disease in Athletes. Journal of Clinical Medicine, 12(10), 3562. https://doi.org/10.3390/jcm12103562












