Potential Ototoxicity of Insulin-like Growth Factor 1 Receptor Signaling Inhibitors: An In Silico Drug Repurposing Study of the Regenerating Cochlear Neuron Transcriptome
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. In Silico Screen
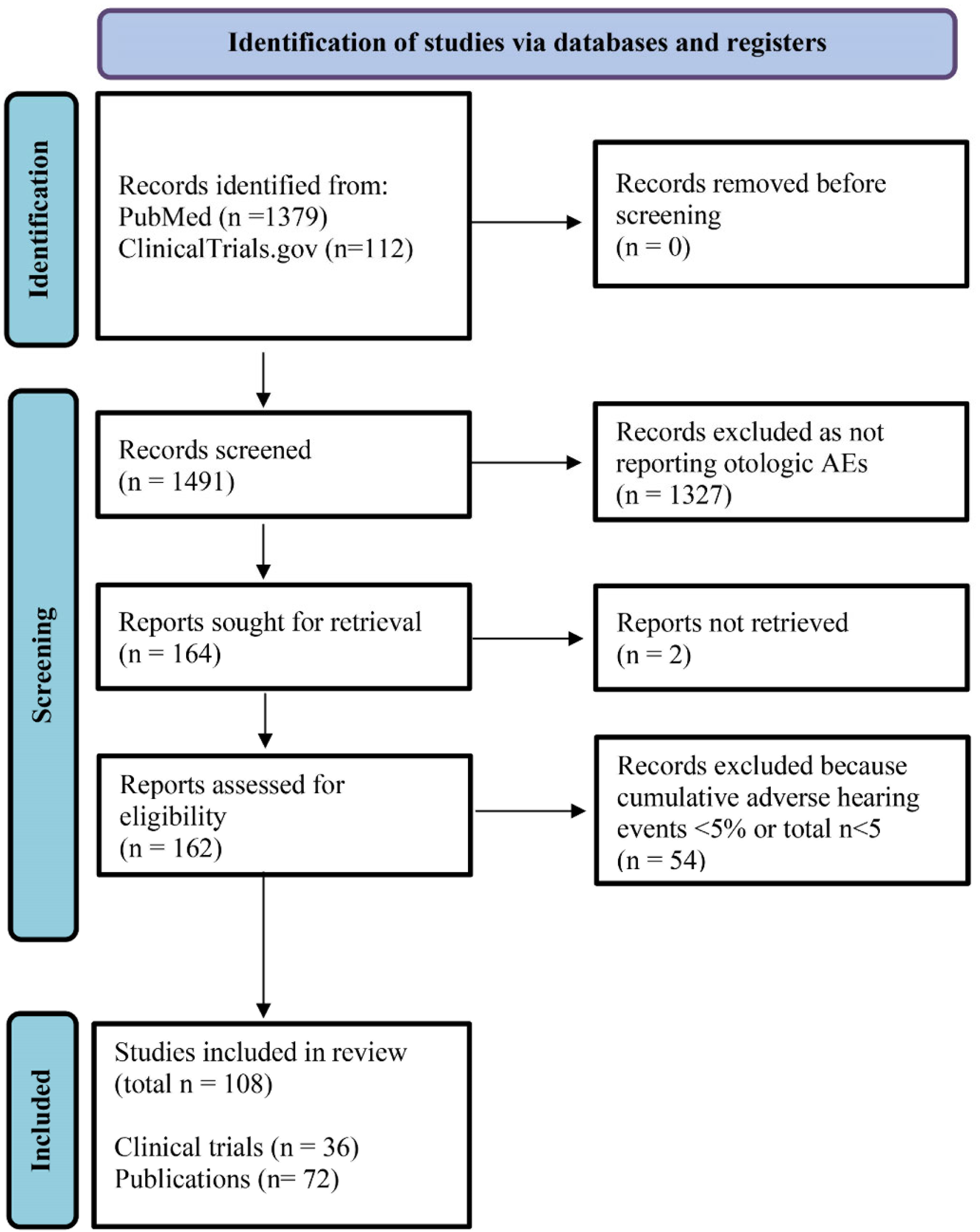
2.3. Systematic Review of Publications Reporting Otologic AEs Associated with IGF-1/R Inhibitors
2.4. Meta-Analysis of Randomized Placebo-Controlled Trials of Teprotumumab for TED
2.5. Statistical Analyses
3. Results
3.1. CLUE Query of Drug Classes Negatively Correlated with SGN Regeneration
3.2. Systematic Literature Review
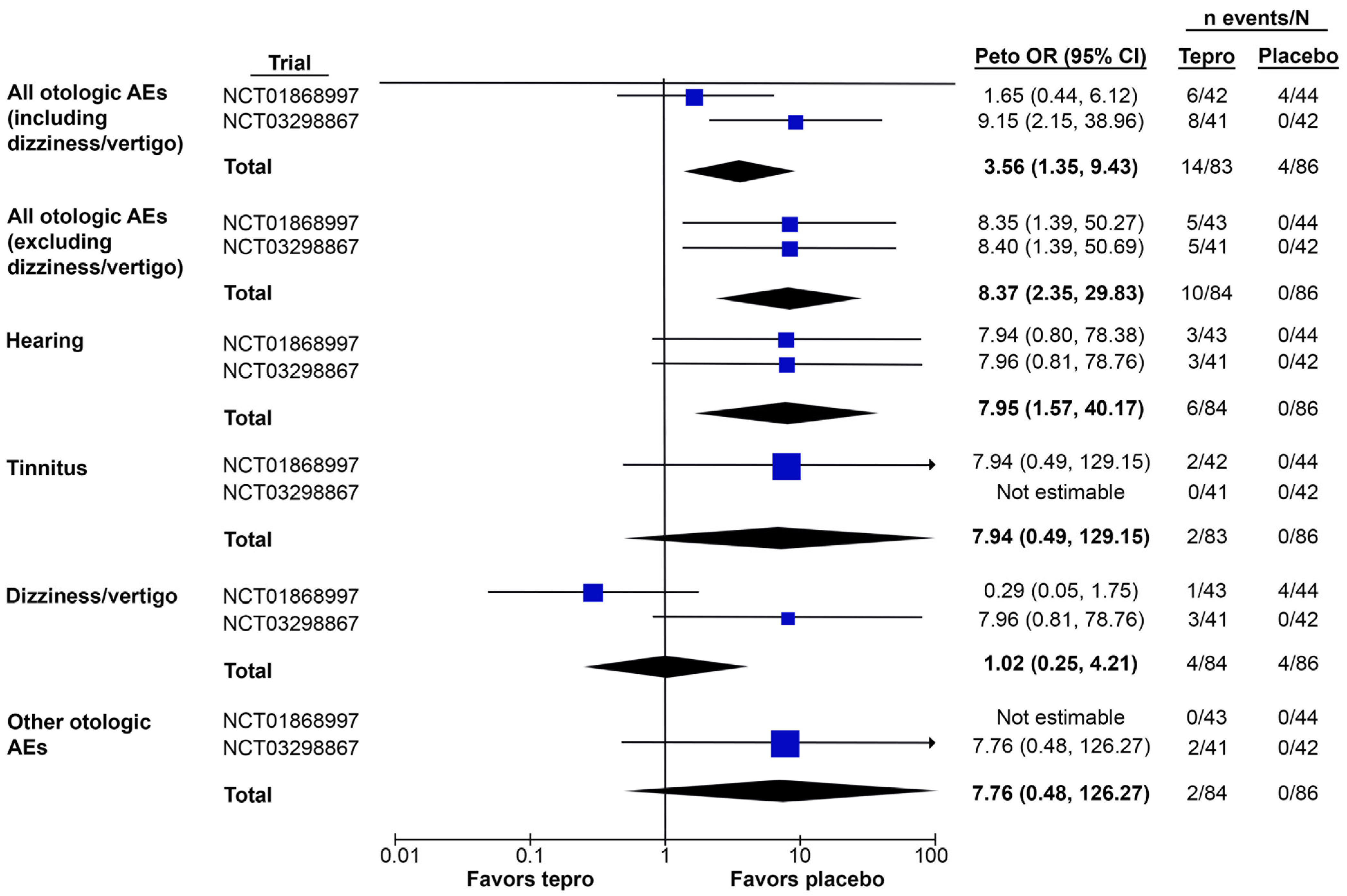
3.3. Meta-Analysis of Otologic AEs Reported in Placebo-Controlled Trials of Teprotumumab
3.3.1. Total Otologic AEs
3.3.2. Otologic AEs by Type
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zetes, D.E.; Tolomeo, J.A.; Holley, M.C. Structure and mechanics of supporting cells in the guinea pig organ of Corti. PLoS ONE 2012, 7, e49338. [Google Scholar] [CrossRef] [PubMed]
- Khimich, D.; Nouvian, R.; Pujol, R.; Tom Dieck, S.; Egner, A.; Gundelfinger, E.D.; Moser, T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature 2005, 434, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, P.A.; Glowatzki, E.; Moser, T. The afferent synapse of cochlear hair cells. Curr. Opin. Neurobiol. 2003, 13, 452–458. [Google Scholar] [CrossRef]
- Ganesan, P.; Schmiedge, J.; Manchaiah, V.; Swapna, S.; Dhandayutham, S.; Kothandaraman, P.P. Ototoxicity: A challenge in diagnosis and treatment. J. Audiol. Otol. 2018, 22, 59–68. [Google Scholar] [CrossRef]
- Lanvers-Kaminsky, C.; Zehnhoff-Dinnesen, A.a.; Parfitt, R.; Ciarimboli, G. Drug-induced ototoxicity: Mechanisms, pharmacogenetics, and protective strategies. Clin. Pharmacol. Ther. 2017, 101, 491–500. [Google Scholar] [CrossRef]
- Rybak, L.P.; Ramkumar, V. Ototoxicity. Kidney Int. 2007, 72, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Rubel, E.W.; Furrer, S.A.; Stone, J.S. A brief history of hair cell regeneration research and speculations on the future. Hear. Res. 2013, 297, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.S.; Cotanche, D.A. Hair cell regeneration in the avian auditory epithelium. Int. J. Dev. Biol. 2007, 51, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xu, L.; Han, Y.; Wang, X.; Chen, F.; Lu, J.; Wang, H.; Liu, W. Regulation of spiral ganglion neuron regeneration as a therapeutic strategy in sensorineural hearing loss. Front. Mol. Neurosci. 2022, 14, 829564. [Google Scholar] [CrossRef]
