Challenges of Pituitary Apoplexy in Pregnancy
Abstract
1. Introduction
Aim
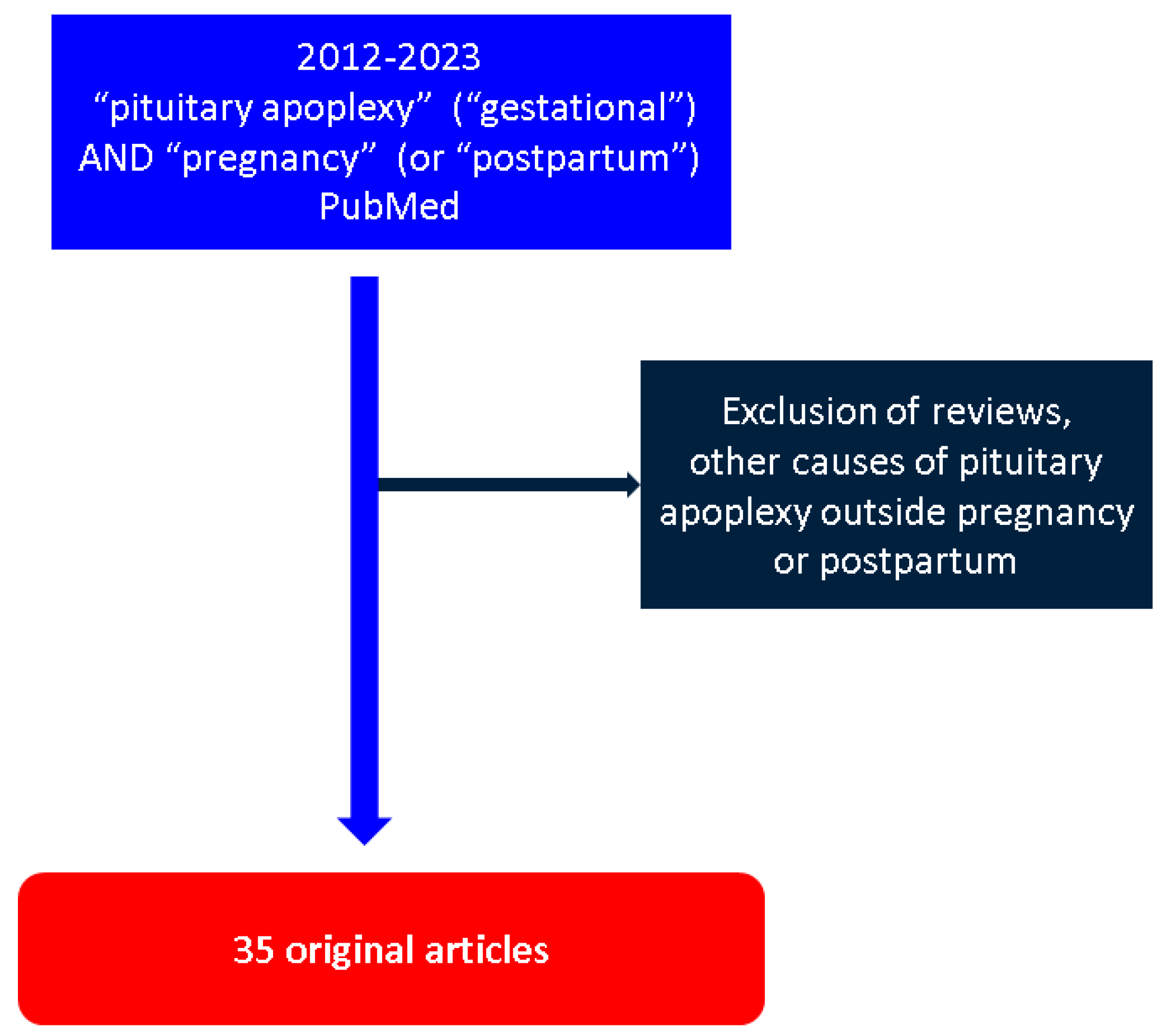
2. Methods
3. Results
3.1. Sample–Case Series Study
3.2. Patients’ Characteristics: Pregnancy Features
3.3. Onset of PA in Pregnancy: Focus on Clinical Panel
3.4. Risk Factors for Developing PA in Pregnancy
3.5. Differentiating PA in Pregnancy from Other Entities
3.6. Conservative Management of PA Amid Pregnancy
3.7. Surgical Management in PA Amid Pregnancy
3.8. PitNET Analysis
3.9. Outcome of PA in Pregnancy
3.10. PA during PP
| Reference (Name, Number, and Year of Publication) | Type of Study | Population | Gravidity and Parity | Days at PP on Presentation | Clinical Presentation | Preexisting Pituitary Lesion | Treatment | Delivery | Maternal Outcome | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| Mathur [93] 2014 | Case report | 34-year-old female | G2P1 | 5 min PP | Persistent headache for 48 h following CS with spinal anesthesia (with onset 5 min after delivery) Nausea Transient DI | No pituitary adenoma | Conservative management with oral hydrocortisone | LB by CS | Normal pituitary function Pituitary enlargement adjacent to the chiasma | Ten days after PA the patient developed RCVS |
| Grand’Maison [20] 2015 | Case series | Four cases of PA related to pregnancy, of which one, a 40-year-old female, was of PA during PP | G4P1A3 | 6 h PP | Headache and unable to lactate | No pituitary mass | Conservative management with cortisol supplementation | LB at 36 WG by VD with forceps | Adrenal insufficiency for 7 months post-partum, GH deficiency, and atrophic pituitary gland | The patient also suffered from type 1 DM and primary hypothyroidism |
| Raina [94] 2015 | Case report | 27-year-old female | G2 | 2 days PP | Headache Blurred vision Ptosis Diplopia | No pituitary adenoma | Conservative management with hydrocortisone 50 mg 6 times hourly Thyroid hormone replacement therapy | LB by VD (home-conducted) | Complete recovery and normalized thyroid function | The patient suffered from PP hemorrhage |
| Dias [95] 2021 | Case report | 37-year-old female | G2 | 12 days PP | Occipital headache Nausea Vomiting Fever | No pituitary adenoma | Conservative (NSAIDs and IV fluids) | LB at term by CS | Hypothyroidism | |
| Hoang [96] 2022 | Case report | 34-year-old female | primigravida | 2 days PP | Right facial paralysis Headache Eye pain Blurred vision | No pituitary adenoma | Conservative | LB at 38th by CS | Complete recovery Normal pituitary function | The patient also suffered from a subdural hematoma |
| Pop [97] 2022 | Case report | 26-year-old female | primigravida | 48 h PP | Headache Nausea Photophobia 3rd cranial nerve palsy: left ptosis and anisocoria Polyuria and polydipsia | NFPA of 3.3 × 1.05 × 1.55 cm without compression on the optic chiasma | Initial conservative management with dexamethasone and LT4 TSS | LB at 40th by CS | Complete neurological recovery at 2 years follow-up: HRT for panhypopituitarism |
4. Discussions
4.1. Integrating PA in Pregnancy and PP to the Larger Frame of PAs
4.2. PA in Pregnancy versus Postpartum
4.3. Integrating PA Amid Other Endocrine Complications of Pregnancy
4.4. COVID-19 Infection Associated with PA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bi, W.L.; Dunn, I.F.; Laws, E.R. Pituitary apoplexy. Endocrine 2015, 48, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Suri, H.; Dougherty, C. Presentation and Management of Headache in Pituitary Apoplexy. Curr. Pain Headache Rep. 2019, 23, 61. [Google Scholar] [CrossRef]
- Barkhoudarian, G.; Kelly, D.F. Pituitary Apoplexy. Neurosurg. Clin. N. Am. 2019, 30, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Mavridis, I.; Meliou, M.; Pyrgelis, E.-S. Presenting Symptoms of Pituitary Apoplexy. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2017, 79, 052–059. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.M. On the origins of pituitary apoplexy. Eur. Neurol. 2015, 74, 18–21. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, E.A. Headache in pregnancy. Neurol. Clin. 2012, 30, 835–866. [Google Scholar] [CrossRef] [PubMed]
- Khoo, C.M.; Lee, K.O. Endocrine emergencies in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2013, 27, 885–891. [Google Scholar] [CrossRef]
