Nasendoscopy to Predict Difficult Videolaryngoscopy: A Multivariable Model Development Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Allocation and Data Collection
2.2. Eligibility Criteria
2.3. Sample Size Analysis
2.4. Outcome Measures
2.5. Co-Variables for Model Fitting
- (i)
- Simplified Airway Risk Index [13]: the SARI score (0 to 12 points) encompasses seven binary or categorized variables: mouth opening, thyromental distance, Mallampati score (modification by Samsoon and Young [41]), neck mobility, mandibular protrusion, body weight and history of difficult intubation.
- (ii)
- Clinical factors (symptom screening): Typical clinical signs for pharyngolaryngeal lesion and demographic data were systematically assessed and subdivided into five sub-groups:
- Dysphagia (self-reported; y/n): dysphagia; pharyngeal pressure or globus sensation; pharyngeal foreign body sensation; excessive salivation; odynophagia; frequent choking; difficulties swallowing liquids; food intake impossible.
- Dysphonia (self-reported and physical examination; y/n): altered voice; lumped speech; frequent throat clearing; weak voice or phonation difficulties; whispering or aphonia; progression of dysphonia in the last 3 months.
- Cough (self-reported and physical examination; y/n): dry cough; productive cough; impaired expectoration.
- Stridor (clinical examination with auscultation; y/n): inspiratory stridor.
- Demographic data: sex (male/female); age (years); height (cm).
- (iii)
- TVE findings: TVE examinations were systematically reviewed as previously reported [9]. Co-variables were subdivided into two sub-groups:
- Location of lesions (y/n): hypopharynx; supraglottic; arytenoids; vocal cords; vestibular folds; epiglottis; base of the tongue; multiple unilateral findings; bilateral findings; no lesions.
- Accompanying findings (y/n): vulnerable mucosa with or without active bleeding; pharyngeal secretion retention; impaired vocal cord mobility; view restrictions on the rima glottidis due to lesions (none/relevant view restriction that cover <50%/≥50% of the glottis cross-sectional area).
2.6. Descriptive Statistics
2.7. Development of Three Multivariable Mixed Logistic Regression Models
2.8. Sensitivity Analysis
2.9. Comparison between Models
3. Results
3.1. Development of Three Multivariable Mixed Logistic Regression Models