- Stevens, S.M.; Xing, Y.; Hensley, C.T.; Zhu, J.; Dubno, J.R.; Lang, H. Heptanol application to the mouse round window: A model for studying cochlear lateral wall regeneration. Otolaryngol. Head Neck Surg. 2014, 150, 659–665. [Google Scholar] [CrossRef]
- Mizutari, K. Spontaneous recovery of cochlear fibrocytes after severe degeneration caused by acute energy failure. Front. Pharmacol. 2014, 5, 198. [Google Scholar] [CrossRef] [PubMed]
- Puel, J.L.; Saffiedine, S.; Gervais d’Aldin, C.; Eybalin, M.; Pujol, R. Synaptic regeneration and functional recovery after excitotoxic injury in the guinea pig cochlea. Comptes Rendus Acad. Sci. III 1995, 318, 67–75. [Google Scholar] [PubMed]
- Hickman, T.T.; Hashimoto, K.; Liberman, L.D.; Liberman, M.C. Cochlear synaptic degeneration and regeneration after noise: Effects of age and neuronal subgroup. Front. Cell. Neurosci. 2021, 15, 684706. [Google Scholar] [CrossRef]
- Wu, C.C.; Brugeaud, A.; Seist, R.; Lin, H.C.; Yeh, W.H.; Petrillo, M.; Coppola, G.; Edge, A.S.B.; Stankovic, K.M. Altered expression of genes regulating inflammation and synaptogenesis during regrowth of afferent neurons to cochlear hair cells. PLoS ONE 2020, 15, e0238578. [Google Scholar] [CrossRef]
- Gao, L.; Kita, T.; Katsuno, T.; Yamamoto, N.; Omori, K.; Nakagawa, T. Insulin-like growth factor 1 on the maintenance of ribbon synapses in mouse cochlear explant cultures. Front. Cell. Neurosci. 2020, 14, 571155. [Google Scholar] [CrossRef]
- Rodríguez-de la Rosa, L.; Lassaletta, L.; Calvino, M.; Murillo-Cuesta, S.; Varela-Nieto, I. The role of insulin-like growth factor 1 in the progression of age-related hearing loss. Front. Aging Neurosci. 2017, 9, 411. [Google Scholar] [CrossRef]
