A Real-World Prognosis in Idiopathic Pulmonary Fibrosis: A Special Reference to the Role of Antifibrotic Agents for the Elderly
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
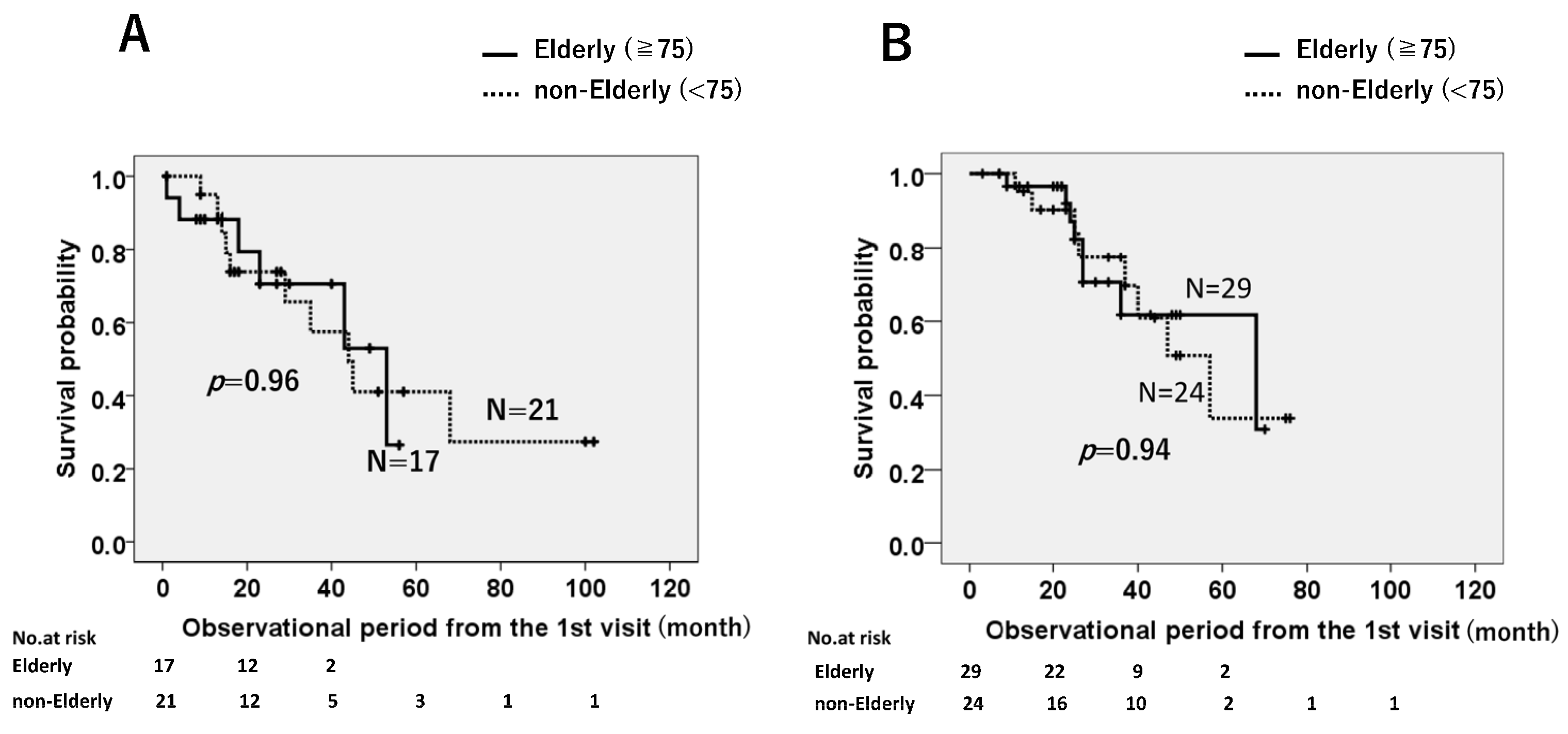
3.1. Analysis of Survival Probabilities
3.1.1. Survival Probabilities of All Enrolled Patients
3.1.2. Survival Probabilities according to Treatment Regimen
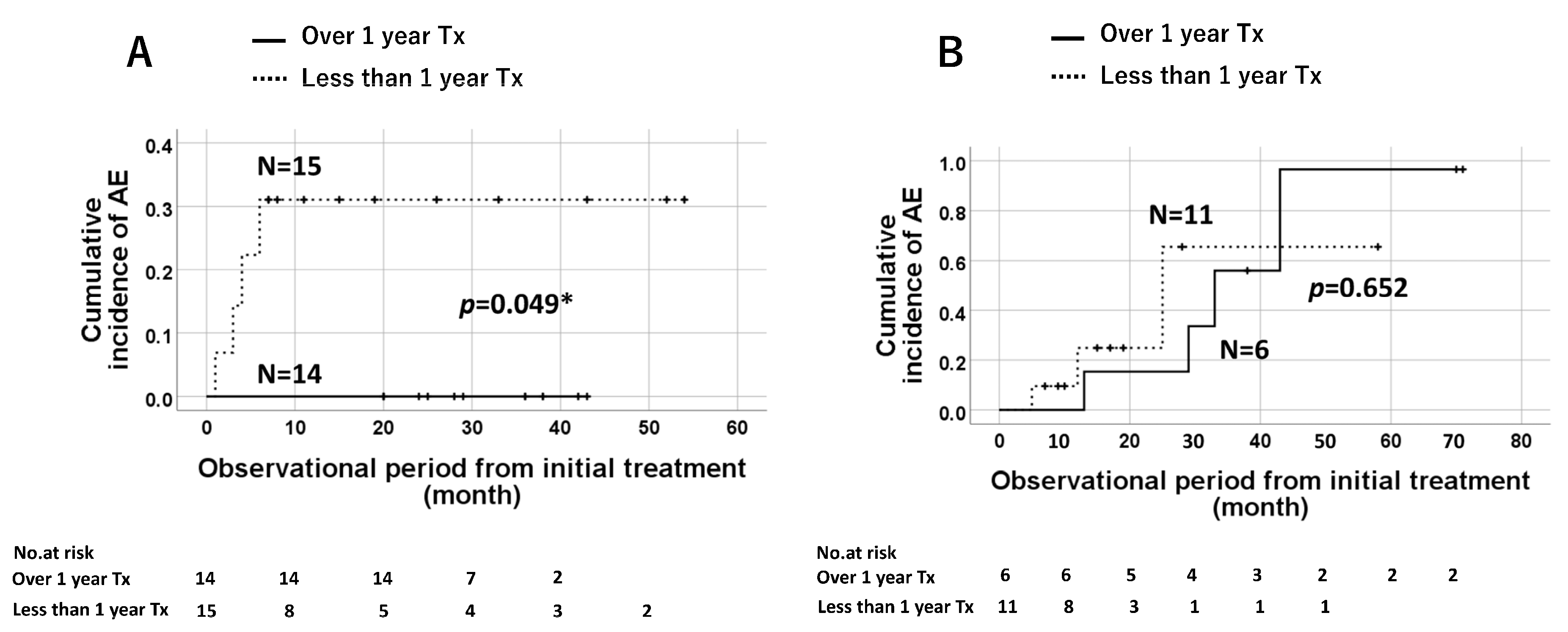
3.1.3. Survival Probabilities of All Enrolled Patients (n = 91) according to the Status of Successful 1-Year Treatment
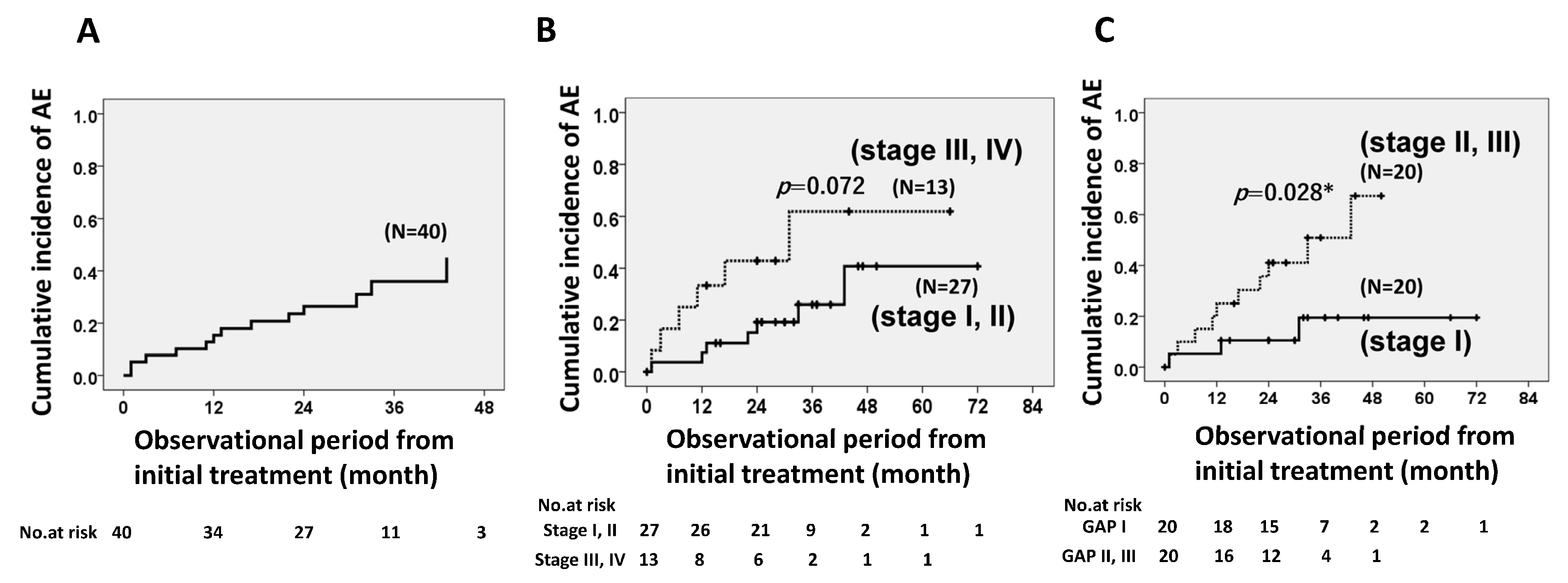
3.2. Cumulative Incidence Ratios of Acute Exacerbation of IPF
3.2.1. Cumulative Incidence Ratio of Acute Exacerbation of IPF with Long-Term Antifibrotic Agent Use (>1 year) Based on JRS or GAP Stages
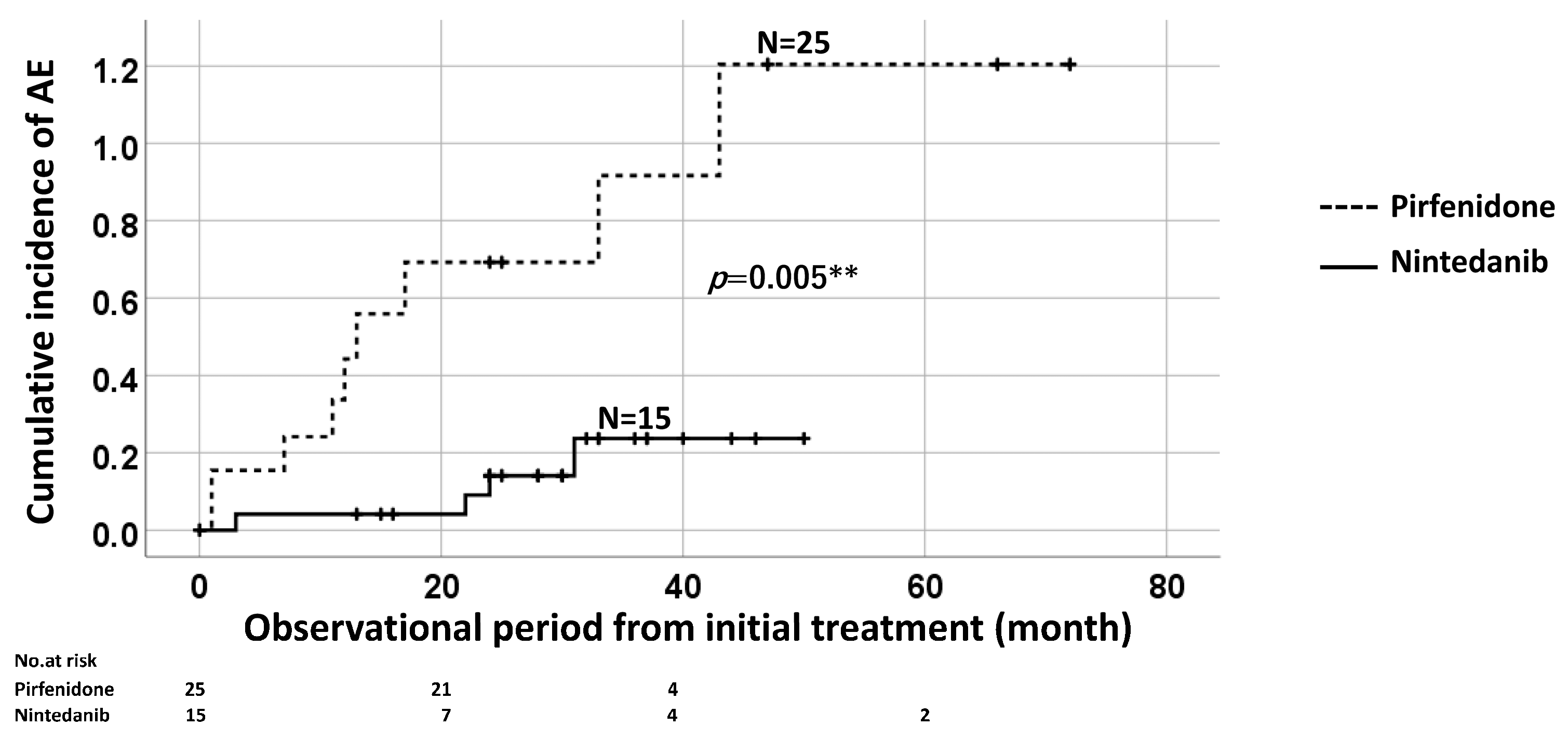
3.2.2. Cumulative Incidence Ratio of AE of IPF with Long-Term Antifibrotic Agent Use (>1 year) according to the Type of Antifibrotic Agent
3.3. Effects of Antifibrotic Agents in Elderly Patients with IPF
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kondoh, Y.; Suda, T.; Hongo, Y.; Yoshida, M.; Hiroi, S.; Iwasaki, K.; Takeshima, T.; Homma, S. Prevalence of idiopathic pulmonary fibrosis in Japan based on a claims database analysis. Respir. Res. 2022, 23, 24. [Google Scholar] [CrossRef] [PubMed]
- Natsuizaka, M.; Chiba, H.; Kuronuma, K.; Otsuka, M.; Kudo, K.; Mori, M.; Bando, M.; Sugiyama, Y.; Takahashi, H. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am. J. Respir. Crit. Care Med. 2014, 190, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R. Overview of idiopathic pulmonary fibrosis (IPF) and evidence-based guidelines. Am. J. Manag. Care 2017, 23 (Suppl. S11), S176–S182. [Google Scholar] [PubMed]