3.2. Comparison between Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cook, T.M.; Woodall, N.; Frerk, C.; Fourth National Audit, P. Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: Anaesthesia. Br. J. Anaesth. 2011, 106, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Cumberworth, A.; Lewith, H.; Sud, A.; Jefferson, H.; Athanassoglou, V.; Pandit, J.J. Major complications of airway management: A prospective multicentre observational study. Anaesthesia 2022, 77, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Huitink, J.M.; Lie, P.P.; Heideman, I.; Jansma, E.P.; Greif, R.; van Schagen, N.; Schauer, A. A prospective, cohort evaluation of major and minor airway management complications during routine anaesthetic care at an academic medical centre. Anaesthesia 2017, 72, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Joffe, A.M.; Aziz, M.F.; Posner, K.L.; Duggan, L.V.; Mincer, S.L.; Domino, K.B. Management of Difficult Tracheal Intubation: A Closed Claims Analysis. Anesthesiology 2019, 131, 818–829. [Google Scholar] [CrossRef]
- Detsky, M.E.; Jivraj, N.; Adhikari, N.K.; Friedrich, J.O.; Pinto, R.; Simel, D.L.; Wijeysundera, D.N.; Scales, D.C. Will This Patient Be Difficult to Intubate?: The Rational Clinical Examination Systematic Review. JAMA 2019, 321, 493–503. [Google Scholar] [CrossRef]
- Roth, D.; Pace, N.L.; Lee, A.; Hovhannisyan, K.; Warenits, A.M.; Arrich, J.; Herkner, H. Airway physical examination tests for detection of difficult airway management in apparently normal adult patients. Cochrane Database Syst. Rev. 2018, 5, CD008874. [Google Scholar] [CrossRef]
- Arne, J.; Descoins, P.; Fusciardi, J.; Ingrand, P.; Ferrier, B.; Boudigues, D.; Aries, J. Preoperative assessment for difficult intubation in general and ENT surgery: Predictive value of a clinical multivariate risk index. Br. J. Anaesth. 1998, 80, 140–146. [Google Scholar] [CrossRef]
- Ayuso, M.A.; Sala, X.; Luis, M.; Carbo, J.M. Predicting difficult orotracheal intubation in pharyngo-laryngeal disease: Preliminary results of a composite index. Can. J. Anaesth. 2003, 50, 81–85. [Google Scholar] [CrossRef]
- Barclay-Steuart, A.; Großhennig, H.L.; Sasu, P.B.; Wünsch, V.A.; Stadlhofer, R.; Berger, J.; Stark, M.; Sehner, S.; Zöllner, C.; Petzoldt, M. Transnasal Videoendoscopy for Preoperative Airway Risk Stratification—Development and Validation of a Multivariable Risk Prediction Model. Anesth. Analg. 2023. [Google Scholar] [CrossRef]
- Descoins, P.; Arne, J.; Bresard, D.; Aries, J.; Fusciardi, J. Proposal for a new multifactor screening score of difficult intubation in ORL and stomatognathic surgery: Preliminary study. Ann. Fr. Anesth. Reanim. 1994, 13, 195–200. [Google Scholar] [CrossRef]