- Briet, C.; Salenave, S.; Bonneville, J.-F.; Laws, E.R.; Chanson, P. Pituitary Apoplexy. Endocr. Rev. 2015, 36, 622–645. [Google Scholar] [CrossRef]
- Kreitschmann-Andermahr, I.; Siegel, S.; Carneiro, R.W.; Maubach, J.M.; Harbeck, B.; Brabant, G. Headache and pituitary disease: A systematic review. Clin. Endocrinol. 2013, 79, 760–769. [Google Scholar] [CrossRef]
- Hage, R.; Eshraghi, S.R.; Oyesiku, N.M.; Ioachimescu, A.G.; Newman, N.J.; Biousse, V.; Bruce, B.B. Third, Fourth, and Sixth Cranial Nerve Palsies in Pituitary Apoplexy. World Neurosurg. 2016, 94, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M. Endocrine Emergencies With Neurologic Manifestations. Contin. Lifelong Learn. Neurol. 2017, 23, 778–801. [Google Scholar] [CrossRef] [PubMed]
- Takeda, R.; Demura, M.; Sugimura, Y.; Miyamori, I.; Konoshita, T.; Yamamoto, H. Pregnancy-associated diabetes insipidus in Japan—A review based on quoting from the literatures reported during the period from 1982 to 2019. Endocr. J. 2021, 68, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.C.; Hamrahian, A.H.; Weil, R.J.; Kennedy, L. Pituitary tumor apoplexy. J. Clin. Neurosci. 2015, 22, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Donegan, D.; Erickson, D. Revisiting Pituitary Apoplexy. J. Endocr. Soc. 2022, 6, bvac113. [Google Scholar] [CrossRef]
- Araujo-Castro, M.; Berrocal, V.R.; Pascual-Corrales, E. Pituitary tumors: Epidemiology and clinical presentation spectrum. Hormones 2020, 19, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Vargas, G.; Gonzalez, B.; Guinto, G.; Mendoza, V.; López-Félix, B.; Zepeda, E.; Mercado, M. Pituitary apoplexy in nonfunctioning pituitary macroadenomas: A case-control study. Endocr. Pract. 2014, 20, 1274–1280. [Google Scholar] [CrossRef]
- Sarwar, K.N.; Huda, M.S.B.; Van De Velde, V.; Hopkins, L.; Luck, S.; Preston, R.; McGowan, B.; Carroll, P.V.; Powrie, J.K. The prevalence and natural history of pituitary hemorrhage in prolactinoma. J. Clin. Endocrinol. Metab. 2013, 98, 2362–2367. [Google Scholar] [CrossRef]
- Nawar, R.N.; AbdelMannan, D.; Selman, W.R.; Arafah, B.M. Pituitary tumor apoplexy: A review. J. Intensiv. Care Med. 2008, 23, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, N. Pituitary Apoplexy: A Comprehensive Review. Neurol. India 2020, 68, S72–S78. [Google Scholar]
- Grand’Maison, S.; Weber, F.; Bédard, M.-J.; Mahone, M.; Godbout, A. Pituitary apoplexy in pregnancy: A case series and literature review. Obstet. Med. 2015, 8, 177–183. [Google Scholar] [CrossRef]
- Semple, P.L.; Jane, J.A., Jr.; Laws, E.R., Jr. Clinical relevance of precipitating factors in pituitary apoplexy. Neurosurgery 2007, 61, 956–962; discussion 961–962. [Google Scholar] [CrossRef] [PubMed]
- Wildemberg, L.E.; Glezer, A.; Bronstein, M.D.; Gadelha, M.R. Apoplexy in nonfunctioning pituitary adenomas. Pituitary 2018, 21, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Wichlińska-Lubińska, M.; Kozera, G. Pituitary apoplexy. Neurol. I Neurochir. Pol. 2019, 53, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, R.; De Martino, M.C.; Auriemma, R.S.; Alviggi, C.; Grasso, L.F.S.; Cozzolino, A.; De Leo, M.; DE Placido, G.; Colao, A.; Lombardi, G. Pituitary tumors and pregnancy: The interplay between a pathologic condition and a physiologic status. J. Endocrinol. Investig. 2014, 37, 99–112. [Google Scholar] [CrossRef]
- Scheithauer, B.W.; Sano, T.; Kovacs, K.T.; Young, W.F.; Ryan, N.; Randall, R.V. The pituitary gland in pregnancy: A clinicopathologic and immunohistochemical study of 69 cases. Mayo Clin. Proc. 1990, 65, 461–474. [Google Scholar] [CrossRef]
- Huang, W.; Molitch, M.E. Pituitary Tumors in Pregnancy. Endocrinol. Metab. Clin. N. Am. 2019, 48, 569–581. [Google Scholar] [CrossRef]
- Cardoso, E.R.; Peterson, E.W. Pituitary apoplexy: A review. Neurosurgery 1984, 14, 363–373. [Google Scholar] [CrossRef]
- Qaiser, R.; Black, P. Neurosurgery in pregnancy. Semin. Neurol. 2007, 27, 476–481. [Google Scholar] [CrossRef]
- Molitch, M.E. Pituitary tumors and pregnancy. Growth Horm. IGF Res. 2003, 13, S38–S44. [Google Scholar] [CrossRef]
- Frise, C.J.; Williamson, C. Endocrine disease in pregnancy. Clin. Med. J. R. Coll. Physicians Lond. 2013, 13, 176–181. [Google Scholar] [CrossRef]
- Araujo, P.B.; Neto, L.V.; Gadelha, M.R. Pituitary Tumor Management in Pregnancy. Endocrinol. Metab. Clin. N. Am. 2015, 44, 181–197. [Google Scholar] [CrossRef]