- Naccache, J.M.; Jouneau, S.; Didier, M.; Borie, R.; Cachanado, M.; Bourdin, A.; Reynaud-Gaubert, M.; Bonniaud, P.; Israël-Biet, D.; Prévot, G.; et al. Cyclophosphamide added to glucocorticoids in acute exacerbation of idiopathic pulmonary fibrosis (EXAFIP): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2022, 10, 26–34. [Google Scholar] [CrossRef]
- Biondini, D.; Balestro, E.; Sverzellati, N.; Cocconcelli, E.; Bernardinello, N.; Ryerson, C.J.; Spagnolo, P. Acute exacerbations of idiopathic pulmonary fibrosis (AE-IPF): An overview of current and future therapeutic strategies. Expert Rev. Respir. Med. 2020, 14, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Homma, S.; Suda, T.; Hongo, Y.; Yoshida, M.; Hiroi, S.; Iwasaki, K.; Takeshima, T.; Kondoh, Y. Incidence and changes in treatment of acute exacerbation of idiopathic pulmonary fibrosis in Japan: A claims-based retrospective study. Respir. Investig. 2022, 60, 798–805. [Google Scholar] [CrossRef]
- Isshiki, T.; Sakamoto, S.; Yamasaki, A.; Shimizu, H.; Miyoshi, S.; Nakamura, Y.; Homma, S.; Kishi, K. Incidence of acute exacerbation of idiopathic pulmonary fibrosis in patients receiving antifibrotic agents: Real-world experience. Respir. Med. 2021, 187, 106551. [Google Scholar] [CrossRef]