- Kohse, E.K.; Siebert, H.K.; Sasu, P.B.; Loock, K.; Dohrmann, T.; Breitfeld, P.; Barclay-Steuart, A.; Stark, M.; Sehner, S.; Zollner, C.; et al. A model to predict difficult airway alerts after videolaryngoscopy in adults with anticipated difficult airways—The VIDIAC score. Anaesthesia 2022, 77, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Bryan, Y.F.; Morgan, A.G.; Johnson, K.N.; Harris, H.M.; May, J.; Whelan, D.M.; Tung, A. Procedural Challenges during Intubation in Patients with Oropharyngeal Masses: A Prospective Observational Study. Anesth. Analg. 2019, 128, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- El-Ganzouri, A.R.; McCarthy, R.J.; Tuman, K.J.; Tanck, E.N.; Ivankovich, A.D. Preoperative airway assessment: Predictive value of a multivariate risk index. Anesth. Analg. 1996, 82, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.E.; Spiegelhalter, D.; Robertson, J.A.; Lesser, P. Predicting difficult intubation. Br. J. Anaesth. 1988, 61, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.M.; De, M.; Foran, B.; Harrington, K.; Mortimore, S. Laryngeal cancer: United Kingdom National Multidisciplinary guidelines. J. Laryngol. Otol. 2016, 130, S75–S82. [Google Scholar] [CrossRef]
- Stachler, R.J.; Francis, D.O.; Schwartz, S.R.; Damask, C.C.; Digoy, G.P.; Krouse, H.J.; McCoy, S.J.; Ouellette, D.R.; Patel, R.R.; Reavis, C.C.W.; et al. Clinical Practice Guideline: Hoarseness (Dysphonia) (Update). Otolaryngol. Head Neck Surg. 2018, 158, S1–S42. [Google Scholar] [CrossRef]
- Gemma, M.; Buratti, L.; Di Santo, D.; Calvi, M.R.; Ravizza, A.; Bondi, S.; Bussi, M.; Beretta, L. Pre-operative transnasal endoscopy as a predictor of difficult airway: A prospective cohort study. Eur. J. Anaesthesiol. 2020, 37, 98–104. [Google Scholar] [CrossRef]
- Guo, Y.; Feng, Y.; Liang, H.; Zhang, R.; Cai, X.; Pan, X. Role of flexible fiberoptic laryngoscopy in predicting difficult intubation. Minerva Anestesiol. 2018, 84, 337–345. [Google Scholar] [CrossRef]
- Apfelbaum, J.L.; Hagberg, C.A.; Connis, R.T.; Abdelmalak, B.B.; Agarkar, M.; Dutton, R.P.; Fiadjoe, J.E.; Greif, R.; Klock, P.A.; Mercier, D.; et al. 2022 American Society of Anesthesiologists Practice Guidelines for Management of the Difficult Airway. Anesthesiology 2022, 136, 31–81. [Google Scholar] [CrossRef]
- Law, J.A.; Duggan, L.V.; Asselin, M.; Baker, P.; Crosby, E.; Downey, A.; Hung, O.R.; Jones, P.M.; Lemay, F.; Noppens, R.; et al. Canadian Airway Focus Group updated consensus-based recommendations for management of the difficult airway: Part 1. Difficult airway management encountered in an unconscious patient. Can. J. Anaesth. 2021, 68, 1373–1404. [Google Scholar] [CrossRef]