- Woodmansee, W.W. Pituitary Disorders in Pregnancy. Neurol. Clin. 2019, 37, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Laway, B.A.; Mir, S.A. Pregnancy and pituitary disorders: Challenges in diagnosis and management. Indian J. Endocrinol. Metab. 2013, 17, 996–1004. [Google Scholar] [CrossRef]
- Valassi, E. Pituitary disease and pregnancy. Endocrinol. Diabetes Nutr. 2021, 68, 184–195. [Google Scholar] [CrossRef]
- Alex, A.; Bhandary, E.; McGuire, K.P. Anatomy and physiology of the breast during pregnancy and lactation. Adv. Exp. Med. Biol. 2020, 1252, 3–7. [Google Scholar] [CrossRef]
- Chrisoulidou, A.; Boudina, M.; Karavitaki, N.; Bili, E.W.J. Pituitary disorders in pregnancy. Hormones 2015, 14, 70–80. [Google Scholar] [CrossRef]
- Grattan, D.R. 60 years of neuroendocrinology: The hypothalamo-prolactin axis. J. Endocrinol. 2015, 226, T101–T122. [Google Scholar] [CrossRef] [PubMed]
- Honegger, J.; Nasi-Kordhishti, I.; Aboutaha, N.; Giese, S. Surgery for prolactinomas: A better choice? Pituitary 2020, 23, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Biagetti, B.; Simò, R. Pituitary Apoplexy: Risk Factors and Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2022, 23, 8721. [Google Scholar] [CrossRef]
- Toossi, S.; Moheet, A.M. Intracerebral Hemorrhage in Women: A Review with Special Attention to Pregnancy and the Post-Partum Period. Neurocritical Care 2019, 31, 390–398. [Google Scholar] [CrossRef]
- Iuliano, S.; Laws, E.R. Management of pituitary tumors in pregnancy. Semin. Neurol. 2011, 31, 423–428. [Google Scholar] [CrossRef]
- Petersenn, S. Pituitary disease management during pregnancy: An overview. Minerva Endocrinol. 2018, 43, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Schoen, J.; Campbell, R.L.; Sadosty, A.T. Headache in pregnancy: An approach to emergency department evaluation and management. West J. Emerg. Med. 2015, 16, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Guryildirim, M.; Kontzialis, M.; Ozen, M.; Kocak, M. Acute Headache in the Emergency Setting. RadioGraphics 2019, 39, 1739–1759. [Google Scholar] [CrossRef] [PubMed]
- Negro, A.; Delaruelle, Z.; Ivanova, T.A.; Khan, S.; Ornello, R.; Raffaelli, B.; Terrin, A.; Reuter, U.; Mitsikostas, D.D.; European Headache Federation School of Advanced Studies (EHF-SAS). Headache and pregnancy: A systematic review. J. Headache Pain 2017, 18, 106. [Google Scholar] [CrossRef]
- Burch, R. Headache in Pregnancy and the Puerperium. Neurol. Clin. 2019, 37, 31–51. [Google Scholar] [CrossRef]
- Bamfo, J.E.A.K.; Sharif, S.; Donnelly, T.; Cohen, M.A.; Golara, M. A case of pituitary apoplexy masquerading as hyperemesis gravidarum. J. Obstet. Gynaecol. 2011, 31, 662. [Google Scholar] [CrossRef] [PubMed]
- Motivala, S.; Gologorsky, Y.; Kostandinov, J.; Post, K.D. Pituitary disorders during pregnancy. Endocrinol. Metab. Clin. N. Am. 2011, 40, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Inder, W.J.; Jang, C. Treatment of Prolactinoma. Medicina 2022, 58, 1095. [Google Scholar] [CrossRef] [PubMed]
- Zak, I.T.; Dulai, H.S.; Kish, K.K. Imaging of neurologic disorders associated with pregnancy and the postpartum period. Radiographics 2007, 27, 95–108. [Google Scholar] [CrossRef]
- Zamora, C.; Castillo, M. Role of MRI and CT in the Evaluation of Headache in Pregnancy and the Postpartum Period. Neurol. Clin. 2022, 40, 661–677. [Google Scholar] [CrossRef] [PubMed]
- E Baldeweg, S.; Vanderpump, M.; Drake, W.; Reddy, N.; Markey, A.; Plant, G.T.; Powell, M.; Sinha, S.; Wass, J.; Society for Endocrinology Clinical Committee. Society for endocrinology endocrine emergency guidance: Emergency management of pituitary apoplexy in adult patients. Endocr. Connect. 2016, 5, G12–G15. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.; Vanderpump, M.; Baldeweg, S.; Drake, W.; Reddy, N.; Lanyon, M.; Markey, A.; Plant, G.; Powell, M.; Sinha, S.; et al. UK guidelines for the management of pituitary apoplexy Pituitary Apoplexy Guidelines Development Group: May 2010. Clin. Endocrinol. 2011, 74, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Chanson, P.; Raverot, G.; Castinetti, F.; Cortet-Rudelli, C.; Galland, F.; Salenave, S.; French Endocrinology Society Non-Functioning Pituitary Adenoma Work-Group. Management of clinically non-functioning pituitary adenoma. Ann. Endocrinol. 2015, 76, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Glezer, A.; Bronstein, M.D. Prolactinomas: How to handle prior to and during pregnancy? Minerva Endocrinol. 2018, 43, 423–429. [Google Scholar] [CrossRef]