- Wei, D.; Jin, Z.; Järlebark, L.; Scarfone, E.; Ulfendahl, M. Survival, synaptogenesis, and regeneration of adult mouse spiral ganglion neurons in vitro. Dev. Neurobiol. 2007, 67, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Naples, J.G.; Rice-Narusch, W.; Watson, N.W.; Ghulam-Smith, M.; Holmes, S.; Li, D.; Jalisi, S. Ototoxicity review: A growing number of non-platinum-based chemo- and immunotherapies. Otolaryngol. Head Neck Surg. 2022, 168, 658–668. [Google Scholar] [CrossRef]
- Cianfrone, G.; Pentangelo, D.; Cianfrone, F.; Mazzei, F.; Turchetta, R.; Orlando, M.P.; Altissimi, G. Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: A reasoned and updated guide. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 601–636. [Google Scholar]
- Mukherjea, D.; Rybak, L.P.; Sheehan, K.E.; Kaur, T.; Ramkumar, V.; Jajoo, S.; Sheth, S. The design and screening of drugs to prevent acquired sensorineural hearing loss. Expert Opin. Drug Discov. 2011, 6, 491–505. [Google Scholar] [CrossRef]
- Sears, C.M.; Azad, A.D.; Amarikwa, L.; Pham, B.H.; Men, C.J.; Kaplan, D.N.; Liu, J.; Hoffman, A.R.; Swanson, A.; Alyono, J.; et al. Hearing dysfunction after treatment with teprotumumab for thyroid eye disease. Am. J. Ophthalmol. 2022, 240, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Laron, Z. Insulin-like growth factor 1 (IGF-1): A growth hormone. Mol. Pathol. 2001, 54, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Kong, Q.; Yin, J.; Zhang, J.; Jiang, Y. Insulin-like growth factor receptor signaling in tumorigenesis and drug resistance: A challenge for cancer therapy. J. Hematol. Oncol. 2020, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Gerber, D.E. ALK alterations and inhibition in lung cancer. Semin. Cancer Biol. 2017, 42, 81–88. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. Highlights of Prescribing Information: ZYKADIA. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205755lbl.pdf (accessed on 23 February 2023).
- United States Food and Drug Administration. Highlights of Prescribing Information: TEPEZZA. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761143s000lbl.pdf (accessed on 23 February 2023).
- Smith, T.J.; Kahaly, G.J.; Ezra, D.G.; Fleming, J.C.; Dailey, R.A.; Tang, R.A.; Harris, G.J.; Antonelli, A.; Salvi, M.; Goldberg, R.A.; et al. Teprotumumab for thyroid-associated ophthalmopathy. N. Engl. J. Med. 2017, 376, 1748–1761. [Google Scholar] [CrossRef]