- Iwata, T.; Yoshida, S.; Fujiwara, T.; Wada, H.; Nakajima, T.; Suzuki, H.; Yoshino, I. Effect of Perioperative Pirfenidone Treatment in Lung Cancer Patients with Idiopathic Pulmonary Fibrosis. Ann. Thorac. Surg. 2016, 102, 1905–1910. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Raghu, G.; Rochwerg, B.; Zhang, Y.; Garcia, C.A.; Azuma, A.; Behr, J.; Brozek, J.L.; Collard, H.R.; Cunningham, W.; Homma, S.; et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2015, 192, e3–e19. [Google Scholar] [CrossRef]
- Kang, J.; Han, M.; Song, J.W. Antifibrotic treatment improves clinical outcomes in patients with idiopathic pulmonary fibrosis: A propensity score matching analysis. Sci. Rep. 2020, 10, 15620. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, T.M.; Sangaralingham, L.R.; Yao, X.; Sanghavi, D.; Shah, N.D.; Limper, A.H. Clinical Effectiveness of Antifibrotic Medications for Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Sugino, K.; Ono, H.; Watanabe, N.; Ando, M.; Tsuboi, E.; Homma, S.; Kishi, K. Efficacy of early antifibrotic treatment for idiopathic pulmonary fibrosis. BMC Pulm. Med. 2021, 21, 218. [Google Scholar] [CrossRef] [PubMed]
- Poletti, V.; Vancheri, C.; Albera, C.; Harari, S.; Pesci, A.; Metella, R.R.; Campolo, B.; Crespi, G.; Rizzoli, S.; FIBRONET Study Group C; et al. Clinical course of IPF in Italian patients during 12 months of observation: Results from the FIBRONET observational study. Respir. Res. 2021, 22, 66. [Google Scholar] [CrossRef]
- Gao, J.; Kalafatis, D.; Carlson, L.; Pesonen, I.H.; Li, C.X.; Wheelock, Å.; Magnusson, J.M.; Sköld, C.M. Baseline characteristics and survival of patients of idiopathic pulmonary fibrosis: A longitudinal analysis of the Swedish IPF Registry. Respir. Res. 2021, 22, 40. [Google Scholar] [CrossRef]