- Glezer, A.; Bronstein, M.D. Prolactinomas in pregnancy: Considerations before conception and during pregnancy. Pituitary 2020, 23, 65–69. [Google Scholar] [CrossRef]
- Luger, A.; A Broersen, L.H.; Biermasz, N.R.; Biller, B.M.K.; Buchfelder, M.; Chanson, P.; Jorgensen, J.O.L.; Kelestimur, F.; Llahana, S.; Maiter, D.; et al. ESE Clinical Practice Guideline on functioning and nonfunctioning pituitary adenomas in pregnancy. Eur. J. Endocrinol. 2021, 185, G1–G33. [Google Scholar] [CrossRef] [PubMed]
- E Molitch, M. Endocrinology in pregnancy: Management of the pregnant patient with a prolactinoma. Eur. J. Endocrinol. 2015, 172, R205–R213. [Google Scholar] [CrossRef]
- Graillon, T.; Cuny, T.; Castinetti, F.; Courbière, B.; Cousin, M.; Albarel, F.; Morange, I.; Bruder, N.; Brue, T.; Dufour, H. Surgical indications for pituitary tumors during pregnancy: A literature review. Pituitary 2020, 23, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Karaca, Z.; Tanriverdi, F.; Unluhizarci, K.; Kelestimur, F. Pregnancy and pituitary disorders. Eur. J. Endocrinol. 2010, 162, 453–475. [Google Scholar] [CrossRef]
- Lamba, N.; Noormohamed, N.; Simjian, T.; Alsheikh, M.Y.; Jamal, A.; Doucette, J.; Zaidi, H.; Smith, T.R.; Mekary, R.A. Fertility after transsphenoidal surgery in patients with prolactinomas: A meta-analysis. Clin. Neurol. Neurosurg. 2019, 176, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.; Chowdhury, M.; Schaller, B.; Cappellani, R.B.; Daya, J. Perioperative considerations for neurosurgical procedures in the gravid patient: Continuing Professional Development. Can. J. Anaesth. 2013, 60, 1139–1155. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Ma, X.; Griffiths, B.B.; Luo, Y. Neurosurgical anesthesia for a pregnant woman with macroprolactinoma: A case report. Medicine 2018, 97, e12360. [Google Scholar] [CrossRef] [PubMed]
- Couture, N.; Aris-Jilwan, N.; Serri, O. Apoplexy of a microprolactinoma during pregnancy: Case report and review of literature. Endocr. Pract. 2012, 18, e147–e150. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.M.; Dreyer, K.; Van Der Weiden, R.M.F. Management of pituitary tumour apoplexy with bromocriptine in pregnancy. JRSM Short Rep. 2012, 3, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kita, D.; Hayashi, Y.; Sano, H.; Takamura, T.; Hayashi, Y.; Tachibana, O.; Hamada, J.-I. Postoperative diabetes insipidus associated with pituitary apoplexy during pregnancy. Neuro Endocrinol. Lett. 2012, 33, 107–112. [Google Scholar]
- Witek, P.; Zieliński, G.; Maksymowicz, M.; Zgliczyński, W. Transsphenoidal surgery for a life-threatening prolactinoma apoplexy during pregnancy. Neuro Endocrinol. Lett. 2012, 33, 483–488. [Google Scholar] [PubMed]
- Chegour, H.; El Ansari, N. Pituitary apoplexy during pregnancy. Pan Afr. Med. J. 2014, 17, 211. [Google Scholar] [CrossRef]
- Hayes, A.R.; O’Sullivan, A.J.; A Davies, M. A case of pituitary apoplexy in pregnancy. Endocrinol. Diabetes Metab. Case Rep. 2014, 2014, 140043. [Google Scholar] [CrossRef]
- Piantanida, E.; Gallo, D.; Lombardi, V.; Tanda, M.L.; Lai, A.; Ghezzi, F.; Minotto, R.; Tabano, A.; Cerati, M.; Azzolini, C.; et al. Pituitary apoplexy during pregnancy: A rare, but dangerous headache. J. Endocrinol. Investig. 2014, 37, 789–797. [Google Scholar] [CrossRef]
- Tandon, A.; Alzate, J.; LaSala, P.; Fried, M.P. Endoscopic Endonasal Transsphenoidal Resection for Pituitary Apoplexy during the Third Trimester of Pregnancy. Surg. Res. Pract. 2014, 2014, 397131. [Google Scholar] [CrossRef] [PubMed]
- Bedford, J.; Dassan, P.; Harvie, M.; Mehta, S. An unusual cause of headache in pregnancy. BMJ 2015, 351, h4681. [Google Scholar] [CrossRef] [PubMed]
- De Ycaza, A.E.; Chang, A.Y.; Jensen, J.R.; Khan, Z.; Erickson, D. Approach to the management of rare clinical presentations of macroprolactinomas in reproductive-aged women. Case Rep. Women’s Health 2015, 8, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Watson, V. An unexpected headache: Pituitary apoplexy in a pregnant woman on anticoagulation. BMJ Case Rep. 2015, 2015, bcr2015210198. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.R.; E Pollitzer, R.; Gokden, M.; A Goulden, P. Spontaneous pituitary apoplexy during the second trimester of pregnancy, with sensory loss. BMJ Case Rep. 2016, 2016, 2015–2017. [Google Scholar] [CrossRef]