- Douglas, R.S.; Kahaly, G.J.; Patel, A.; Sile, S.; Thompson, E.H.Z.; Perdok, R.; Fleming, J.C.; Fowler, B.T.; Marcocci, C.; Marinò, M.; et al. Teprotumumab for the treatment of active thyroid eye disease. N. Engl. J. Med. 2020, 382, 341–352. [Google Scholar] [CrossRef]
- Belinsky, I.; Creighton, F.X., Jr.; Mahoney, N.; Petris, C.K.; Callahan, A.B.; Campbell, A.A.; Kazim, M.; Lee, H.B.H.; Yoon, M.K.; Dagi Glass, L.R. Teprotumumab and hearing loss: Case series and proposal for audiologic monitoring. Ophthalmic Plast. Reconstr. Surg. 2022, 38, 73–78. [Google Scholar] [CrossRef]
- Ding, A.S.; Mahoney, N.R.; Campbell, A.A.; Creighton, F.X. Sensorineural hearing loss after teprotumumab therapy for thyroid eye disease: A case report. Otol. Neurotol. 2022, 43, e148–e152. [Google Scholar] [CrossRef]
- García-Mato, Á.; Cervantes, B.; Murillo-Cuesta, S.; Rodríguez-de la Rosa, L.; Varela-Nieto, I. Insulin-like growth factor 1 signaling in mammalian hearing. Genes 2021, 12, 1553. [Google Scholar] [CrossRef]
- Celaya, A.M.; Rodríguez-de la Rosa, L.; Bermúdez-Muñoz, J.M.; Zubeldia, J.M.; Romá-Mateo, C.; Avendaño, C.; Pallardó, F.V.; Varela-Nieto, I. IGF-1 haploinsufficiency causes age-related chronic cochlear inflammation and increases noise-induced hearing loss. Cells 2021, 10, 1686. [Google Scholar] [CrossRef]
- Riva, C.; Donadieu, E.; Magnan, J.; Lavieille, J.P. Age-related hearing loss in CD/1 mice is associated to ROS formation and HIF target proteins up-regulation in the cochlea. Exp. Gerontol. 2007, 42, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Woods, K.A.; Camacho-Hübner, C.; Savage, M.O.; Clark, A.J. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N. Engl. J. Med. 1996, 335, 1363–1367. [Google Scholar] [CrossRef]
- Bonapace, G.; Concolino, D.; Formicola, S.; Strisciuglio, P. A novel mutation in a patient with insulin-like growth factor 1 (IGF1) deficiency. J. Med. Genet. 2003, 40, 913–917. [Google Scholar] [CrossRef]
- Walenkamp, M.J.; Karperien, M.; Pereira, A.M.; Hilhorst-Hofstee, Y.; van Doorn, J.; Chen, J.W.; Mohan, S.; Denley, A.; Forbes, B.; van Duyvenvoorde, H.A.; et al. Homozygous and heterozygous expression of a novel insulin-like growth factor-I mutation. J. Clin. Endocrinol. Metab. 