- De Carvalho, C.C.; da Silva, D.M.; Lemos, V.M.; Dos Santos, T.G.B.; Agra, I.C.; Pinto, G.M.; Ramos, I.B.; Costa, Y.S.C.; Santos Neto, J.M. Videolaryngoscopy vs. direct Macintosh laryngoscopy in tracheal intubation in adults: A ranking systematic review and network meta-analysis. Anaesthesia 2022, 77, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, T. Management of the Difficult Airway. N. Engl. J. Med. 2021, 384, 1836–1847. [Google Scholar] [CrossRef] [PubMed]
- Hansel, J.; Rogers, A.M.; Lewis, S.R.; Cook, T.M.; Smith, A.F. Videolaryngoscopy versus direct laryngoscopy for adults undergoing tracheal intubation. Cochrane Database Syst. Rev. 2022, 4, CD011136. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.F.; Bayman, E.O.; Van Tienderen, M.M.; Todd, M.M.; Brambrink, A.M. Predictors of difficult videolaryngoscopy with GlideScope (R) or C-MAC (R) with D-blade: Secondary analysis from a large comparative videolaryngoscopy trial. Br. J. Anaesth. 2016, 117, 118–123. [Google Scholar] [CrossRef]
- Caldiroli, D.; Cortellazzi, P. A new difficult airway management algorithm based upon the El Ganzouri Risk Index and GlideScope(R) videolaryngoscope. A new look for intubation? Minerva Anestesiol. 2011, 77, 1011–1017. [Google Scholar]
- Cook, F.; Lobo, D.; Martin, M.; Imbert, N.; Grati, H.; Daami, N.; Cherait, C.; Saidi, N.E.; Abbay, K.; Jaubert, J.; et al. Prospective validation of a new airway management algorithm and predictive features of intubation difficulty. Br. J. Anaesth. 2019, 122, 245–254. [Google Scholar] [CrossRef]
- Cortellazzi, P.; Minati, L.; Falcone, C.; Lamperti, M.; Caldiroli, D. Predictive value of the El-Ganzouri multivariate risk index for difficult tracheal intubation: A comparison of Glidescope videolaryngoscopy and conventional Macintosh laryngoscopy. Br. J. Anaesth. 2007, 99, 906–911. [Google Scholar] [CrossRef]
- Lundstrom, L.H.; Moller, A.M.; Rosenstock, C.; Astrup, G.; Gatke, M.R.; Wetterslev, J.; Danish Anaesthesia, D. A documented previous difficult tracheal intubation as a prognostic test for a subsequent difficult tracheal intubation in adults. Anaesthesia 2009, 64, 1081–1088. [Google Scholar] [CrossRef]
- Sajayan, A.; Nair, A.; McNarry, A.F.; Mir, F.; Ahmad, I.; El-Boghdadly, K. Analysis of a national difficult airway database. Anaesthesia 2022, 77, 1081–1088. [Google Scholar] [CrossRef]