- Annamalai, A.K.; Jeyachitra, G.; Jeyamithra, A.; Ganeshkumar, M.; Srinivasan, K.G.; Gurnell, M. Gestational Pituitary Apoplexy Prevalence of Islet Autoantibodies in Type 1 Diabetes. Indian J. Endocrinol. Metab. 2017, 21, 484–485. [Google Scholar] [CrossRef] [PubMed]
- Galvão, A.; Gonçalves, D.; Moreira, M.; Inocêncio, G.; Silva, C.; Braga, J. Prolactinoma and pregnancy—A series of cases including pituitary apoplexy. J. Obstet. Gynaecol. 2017, 37, 284–287. [Google Scholar] [CrossRef]
- Lambert, K.; Rees, K.; Seed, P.T.; Dhanjal, M.K.; Knight, M.; McCance, D.R.; Williamson, C. Macroprolactinomas and Nonfunctioning Pituitary Adenomas and Pregnancy Outcomes. Obstet. Gynecol. 2017, 129, 185–194. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, M.A. Headaches complicating pregnancy and the postpartum period. Pract. Neurol. 2017, 17, 191–202. [Google Scholar] [CrossRef]
- Bachmeier, C.A.E.; Snell, C.; Morton, A. Visual loss in pregnancy. BMJ Case Rep. 2019, 12, e228323. [Google Scholar] [CrossRef]
- Jemel, M.; Kandara, H.; Riahi, M.; Gharbi, R.; Nagi, S.; Kamoun, I. Gestational pituitary apoplexy: Case series and review of the literature. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Barraud, S.; Guédra, L.; Delemer, B.; Raverot, G.; Ancelle, D.; Fèvre, A.; Jouanneau, E.; Litré, C.; Wolak-Thierry, A.; Borson-Chazot, F.; et al. Evolution of macroprolactinomas during pregnancy: A cohort study of 85 pregnancies. Clin. Endocrinol. 2020, 92, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Bichard, L.K.; Torpy, D.J. Diabetes insipidus complicating apoplexy during pregnancy: The potential use of copeptin. Intern. Med. J. 2020, 50, 877–879. [Google Scholar] [CrossRef]
- Chan, J.; Gregory, K.D.; Smithson, S.S.; Naqvi, M.; Mamelak, A.N. Pituitary apoplexy associated with acute COVID-19 infection and pregnancy. Pituitary 2020, 23, 716–720. [Google Scholar] [CrossRef]
- Oguz, S.H.; Soylemezoglu, F.; Dagdelen, S.; Erbas, T. A case of atypical macroprolactinoma presenting with pituitary apoplexy during pregnancy and review of the literature. Gynecol. Endocrinol. 2020, 36, 109–116. [Google Scholar] [CrossRef]
- Geissler, F.; Hoesli, I.; Bernasconi, M.T. Recurrent pituitary apoplexy in pregnancy. BMJ Case Rep. 2021, 14, e242353. [Google Scholar] [CrossRef] [PubMed]
- Kanneganti, A.; Lwin, S.; Su, L.L. Pituitary Apoplexy in Pregnancy. J. Obstet. Gynaecol. Can. 2021, S1701–S2163. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ogawa, Y.; Tominaga, T. Treatment and therapeutic strategies for pituitary apoplexy in pregnancy: A case series. J. Med. Case Rep. 2021, 15, 289. [Google Scholar] [CrossRef] [PubMed]
- Khaldi, S.; Saad, G.; Elfekih, H.; Ben Abdelkrim, A.; Ach, T.; Kacem, M.; Chaieb, M.; Maaroufi, A.; Hasni, Y.; Ach, K. Pituitary apoplexy of a giant prolactinoma during pregnancy. Gynecol. Endocrinol. 2021, 37, 863–866. [Google Scholar] [CrossRef]
- Kuhn, E.; A Weinreich, A.; Biermasz, N.R.; Jorgensen, J.O.L.; Chanson, P. Apoplexy of microprolactinomas during pregnancy: Report of five cases and review of the literature. Eur. J. Endocrinol. 2021, 185, 99–108. [Google Scholar] [CrossRef]
- Ye, W.; Huang, W.; Chen, L.; Yao, C.; Sheng, S.; Liu, Z.; Xue, C.; Xing, W. Pituitary tumor apoplexy associated with extrapontine myelinolysis during pregnancy: A case report. Medicine 2021, 100, e25075. [Google Scholar] [CrossRef] [PubMed]
- Sedai, H.; Shrestha, S.; Poddar, E.; Sharma, P.; Dahal, D.; Khatiwada, P.; Pradhanang, A. Delayed identification of massive pituitary apoplexy in pregnancy: A case report. Int. J. Surg. Case Rep. 2022, 99, 107706. [Google Scholar] [CrossRef] [PubMed]
- Mathur, D.; Lim, L.F.M.; Mathur, M.; Sng, B.L. pituitary apoplexy with reversible cerebral vasoconstrictive syndrome after spinal anaesthesia for emergency caesarean section: An uncommon cause for postpartum headache. Anaesth. Intensiv. Care 2014, 42, 99–105. [Google Scholar] [CrossRef]