2005, 90, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Corsello, S.M.; Nagari, R.T.; Spangler, R.D.; Rossen, J.; Kocak, M.; Bryan, J.G.; Humeidi, R.; Peck, D.; Wu, X.; Tang, A.A.; et al. Discovering the anti-cancer potential of non-oncology drugs by systematic viability profiling. Nat. Cancer 2020, 1, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Narayan, R.; Corsello, S.M.; Peck, D.D.; Natoli, T.E.; Lu, X.; Gould, J.; Davis, J.F.; Tubelli, A.A.; Asiedu, J.K.; et al. A next generation connectivity map: L1000 platform and the first 1,000,000 profiles. Cell 2017, 171, 1437–1452.e1417. [Google Scholar] [CrossRef]
- Lamb, J.; Crawford, E.D.; Peck, D.; Modell, J.W.; Blat, I.C.; Wrobel, M.J.; Lerner, J.; Brunet, J.P.; Subramanian, A.; Ross, K.N.; et al. The Connectivity Map: Using gene-expression signatures to connect small molecules, genes, and disease. Science 2006, 313, 1929–1935. [Google Scholar] [CrossRef]
- Seist, R.; Tong, M.; Landegger, L.D.; Vasilijic, S.; Hyakusoku, H.; Katsumi, S.; McKenna, C.E.; Edge, A.S.B.; Stankovic, K.M. Regeneration of cochlear synapses by systemic administration of a bisphosphonate. Front. Mol. Neurosci. 2020, 13, 87. [Google Scholar] [CrossRef]
- Landegger, L.D.; Dilwali, S.; Stankovic, K.M. Neonatal murine cochlear explant technique as an in vitro screening tool in hearing research. J. Vis. Exp. 2017, 8, 55704. [Google Scholar] [CrossRef]
- Pujol, R.; Lenoir, M.; Robertson, D.; Eybalin, M.; Johnstone, B.M. Kainic acid selectively alters auditory dendrites connected with cochlear inner hair cells. Hear. Res. 1985, 18, 145–151. [Google Scholar] [CrossRef]
- Fernandez, K.A.; Watabe, T.; Tong, M.; Meng, X.; Tani, K.; Kujawa, S.G.; Edge, A.S. Trk agonist drugs rescue noise-induced hidden hearing loss. JCI Insight 2021, 6, e142572. [Google Scholar] [CrossRef]
- Corsello, S.M.; Bittker, J.A.; Liu, Z.; Gould, J.; McCarren, P.; Hirschman, J.E.; Johnston, S.E.; Vrcic, A.; Wong, B.; Khan, M.; et al. The Drug Repurposing Hub: A next-generation drug library and information resource. Nat. Med. 2017, 23, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Horizon Pharma USA, Inc. Teprotumumab (RV 001) Treatment in Patients With Active Thyroid Eye Disease. 2013. Available online: https://ClinicalTrials.gov/show/NCT01868997 (accessed on 22 February 2023).