- Siebert, H.K.; Kohse, E.K.; Petzoldt, M. A universal classification for videolaryngoscopy using the VIDIAC score requires real world conditions: A reply. Anaesthesia 2023, 78, 126. [Google Scholar] [CrossRef]
- Cook, T.M.; Boniface, N.J.; Seller, C.; Hughes, J.; Damen, C.; MacDonald, L.; Kelly, F.E. Universal videolaryngoscopy: A structured approach to conversion to videolaryngoscopy for all intubations in an anaesthetic and intensive care department. Br. J. Anaesth. 2018, 120, 173–180. [Google Scholar] [CrossRef] [PubMed]
- De Jong, A.; Sfara, T.; Pouzeratte, Y.; Pensier, J.; Rolle, A.; Chanques, G.; Jaber, S. Videolaryngoscopy as a first-intention technique for tracheal intubation in unselected surgical patients: A before and after observational study. Br. J. Anaesth. 2022, 129, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Chrimes, N.; Higgs, A.; Hagberg, C.A.; Baker, P.A.; Cooper, R.M.; Greif, R.; Kovacs, G.; Law, J.A.; Marshall, S.D.; Myatra, S.N.; et al. Preventing unrecognised oesophageal intubation: A consensus guideline from the Project for Universal Management of Airways and international airway societies. Anaesthesia 2022, 77, 1395–1415. [Google Scholar] [CrossRef] [PubMed]
- Elm, E.v.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef]
- Riley, R.D.; Ensor, J.; Snell, K.I.E.; Harrell, F.E., Jr.; Martin, G.P.; Reitsma, J.B.; Moons, K.G.M.; Collins, G.; van Smeden, M. Calculating the sample size required for developing a clinical prediction model. BMJ 2020, 368, m441. [Google Scholar] [CrossRef]
- Moorthy, S.S.; Gupta, S.; Laurent, B.; Weisberger, E.C. Management of airway in patients with laryngeal tumors. J. Clin. Anesth. 2005, 17, 604–609. [Google Scholar] [CrossRef]
- Rosenblatt, W.; Ianus, A.I.; Sukhupragarn, W.; Fickenscher, A.; Sasaki, C. Preoperative endoscopic airway examination (PEAE) provides superior airway information and may reduce the use of unnecessary awake intubation. Anesth. Analg. 2011, 112, 602–607. [Google Scholar] [CrossRef]
- Tasli, H.; Karakoc, O.; Birkent, H. A Grading System for Transnasal Flexible Laryngoscopy. J. Voice 2019, 33, 712–715. [Google Scholar] [CrossRef]
- Yamamoto, K.; Tsubokawa, T.; Shibata, K.; Ohmura, S.; Nitta, S.; Kobayashi, T. Predicting difficult intubation with indirect laryngoscopy. Anesthesiology 1997, 86, 316–321. [Google Scholar] [CrossRef]
- Grensemann, J.; Mohlenkamp, E.; Breitfeld, P.; Tariparast, P.A.; Peters, T.; Punke, M.A.; Kluge, S.; Petzoldt, M. Tracheal Tube-Mounted Camera Assisted Intubation vs. Videolaryngoscopy in Expected Difficult Airway: A Prospective, Randomized Trial (VivaOP Trial). Front. Med. 2021, 8, 767182. [Google Scholar] [CrossRef]