- Brown, A.W.; Fischer, C.P.; Shlobin, O.A.; Buhr, R.G.; Ahmad, S.; Weir, N.A.; Nathan, S.D. Outcomes after hospitalization in idiopathic pulmonary fibrosis: A cohort study. Chest 2015, 147, 173–179. [Google Scholar] [CrossRef]
- Ley, B.; Swigris, J.; Day, B.M.; Stauffer, J.L.; Raimundo, K.; Chou, W.; Collard, H.R. Pirfenidone Reduces Respiratory-related Hospitalizations in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2017, 196, 756–761. [Google Scholar] [CrossRef]
- Vianello, A.; Molena, B.; Turato, C.; Braccioni, F.; Arcaro, G.; Paladini, L.; Andretta, M.; Saetta, M. Pirfenidone improves the survival of patients with idiopathic pulmonary fibrosis hospitalized for acute exacerbation. Curr. Med. Res. Opin. 2019, 35, 1187–1190. [Google Scholar] [CrossRef]
- Collard, H.R.; Richeldi, L.; Kim, D.S.; Taniguchi, H.; Tschoepe, I.; Luisetti, M.; Roman, J.; Tino, G.; Schlenker-Herceg, R.; Hallmann, C.; et al. Acute exacerbations in the INPULSIS trials of nintedanib in idiopathic pulmonary fibrosis. Eur. Respir. J. 2017, 49, 1601339. [Google Scholar] [CrossRef]
- Cameli, P.; Refini, R.M.; Bergantini, L.; d’Alessandro, M.; Alonzi, V.; Magnoni, C.; Rottoli, P.; Sestini, P.; Bargagli, E. Long-Term Follow-Up of Patients with Idiopathic Pulmonary Fibrosis Treated with Pirfenidone or Nintedanib: A Real-Life Comparison Study. Front. Mol. Biosci. 2020, 7, 581828. [Google Scholar] [CrossRef]
- Uchida, Y.; Ikeda, S.; Sekine, A.; Tabata, E.; Oda, T.; Okuda, R.; Kitamura, H.; Baba, T.; Komatsu, S.; Ogura, T. Tolerability and safety of nintedanib in elderly patients with idiopathic pulmonary fibrosis. Respir. Investig. 2021, 59, 99–105. [Google Scholar] [CrossRef] [PubMed]