- Horizon Pharma USA, Inc. Treatment of Graves’ Orbitopathy (Thyroid Eye Disease) to Reduce Proptosis With Teprotumumab Infusions in a Randomized, Placebo-Controlled, Clinical Study. 2017. Available online: https://ClinicalTrials.gov/show/NCT03298867 (accessed on 22 February 2023).
- Yusuf, S.; Peto, R.; Lewis, J.; Collins, R.; Sleight, P. Beta blockade during and after myocardial infarction: An overview of the randomized trials. Prog. Cardiovasc. Dis. 1985, 27, 335–371. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration; Center for Drug Evaluation and Research. Application Number: 761143Orig1s000 Summary Review [Teprezza]. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/761143Orig1s000SumR.pdf (accessed on 22 February 2023).
- Foulstone, E.; Prince, S.; Zaccheo, O.; Burns, J.L.; Harper, J.; Jacobs, C.; Church, D.; Hassan, A.B. Insulin-like growth factor ligands, receptors, and binding proteins in cancer. J. Pathol. 2005, 205, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Rabinovsky, E.D. The multifunctional role of IGF-1 in peripheral nerve regeneration. Neurol. Res. 2004, 26, 204–210. [Google Scholar] [CrossRef]
- Musarò, A.; McCullagh, K.; Paul, A.; Houghton, L.; Dobrowolny, G.; Molinaro, M.; Barton, E.R.; L Sweeney, H.; Rosenthal, N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat. Genet. 2001, 27, 195–200. [Google Scholar] [CrossRef]
- Sanchez-Calderon, H.; Rodriguez-de la Rosa, L.; Milo, M.; Pichel, J.G.; Holley, M.; Varela-Nieto, I. RNA microarray analysis in prenatal mouse cochlea reveals novel IGF-I target genes: Implication of MEF2 and FOXM1 transcription factors. PLoS ONE 2010, 5, e8699. [Google Scholar] [CrossRef]
- Okano, T.; Xuan, S.; Kelley, M.W. Insulin-like growth factor signaling regulates the timing of sensory cell differentiation in the mouse cochlea. J. Neurosci. 2011, 31, 18104–18118. [Google Scholar] [CrossRef]
- Cediel, R.; Riquelme, R.; Contreras, J.; Díaz, A.; Varela-Nieto, I. Sensorineural hearing loss in insulin-like growth factor I-null mice: A new model of human deafness. Eur. J. Neurosci. 2006, 23, 587–590. [Google Scholar] [CrossRef]
- Reed, D.S.; Kostosky, N.; Davies, B.W.; Epstein, A.; Durairaj, V.D. Rifle blast exacerbating hearing loss in a patient treated with teprotumumab for thyroid eye disease. Ophthalmic Plast. Reconstr. Surg. 2022, 38, e41–e43. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Nakagawa, T.; Endo, T.; Matsuoka, Y.; Kita, T.; Kim, T.S.; Tabata, Y.; Ito, J. Cochlear protection by local insulin-like growth factor-1 application using biodegradable hydrogel. Laryngoscope 2006, 116, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Nakagawa, T.; Okano, T.; Hori, R.; Ono, K.; Tabata, Y.; Lee, S.H.; Ito, J. Novel therapy for hearing loss: Delivery of insulin-like growth factor 1 to the cochlea using gelatin hydrogel. Otol. Neurotol. 