- Samsoon, G.L.; Young, J.R. Difficult tracheal intubation: A retrospective study. Anaesthesia 1987, 42, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Soc. Ser. B (Methodol.) 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Groll, A.; Trutz, G. Variable selection for generalized linear mixed models by L1-penalized estimation. Stat. Comput. 2014, 24, 137–154. [Google Scholar] [CrossRef]
- Cavanaugh, J.E.; Neath, A.A. The Akaike information criterion: Background, derivation, properties, application, interpretation, and refinements. WIREs Comput. Stat. 2019, 11, e1460. [Google Scholar] [CrossRef]
- Gaszynski, T. A comparison of pre-operative transnasal flexible endoscopic laryngoscopy and actual laryngeal view obtained with videolaryngoscopy in predicted difficult intubations. Eur. J. Anaesthesiol. 2021, 38, 201–202. [Google Scholar] [CrossRef]
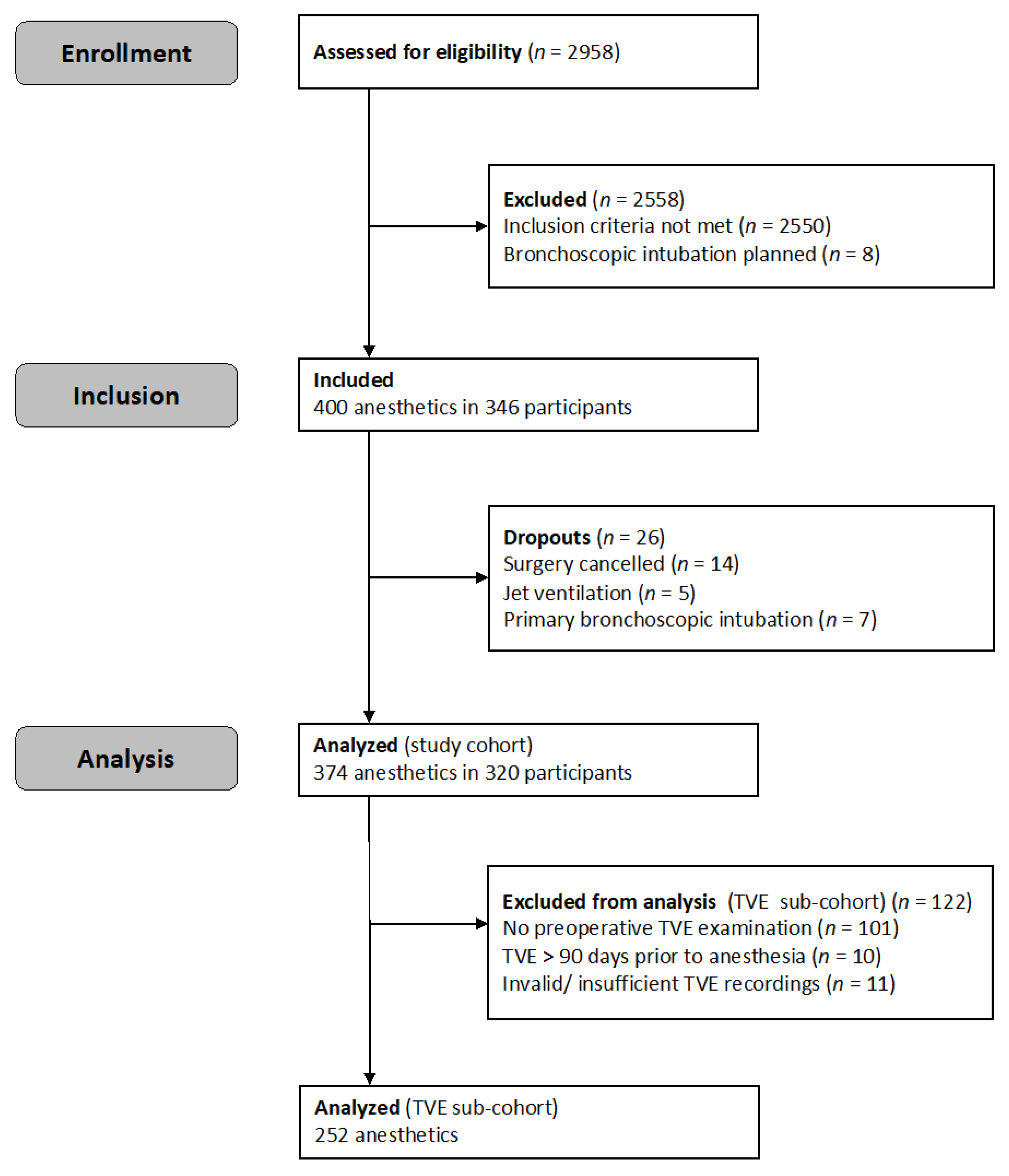
| Characteristics | Study Cohort n = 374 | TVE Sub-Cohort n = 252 |
|---|---|---|
| Age [years], mean (SD) | 61.5 (13.8) | 61.8 (13.5) |
| Sex [male] | 69.5% [260/374] | 73.4% [185/252] |
| ASA physical status classification [grade] | ||
| 1 | 5.6% [21/374] | 5.6% [14/252] |
| 2 | 33.7% [126/374] | 31.7% [80/252] |
| 3 | 57.8% [216/374] | 58.7% [148/252] |
| 4 | 2.9% [11/374] | 4.0% [10/252] |
| Previous neck dissection | 29.9% [112/374] | 26.6% [67/252] |
| Previous tracheostomy | 27.0% [101/374] | 27.4% [69/252] |
| Previous neck radiotherapy | 25.4% [95/374] | 25.4% [64/252] |
| Previous awake tracheal intubation | 17.1% [64/374] | 15.5% [39/252] |
| Previous mouth floor resection | 14.4% [54/374] | 11.5% [29/252] |
| Existing anesthesia alert card | 12.8% [48/374] | 15.9% [40/252] |
| Mallampati class | ||
| 1 | 11.2% [42/374] | 11.1% [28/252] |
| 2 | 21.4% [80/374] | 23.8% [60/252] |
| 3 | 31.8% [119/374] | 29.4% [74/252] |
| 4 | 35.6% [133/374] | 35.7% [90/252] |
| Supraglottic tumor | 25.1% [94/374] | 28.6% [72/252] |
| Glottic tumor | 9.6% [36/374] | 11.1% [28/252] |
| SARI [0–12], median (IQR) | 4 (3–6) | 4 (2.5–6) |
| Could not bite upper lip | 38.2% [143/374] | 33.3% [84/252] |
| Operation | ||
| Laryngopharyngeal | 40.4% [151/374] | 44.0% [111/252] |
| Lower jaw | 23.8% [89/374] | 24.6% [62/252] |
| Neck, maxillofacial | 20.3% [76/374] | 16.7% [42/252] |
| Ear, nose | 9.4% [35/374] | 9.9% [25/252] |
| Dentoalveolar | 6.1% [23/374] | 4.8% [12/252] |
| Nasal intubation | 30.2% [113/374] | 26.6% [67/252] |
| Rapid sequence intubation | 7.8% [29/374] | 7.1% [18/252] |