| All Patients (n = 91) | Elderly Group (n = 46) | Non-Elderly Group (n = 45) | p-Value | |
|---|---|---|---|---|
| Gender (male/female) | 63/28 | 32/14 | 31/14 | 1.0 |
| Age | 75 (68–79) | 79 (76–81) | 68 (63–72) | <0.001 *** |
| Comorbidity | ||||
| Malignancy | 17 | 10 | 7 | 0.592 |
| Cardiovascular disease | 7 | 2 | 5 | 0.267 |
| Diabetes mellitus | 15 | 9 | 6 | 0.574 |
| COPD | 6 | 2 | 4 | 0.434 |
| JRS classification (I/II/III/IV) | (38/6/17/20) | (17/5/6/10) | (21/1/11/10) | 1 |
| GAP stage (I/II/III) | (39/36/6) | (16/22/2) | (23/14/4) | 0.141 |
| Duration from 1st visit to initial therapy (month) | 6 (1–17) | 4 (1–13) | 8 (3–25) | 0.123 |
| Pirfenidone/Nintedanib | 37/54 | 16/30 | 21/24 | 0.29 |
| KL-6 (IU/mL) | 1004 (644–1745) | 816 (500–1957) | 868 (465–1342) | 0.948 |
| SP-D (ng/mL) | 249 (165–358) | 207 (142–388) | 209 (143–353) | 0.378 |
| BMI | 23.4 (20.9–25.9) | 22.8 (21.1–24.9) | 24.9 (21.7–28.6) | 0.07 |
| Alb (g/dL) | 4.1 (3.9–4.4) | 4.1 (3.8–4.4) | 4.2 (3.9–4.4) | 0.238 |
| LDH (U/mL) | 233 (201–262) | 222 (201–254) | 215 (197–265) | 0.984 |
| %FVC | 75.2 (61.3–88.3) | 76.8 (61.0–85.5) | 81.8 (58.4–94.5) | 0.824 |
| %DLco | 54.1 (40.8–71.8) | 55.4 (41.5–70.1) | 57.7 (40.8–80.9) | 0.898 |
| %DLco/VA | 69.7 (48.5–83.8) | 68.9 (53.1–83.5) | 76.8 (51.2–90.6) | 0.501 |
| All Patients (n = 40) | Pirfenidone (n = 15) | Nintedanib (n = 25) | p-Value | |
|---|---|---|---|---|
| Gender (male/female) | 28/12 | 10/5 | 18/7 | 0.736 |