2007, 28, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Yamamoto, N.; Nakagawa, T.; Omori, K.; Ito, J. Activation of IGF1 signaling in the cochlea induces the transcription of its mediators during the protection of cochlear hair cells against aminoglycoside. Otol. Neurotol. 2017, 38, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Hato, N.; Nakagawa, T.; Tabata, Y.; Yoshida, T.; Komobuchi, H.; Takeda, S.; Hyodo, J.; Hakuba, N.; Gyo, K. Insulin-like growth factor 1 treatment via hydrogels rescues cochlear hair cells from ischemic injury. Neuroreport 2008, 19, 1585–1588. [Google Scholar] [CrossRef]
- Yamahara, K.; Nishimura, K.; Ogita, H.; Ito, J.; Nakagawa, T.; Furuta, I.; Kita, T.; Omori, K.; Yamamoto, N. Hearing preservation at low frequencies by insulin-like growth factor 1 in a guinea pig model of cochlear implantation. Hear. Res. 2018, 368, 92–108. [Google Scholar] [CrossRef]
- Nair, T.S.; Raphael, Y.; Dolan, D.F.; Parrett, T.J.; Perlman, L.S.; Brahmbhatt, V.R.; Wang, Y.; Hou, X.; Ganjei, G.; Nuttall, A.L.; et al. Monoclonal antibody induced hearing loss. Hear. Res. 1995, 83, 101–113. [Google Scholar] [CrossRef]
- Disher, M.J.; Ramakrishnan, A.; Nair, T.S.; Miller, J.M.; Telian, S.A.; Arts, H.A.; Sataloff, R.T.; Altschuler, R.A.; Raphael, Y.; Carey, T.E. Human autoantibodies and monoclonal antibody KHRI-3 bind to a phylogenetically conserved inner-ear-supporting cell antigen. Ann. N. Y. Acad. Sci. 1997, 830, 253–265. [Google Scholar] [CrossRef]
- Katsumi, S.; Sahin, M.I.; Lewis, R.M.; Iyer, J.S.; Landegger, L.D.; Stankovic, K.M. Intracochlear perfusion of tumor necrosis factor-alpha induces sensorineural hearing loss and synaptic degeneration in guinea pigs. Front. Neurol. 2019, 10, 1353. [Google Scholar] [CrossRef]
- Hirose, K.; Li, S.Z. The role of monocytes and macrophages in the dynamic permeability of the blood-perilymph barrier. Hear. Res. 2019, 374, 49–57. [Google Scholar] [CrossRef]
- Ishiyama, G.; Lopez, I.A.; Ishiyama, P.; Vinters, H.V.; Ishiyama, A. The blood labyrinthine barrier in the human normal and Meniere’s disease macula utricle. Sci. Rep. 2017, 7, 253. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Nakagawa, T. Insulin-like growth factor 1: Role in the auditory system and therapeutic potential in otology. Curr. Opin. Otolaryngol. Head Neck Surg. 2020, 28, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Horizon Pharma USA, Inc. Treatment of Graves’ Orbitopathy to Reduce Proptosis with Teprotumumab Infusions in an Open-Label Clinical Extension Study. 2018. Available online: https://ClinicalTrials.gov/show/NCT03461211 (accessed on 22 February 2023).