- Raina, S.; Jearth, V.; Sharma, A.; Sharma, R.; Mistry, K. Postpartum pituitary apoplexy with isolated oculomotor nerve palsy: A rare medical emergency. J. Neurosci. Rural. Pract. 2015, 6, 598–600. [Google Scholar] [CrossRef]
- Dias, R.; Ferreira, C.; Mendes, B.; Marvão, J.; Lages, N.M.H. Postpartum headache after epidural anaesthesia: Who to blame? Rev. Esp. Anestesiol. Reanim. 2021, 68, 531–536. [Google Scholar] [CrossRef]
- Hoang, V.T.; Hoang, T.H.; Nguyen, T.T.T.; Chansomphou, V.; Hoang, D.T. Pituitary Apoplexy and Subdural Hematoma after Caesarean Section. Case Rep. Obstet. Gynecol. 2022, 2022, 3097949. [Google Scholar] [CrossRef]
- Pop, L.G.; Ilian, A.; Georgescu, T.; Bacalbasa, N.; Balescu, I.; Toader, O.D. Pituitary adenoma apoplexy in pregnancy: Case report and literature review. Exp. Ther. Med. 2022, 23, 218. [Google Scholar] [CrossRef] [PubMed]
- Ducros, A.; Bousser, M.-G. Reversible cerebral vasoconstriction syndrome. Pract. Neurol. 2009, 9, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, S. Gestational diabetes insipidus: Diagnosis and management. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101384. [Google Scholar] [CrossRef] [PubMed]
- Trandafir, A.I.; Petrova, E.; Ghemigian, A.; Valea, A.; Carsote, M.; Sandru, F. Emergency hypophysectomy for pituitary apoplexy in a previously undiagnosed case of acromegaly. Rom. J. Emerg. Surg. 2021, 3, 55–61. [Google Scholar]
- Carsote, M.; Valea, A.; Dumitrascu, M.C.; Albu, S.E.; Sandru, F. Pituitary non-functioning macroadenomas: If and when to recommend surgery. Rom. Med. J. 2019, 66, 430–433. [Google Scholar] [CrossRef]
- Arnold, M.A.; Barbero, J.M.R.; Pradilla, G.; Wise, S.K. Pituitary Gland Surgical Emergencies: The Role of Endoscopic Intervention. Otolaryngol. Clin. N. Am. 2022, 55, 397–410. [Google Scholar] [CrossRef]
- Diabetes insipidus in pregnancy: How to advice the patient? Minerva Endocrinol. 2018, 43, 458–464. [CrossRef]
- Tomkins, M.; Lawless, S.; Martin-Grace, J.; Sherlock, M.; Thompson, C.J. Diagnosis and Management of Central Diabetes Insipidus in Adults. J. Clin. Endocrinol. Metab. 2022, 107, 2701–2715. [Google Scholar] [CrossRef] [PubMed]
- Mutter, C.M.; Smith, T.; Menze, O.; Zakharia, M.; Nguyen, H. Diabetes Insipidus: Pathogenesis, Diagnosis, and Clinical Management. Cureus 2021, 13, e13523. [Google Scholar] [CrossRef]
- Ribas, M.Z.; Paticcié, G.F.; de Medeiros, S.D.P.; Veras, A.D.O.; Noleto, F.M.; dos Santos, J.C.C. Reversible cerebral vasoconstriction syndrome: Literature review. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 5. [Google Scholar] [CrossRef]
- Chen, S.-P.; Wang, S.-J. Pathophysiology of reversible cerebral vasoconstriction syndrome. J. Biomed. Sci. 2022, 29, 72. [Google Scholar] [CrossRef]
- Perillo, T.; Paolella, C.; Perrotta, G.; Serino, A.; Caranci, F.; Manto, A. Reversible cerebral vasoconstriction syndrome: Review of neuroimaging findings. La Radiol. Med. 2022, 127, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, G.; Pergialiotis, V.; Domellöf, M.; Ehrhardt, H.; Di Renzo, G.C.; Koç, E.; Malamitsi-Puchner, A.; Kacerovsky, M.; Modi, N.; Shennan, A.; et al. European guidelines on perinatal care: Corticosteroids for women at risk of preterm birth. J. Matern. Neonatal Med. 2023, 36, 2160628. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, N.; Panait, C.G.; Cocolos, A.; Sandru, F.; Valea, A.; Carsote, M.; Ghemigian, A. Autoimmune thyroid disease and pregnancy, what could be a common factor? Rom. J. Clin. Res. 2023, 6, 78–83. [Google Scholar]
- Drui, D.; Briet, C.; Guerin, C.; Lugat, A.; Borson-Chazot, F.; Grunenwald, S. SFE-AFCE-SFMN 2022 Consensus on the management of thyroid nodules: Thyroid nodules and pregnancy. Ann. Endocrinol. 2022, 83, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Honegger, J.; Giese, S.; Nasi-Kordhishti, I.; Donegan, D.M. Pregnancy-related hypophysitis revisited. Eur. J. Endocrinol. 2023, 188, lvad003. [Google Scholar] [CrossRef] [PubMed]
- Laway, B.A. Sheehan syndrome: Cardiovascular and metabolic comorbidities. Front. Endocrinol. 2023, 14, 1086731. [Google Scholar] [CrossRef]
- Zain, A.; Sivakumar, A.; Akah, O.; Shiza, S.T.; Mahadevaiah, A.; Khan, A. A Rare Case of Sheehan Syndrome with Cardiac Tamponade. Cureus 2022, 14, e24329. [Google Scholar] [CrossRef] [PubMed]