| Age | 75 (67–79) | 68 (56–75) | 76 (67–81) | 0.737 |
| BMI | 23.9 (22.3–26.9) | 24.9 (23.8–43.6) | 23.3 (21.2–26.4) | 0.371 |
| JRS classification (I–II/III–IV) | (27/13) | (8/7) | (19/6) | 0.175 |
| GAP stage (I/II–III) | (20/20) | (6/9) | (14/11) | 0.514 |
| Duration from 1st visit to initial treatment (month) | 6.0 (1.0–16.0) | 6.0 (0–19.0) | 6.0 (2.0–15.0) | 0.510 |
| KL-6 (IU/mL) | 956 (576–1445) | 748 (395–1479) | 868 (540–1186) | 0.410 |
| SP-D (ng/mL) | 209 (142–311) | 146 (138–323) | 191 (137–266) | 0.808 |
| Alb(g/dL) | 4.1 (3.9–4.3) | 4.1 (3.8–4.3) | 4.0 (3.9–4.4) | 0.676 |
| LDH (U/mL) | 217 (198–246) | 208 (187–234) | 212 (200–235) | 0.806 |
| %FVC | 73.4 (61.7–87.5) | 81.8 (53.6–93.8) | 77.4 (60.3–103.8) | 0.530 |
| %DLco | 55.5 (46.5–70.1) | 62.8 (51.2–77.7) | 58.9 (48.0–70.2) | 0.365 |
| %DLco/VA | 76.7 (52.5–86.3) | 70.9 (46.1–94.2) | 80.9 (63.8–89.9) | 0.038 * |
| Over 1 Year Tx | Less than 1 Year Tx | p Value | |
|---|---|---|---|
| Number of patients | 20 | 26 | |
| Male | 12 | 19 | 0.333 |
| Age (years) | 79.5 (76.0–81.0) | 78.0 (77.0–79.3) | 0.180 |
| JRS classification (Ⅰ/Ⅱ/Ⅲ/Ⅳ) | 9/3/1/5 | 10/3/5/5 | 0.561 a |
| GAP stage (Ⅰ/Ⅱ/Ⅲ) | 7/13/0 | 8/18/0 | 1.0 b |
| Comorbidity | |||
| Malignancy | 3 | 7 | 0.476 |
| lung cancer | 1 | 1 | |
| prostate cancer | 1 | 2 | |
| lymphoma | 1 | 0 | |
| rectal cancer | 0 | 1 | |
| gastric cancer | 0 | 1 | |
| renal cell cancer | 0 | 1 | |
| unknown | 0 | 1 | |
| Cardiovascular disease | 0 | 2 | 0.498 |
| Diabetes mellitus | 5 | 4 | 0.472 |
| COPD | 0 | 2 | 0.498 |