| Outcome Measures | Study Cohort n = 374 | TVE Sub-Cohort n = 252 |
|---|---|---|
| Difficult videolaryngoscopic intubation alert | 48.9% [183/374] | 55.2% [139/252] |
| Difficult intubation * | 30.5% [114/374] | 32.1% [81/252] |
| Difficult videolaryngoscopy * | 19.3% [72/374] | 22.2% [56/252] |
| Transition to a hyperangulated blade | 20.3% [76/374] | 23.8% [60/252] |
| Transition to bronchoscopic intubation | 1.3% [5/374] | 1.2% [3/252] |
| Laryngoscopy attempts | ||
| 1 | 67.1% [251/374] | 63.5% [160/252] |
| 2 | 24.3% [91/374] | 27.4% [69/252] |
| >2 | 8.6% [32/374] | 9.1% [23/252] |
| Intubation attempts | ||
| 1 | 69.5% [260/374] | 67.9% [171/252] |
| 2 | 12.8% [48/374] | 13.1% [33/252] |
| >2 | 17.6% [66/374] | 19.0% [48/252] |
| First pass success † | 52.1% [195/374] | 50.4% [127/252] |
| Time to tracheal intubation [s], median (IQR) | 86 (42–175) | 90 (43–177) |
| End-tidal pCO2 after intubation [mmHg], mean (SD) | 36 (8.4) | 36 (8.5) |
| Airway-related adverse events | 18.2% [68/374] | 18.7% [47/252] |
| Length of hospital stay (days), median (IQR) | 3 (2–7) | 3 (2–7) |
| Deaths in hospital | 0.5% [2/374] | 0.4% [1/252] |
| Characteristics | |
|---|---|
| SARI | (n = 374) |
| SARI [0–12], median (IQR) | 4 (3–6) |
| Clinical signs | (n = 374) |
| Age [years], mean (SD) | 61.5 (13.8) |
| Height [cm], mean (SD) | 174 (9.6) |
| Dysphagia | 35.3% [132/374] |
| Weak voice or phonation difficulties | 18.7% [70/374] |
| Whispering or aphonia | 8.3% [31/374] |
| Dry cough | 17.9% [67/374] |
| Productive cough | 19.5% [73/374] |
| Impaired expectoration | 13.1% [49/374] |
| Stridor | 1.9% [7/374] |
| Transnasal videoendoscopy findings | (n = 252) |
| Pharyngeal secretion retention | 47.6% [120/252] |
| Lesions | |
| Vocal cords | 2.8% [7/252] |
| Vestibular folds | 17.5% [44/252] |
| Epiglottis | 17.5% [44/252] |
| Multiple unilateral lesions | 21.8% [55/252] |
| View restriction on rima glottidis | |
| Relevant, covers < 50% of the glottis area | 13.9% [35/252] |
| Covers ≥ 50% of the glottis area | 11.5% [29/252] |
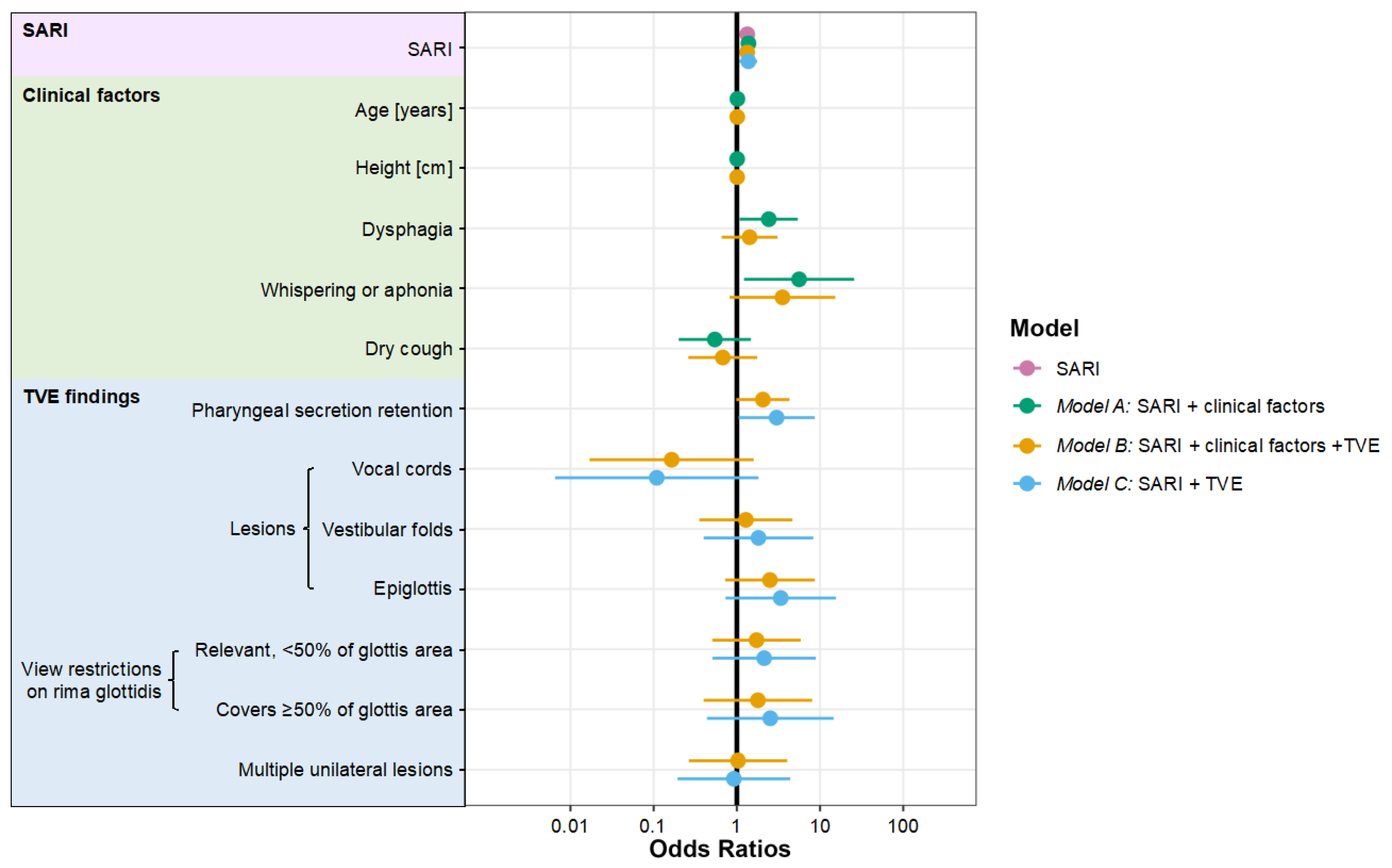
| Characteristics | SARI OR (95% CI) | Model A SARI with Clinical Factors OR (95% CI) | Model B SARI, Clinical Factors and TVE OR (95% CI) | Model C SARI with TVE OR (95% CI) |
|---|---|---|---|---|
| Likelihood ratio test; compared with SARI, p-values | - | p = 0.01 | p < 0.001 | p < 0.001 |
| Likelihood ratio test; compared with model C, p-values | p < 0.001 | p < 0.001 | p = 0.37 | - |
| SARI | ||||