- Williams, G.; Gatt, A.; Clarke, E.; Corcoran, J.; Doherty, P.; Chambers, D.; Ballard, C. Drug repurposing for Alzheimer’s disease based on transcriptional profiling of human iPSC-derived cortical neurons. Transl. Psychiatry 2019, 9, 220. [Google Scholar] [CrossRef] [PubMed]

| Drug Class | CMap Connectivity Score |
|---|---|
| IMPDH inhibitor | −98.91 |
| IGF-1 inhibitor | −98.87 |
| mTOR inhibitor | −97.46 |
| PI3K inhibitor | −96.33 |
| DNA-dependent protein kinase inhibitor | −92.91 |
| HMGCR inhibitor | −90.86 |
| Publication/Trial Data | Reported Otologic AEs, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| Drug | N of Trials/Publications | Total N of Patients | Hearing | Tinnitus | Dizziness/Vertigo | Other | Total |
| Teprotumumab a | 3/4 | 161 | 21 (13.0%) | 14 (8.7%) | 7 (4.3%) | 27 (16.8%) | 69 (42.9%) |
| R1507 | 4/2 | 473 | 0 (0%) | 5 (1.1%) | 25 (5.3%) | 11 (2.3%) | 41 (8.7%) |
| Cixutumumab | 26/10 | 1566 | 14 (0.9%) | 24 (1.5%) | 210 (13.4%) | 32 (2%) | 280 (17.9%) |
| Ganitumab | 4/1 | 255 | 3 (1.2%) | 4 (1.6%) | 38 (14.9%) | 4 (1.6%) | 49 (19.2%) |
| Figitumumab | 9/5 | 1389 | 48 (3.5%) | 32 (2.3%) | 142 (10.2%) | 38 (2.7%) | 260 (18.7%) |
| Dalotuzumab | 9/6 | 616 | 9 (1.5%) | 4 (0.6%) | 57 (9.3%) | 8 (1.3%) | 78 (12.7%) |
| Ceritinib a | 10/7 | 1253 | 4 (0.3%) | 19 (1.5%) | 185 (14.8%) | 10 (0.8%) | 218 (17.4%) |
| Ganetespib | 7/1 | 428 | 2 (0.5%) | 2 (0.5%) | 35 (8.2%) | 3 (0.7%) | 42 (9.8%) |
| Total | 72/36 | 6141 | 101 (1.6%) | 104 (1.7%) | 699 (11.4%) | 133 (2.2%) | 1037 (16.9%) |
| Heterogeneity | Overall Effect | |||||
|---|---|---|---|---|---|---|
| Otologic AE | Chi2 | df | p | I2 | Z-Score | p |
| All otologic AEs | ||||||
| Including dizziness/vertigo | 2.95 | 1 | 0.09 | 66% | 2.56 | 0.01 * |
| Excluding dizziness/vertigo | 0.00 | 1 | 1.00 | 0% | 3.28 | 0.001 * |
| Hearing | 0.00 | 1 | 1.00 | 0% | 2.51 | 0.01 * |
| Tinnitus | NE | 1.46 | 0.15 | |||
| Dizziness/vertigo | 4.97 | 1 | 0.03 | 80% | 0.03 | 0.97 |
| Other otologic AEs | NE | 1.44 | 0.15 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertagnoli, L.E.; Seist, R.; Batts, S.; Stankovic, K.M. Potential Ototoxicity of Insulin-like Growth Factor 1 Receptor Signaling Inhibitors: An In Silico Drug Repurposing Study of the Regenerating Cochlear Neuron Transcriptome. J. Clin. Med. 2023, 12, 3485. https://doi.org/10.3390/jcm12103485
Bertagnoli LE, Seist R, Batts S, Stankovic KM. Potential Ototoxicity of Insulin-like Growth Factor 1 Receptor Signaling Inhibitors: An In Silico Drug Repurposing Study of the Regenerating Cochlear Neuron Transcriptome. Journal of Clinical Medicine. 2023; 12(10):3485. https://doi.org/10.3390/jcm12103485
Chicago/Turabian StyleBertagnoli, Lino E., Richard Seist, Shelley Batts, and Konstantina M. Stankovic. 2023. "Potential Ototoxicity of Insulin-like Growth Factor 1 Receptor Signaling Inhibitors: An In Silico Drug Repurposing Study of the Regenerating Cochlear Neuron Transcriptome" Journal of Clinical Medicine 12, no. 10: 3485. https://doi.org/10.3390/jcm12103485
APA StyleBertagnoli, L. E., Seist, R., Batts, S., & Stankovic, K. M. (2023). Potential Ototoxicity of Insulin-like Growth Factor 1 Receptor Signaling Inhibitors: An In Silico Drug Repurposing Study of the Regenerating Cochlear Neuron Transcriptome. Journal of Clinical Medicine, 12(10), 3485. https://doi.org/10.3390/jcm12103485