| Pirfenidone/Nintedanib | 6/14 | 11/15 | 0.534 |
| Duration of antifibrotic Tx (months) | 24.5 (18.0–34.3) | 5.0 (2.3–7.8) | 0.001 |
| AE | 4 | 7 | 0.728 |
| Number of AEs after Tx | |||
| Duration of initial treatment to AE (months) | 34.5 (24.3–41.0) | 13.5 (7.0–26.5) | <0.001 |
| Adverse effects | 5 (1) | 8 (0) | 0.749 |
| appetite loss | 2 | 4 | 0.684 |
| diarrhea | 2 (1) | 3 | 1.0 |
| liver dysfunction | 1 | 0 | 0.435 |
| dysgeusia | 0 | 2 | 0.498 |
| Discontinuation of Tx | 3 | 5 | 1.0 |
| Dose reduction of Tx | 2 | 3 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honda, K.; Saraya, T.; Ishii, H. A Real-World Prognosis in Idiopathic Pulmonary Fibrosis: A Special Reference to the Role of Antifibrotic Agents for the Elderly. J. Clin. Med. 2023, 12, 3564. https://doi.org/10.3390/jcm12103564
Honda K, Saraya T, Ishii H. A Real-World Prognosis in Idiopathic Pulmonary Fibrosis: A Special Reference to the Role of Antifibrotic Agents for the Elderly. Journal of Clinical Medicine. 2023; 12(10):3564. https://doi.org/10.3390/jcm12103564
Chicago/Turabian StyleHonda, Kojiro, Takeshi Saraya, and Haruyuki Ishii. 2023. "A Real-World Prognosis in Idiopathic Pulmonary Fibrosis: A Special Reference to the Role of Antifibrotic Agents for the Elderly" Journal of Clinical Medicine 12, no. 10: 3564. https://doi.org/10.3390/jcm12103564
APA StyleHonda, K., Saraya, T., & Ishii, H. (2023). A Real-World Prognosis in Idiopathic Pulmonary Fibrosis: A Special Reference to the Role of Antifibrotic Agents for the Elderly. Journal of Clinical Medicine, 12(10), 3564. https://doi.org/10.3390/jcm12103564