| SARI [0–12] | 1.33 (1.13 to 1.58) | 1.38 (1.15 to 1.65) | 1.33 (1.14 to 1.56) | 1.37 (1.08 to 1.76) |
| Clinical factors | ||||
| Age [years] | - | 1.02 (0.99 to 1.04) | 1.01 (0.99 to 1.04) | - |
| Height [cm] | - | 1.01 (0.97 to 1.05) | 1.01 (0.97 to 1.05) | - |
| Dysphagia | - | 2.42 (1.08 to 5.41) | 1.42 (0.66 to 3.09) | - |
| Whispering or aphonia | - | 5.60 (1.22 to 25.74) | 3.53 (0.82 to 15.25) | - |
| Dry cough | - | 0.54 (0.20 to 1.47) | 0.68 (0.26 to 1.76) | - |
| TVE findings | ||||
| Pharyngeal secretion retention | - | - | 2.06 (0.98 to 4.29) | 3.01 (1.05 to 8.63) |
| Lesions | ||||
| Vocal cords | - | - | 0.16 (0.02 to 1.60) | 0.11 (0.01 to 1.82) |
| Vestibular folds | - | - | 1.28 (0.35 to 4.66) | 1.82 (0.40 to 8.29) |
| Epiglottis | - | - | 2.50 (0.72 to 8.63) | 3.37 (0.73 to 15.54) |
| View restriction on rima glottidis | ||||
| Relevant, <50% of glottis area | - | - | 1.72 (0.51 to 5.87) | 2.13 (0.51 to 8.89) |
| Covers ≥50% of glottis area | - | - | 1.79 (0.40 to 8.04) | 2.52 (0.44 to 14.56) |
| Multiple unilateral lesions | - | - | 1.03 (0.26 to 4.03) | 0.92 (0.19 to 4.38) |
| ICC | 0.30 | 0.29 | 0.21 | 0.38 |
| AICc | 327.1 | 323.2 | 316.8 | 311.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasu, P.B.; Pansa, J.-I.; Stadlhofer, R.; Wünsch, V.A.; Loock, K.; Buscher, E.K.; Dankert, A.; Ozga, A.-K.; Zöllner, C.; Petzoldt, M. Nasendoscopy to Predict Difficult Videolaryngoscopy: A Multivariable Model Development Study. J. Clin. Med. 2023, 12, 3433. https://doi.org/10.3390/jcm12103433
Sasu PB, Pansa J-I, Stadlhofer R, Wünsch VA, Loock K, Buscher EK, Dankert A, Ozga A-K, Zöllner C, Petzoldt M. Nasendoscopy to Predict Difficult Videolaryngoscopy: A Multivariable Model Development Study. Journal of Clinical Medicine. 2023; 12(10):3433. https://doi.org/10.3390/jcm12103433
Chicago/Turabian StyleSasu, Phillip Brenya, Jennifer-Isabel Pansa, Rupert Stadlhofer, Viktor Alexander Wünsch, Karolina Loock, Eva Katharina Buscher, André Dankert, Ann-Kathrin Ozga, Christian Zöllner, and Martin Petzoldt. 2023. "Nasendoscopy to Predict Difficult Videolaryngoscopy: A Multivariable Model Development Study" Journal of Clinical Medicine 12, no. 10: 3433. https://doi.org/10.3390/jcm12103433
APA StyleSasu, P. B., Pansa, J.-I., Stadlhofer, R., Wünsch, V. A., Loock, K., Buscher, E. K., Dankert, A., Ozga, A.-K., Zöllner, C., & Petzoldt, M. (2023). Nasendoscopy to Predict Difficult Videolaryngoscopy: A Multivariable Model Development Study. Journal of Clinical Medicine, 12(10), 3433. https://doi.org/10.3390/jcm12103433