- Cherian, K.E.; Kapoor, N.; Paul, T.V.; Asha, H.S. Functioning Endocrine Tumors in Pregnancy: Diagnostic and Therapeutic Challenges. Indian J. Endocrinol. Metab. 2021, 25, 299–304. [Google Scholar] [PubMed]
- Nistor, C.-E.; Pantile, D.; Stanciu-Gavan, C.; Ciuche, A.; Moldovan, H. Diagnostic and Therapeutic Characteristics in Patients with Pneumotorax Associated with COVID-19 versus Non-COVID-19 Pneumotorax. Medicina 2022, 58, 1242. [Google Scholar] [CrossRef]
- Mirza, S.A.; Sheikh, A.A.E.; Barbera, M.; Ijaz, Z.; Javaid, M.A.; Shekhar, R.; Pal, S.; Sheikh, A.B. COVID-19 and the Endocrine System: A Review of the Current Information and Misinformation. Infect. Dis. Rep. 2022, 14, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.E.; Gavan, C.S.; Pantile, D.; Tanase, N.V.; Ciuche, A. Cervico-Thoracic Air Collections in COVID-19 Pneumonia Patients—Our Experience and Brief Review. Chirurgia 2022, 117, 317. [Google Scholar] [CrossRef]
- Garg, M.K.; Gopalakrishnan, M.; Yadav, P.; Misra, S. Endocrine Involvement in COVID-19: Mechanisms, Clinical Features, and Implications for Care. Indian J. Endocrinol. Metab. 2020, 24, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Liu, L.; Huang, X.; Li, D.; Zhu, Y.; Lu, X.; Du, M. The risk of intrauterine exposure to SARS-CoV-2 in female COVID-19 patients: A comprehensive review. Am. J. Reprod. Immunol. 2022, e13528. [Google Scholar] [CrossRef]
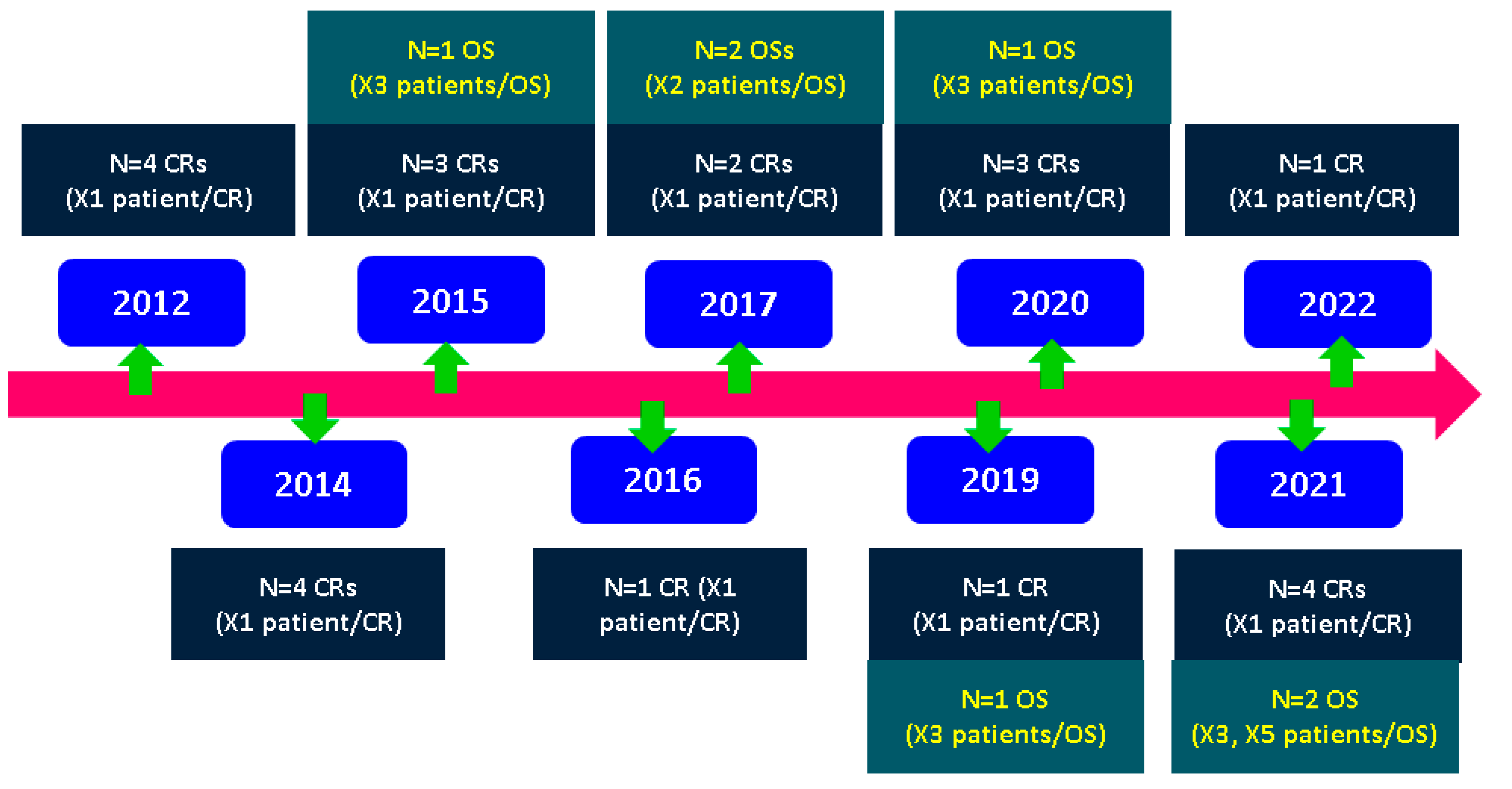
| Reference (Name, Number, and Year of Publication) and Type of Study | Population | WG on Presentation | Clinical Presentation | Preexisting Pituitary Lesion |
|---|---|---|---|---|
| Couture [64] 2012 Case report | 37 y F | 16 WG | Headache Nausea Vomiting Blurred vision | Lactotroph PitNET < 1 cm—diagnosed before pregnancy and treated with DA (bromocriptine switched to cabergoline) until pregnancy was confirmed |
| Jansssen [65] 2012 Case report | 27 y F | 10 WG (G1) | Headache Visual disturbance | Lactotroph PitNET > 1 cm—diagnosed before pregnancy and treated with DA (bromocriptine) |
| Kita [66] 2012 Case report | 26 y F | 26 WG | Headache Bitemporal hemianopsia | NFPA > 1 cm |
| Witek [67] 2012 Case report | 26 y F | 14 WG | Headache Visual field abnormalities | Lactotroph PitNET > 1 cm—diagnosed before pregnancy and treated with DA (bromocriptine) |
| Chegour [68] 2014 Case report | 29 y F | 19 WG | Headache Visual disturbances (unilateral vision loss) | Lactotroph PitNET > 1 cm—undiagnosed before pregnancy The patient received treatment with DA (bromocriptine) before pregnancy for hyperprolactinemia of uninvestigated etiology |
| Hayes [69] 2014 Case report | 41 y F | 18 WG | Headache Visual disturbances (visual field defects) | Pituitary adenoma—diagnosed before pregnancy (Lactotroph PitNET) |
| Piantanida [70] 2014 Case report | 27 y F | 35 WG (G1) | Headache Photophobia Bitemporal hemianopsia | Pituitary adenoma—undiagnosed before pregnancy |
| Tandon [71] 2014 Case report | 27 y F | 36 WG | Headache Unilateral vision loss | Lactotroph PitNET—diagnosed during pregnancy at 19 WG and treated with bromocriptine |
| Bedford [72] 2015 Case report | 35 y F | NA | Headache | Pituitary macroadenoma |
| De Ycaza [73] 2015 Case report | 26 y F | 28 WG (G1) | Headache | Macroprolactinoma—diagnosed before pregnancy |
| Grand’Maison [20] 2015 Case series | 4 F with PA # | Patient 1: 39 WG (G6P3A2) Patient 2: 20 WG (G1) Patient 4: G4P1A3 | Patient 1: Headache, nausea, blurred vision, and neck stiffness Patient 2: Headache | Patient 1: Pituitary hyperplasia without preexisting lesion Patient 2: Lactotroph PitNET with regression after DA (cabergoline) treatment |
| Watson [74] 2015 Case report | 30 y F | 37 WG (G5P4) | Headache Visual disturbances Numbness and weakness of the left side of the body/transient left-sided facial numbness | Pituitary adenoma—undiagnosed before pregnancy |
| Abraham [75] 2016 Case report | 32 y F | 23 WG (G6P4) | Headache Photophobia Right-sided numbness Diplopia Superotemporal hemianopsia | No pituitary adenoma |
| Annamalai [76] 2017 Case report | 25 y F | 37 WG | Headache | Lactotroph PitNET—treated with DA for three months |
| Galvão [77] 2017 Retrospective, observational study | 35 F ## | Patient 1: 28 WG (G1) Patient 2: 25 WG | Patient 1: Headache, blurred vision, and loss of consciousness Patient 2: Headache, blurred vision, and visual field defects | Patient 1: Lactotroph PitNET >1 cm Patient 2: Lactotroph PitNET |
| Lambert [78] 2017 Prospective, observational study | 71 F ### | NA | Headache | Patient 1: Macroprolactinoma—diagnosed before pregnancy Patient 2: Non-functioning adenoma—diagnosed before pregnancy |
| O’Neal [79] 2017 Case report | 27 y F | 29 WG | Headache Visual field defects (2 days after start of conservative management) | Pituitary adenoma—undiagnosed before pregnancy |
| Bachmeier [80] 2019 Case report | 30 y F | 36 WG (G1) | Headaches Unilateral visual loss | Lactotroph PitNET—clinically asymptomatic and previously undiagnosed |
| Jemel [81] 2019 Case series | 3 F with PA #### | Patient 1: 37 WG (G2P2A0) Patient 2: 22 WG (G1) Patient 3: 24 WG | Patient 1: Headache and blurred vision Patient 2: Headache, nausea, and vomiting Patient 3: Headache and visual disturbances | Patient 1: Pituitary adenoma—undiagnosed before pregnancy Patient 2: Non-secretory pituitary adenoma—the patient underwent treatment with DA for two years Patient 3: Pituitary macroadenoma—undiagnosed before pregnancy |
| Barraud [82] 2020 Retrospective, observational study | 46 F ##### (3 F with PA) | Patient 1: NA Patient 2: 4th month of gestation Patient 3: 36 WG | Symptoms of PA, including visual field defects | Lactotroph PitNET ≥ 1 cm |
| Bichard [83] 2020 Case report | 29 y F | 30 WG | Headache Nausea Vomiting Anisocoria DI (Polydipsia 10 L/day and polyuria) | Pituitary adenoma—undiagnosed before pregnancy |
| Chan [84] 2020 Case report | 28 y F | 38 WG (G5P1) | Headache Decrease in visual acuity Anisocoria | Pituitary adenoma—undiagnosed before pregnancy |
| Oguz [85] 2020 Case report | 26 y F | 22 WG(G0) | Headache Nausea Visual disturbances (including visual field deficit) | Lactotroph pituitary macroadenoma |
| Geissler [86] 2021 Case report | 27 y F | 1st pregnancy: 34 WG (G3P0) 2nd pregnancy: 32 WG | Similar presentation for both pregnancies: Headache Visual disturbance (repetitive flashes of light) | Pituitary adenoma—undiagnosed before pregnancy |
| Kanneganti [87] 2021 Case report | 26 y F | 37 WG (G1) | Headache Visual disturbance Non-vertiginous giddiness Breast discharge | PA with optic chiasma compression |
| Kato [88] 2021 Case series | 3 F with PA ###### | Patient 1: 35 WG (P1) Patient 2: 32 WG Patient 3: 28 WG | Patient 1: Visual field defects (temporal hemianopia) Patient 2: Headache and visual field defects (temporal hemianopia) Patient 3: Headache | Patient 1: Lactotroph PitNET with compression on the optical chiasm (known before pregnancy) Patient 2: Lactotroph and gonadotroph PitNET with compression on the optical chiasm (undiagnosed before pregnancy) Patient 3: Lactotroph adenoma with compression on the optical chiasm (undiagnosed before pregnancy) |
| Khaldi [89] 2021 Case report | 30 y F | 22 WG | Headache Nausea Visual disturbance | Giant lactotroph PitNET—diagnosed before pregnancy and treated with DA and TSS with a 50% residual tumor |
| Kuhn [90] 2021 Case series | 5 females with PA ####### | Patient 1: 36 WG Patient 2: 26 WG Patient 3: 35 WG (G3) Patient 4: 16 WG Patient 5: 24 WG | Patient 1: Headache, visual impairment, bitemporal hemianopia, and photophobia Patient 2: Transient DI Patient 3: Headache and unilateral vision loss Patient 4: Headaches and visual field defects Patient 5: Headache | Patient 1: Lactotroph PitNET Patient 2: Lactotroph PitNET Patient 3: Lactotroph PitNET Patient 4: Lactotroph PitNET Patient 5: Lactotroph PitNET All patients were diagnosed with pituitary adenomas before pregnancy |
| Ye [91] 2021 Case report | 24 y F | 32 WG | Headache throughout pregnancy without remission under analgetic treatment Vomiting Dysarthria and hemiplegia after sodium supplementation Low-grade fever | Pituitary adenoma—undiagnosed before pregnancy |
| Sedai [92] 2022 Case report | 40 y F | 21 WG (G2P0A1L0) | Headache Projectile vomiting Ptosis Decreased visual acuity Altered consciousness | Pituitary adenoma—undiagnosed before pregnancy |
| Reference (Name and Number) | Additional Risk Factors | Treatment | Delivery | Maternal Outcome |
|---|---|---|---|---|
| Couture [64] | None | Conservative management with DA (cabergoline) | LB at 38 WG by CS | Complete recovery Resolution of pituitary adenoma |
| Jansssen [65] | None | Conservative treatment with DA (bromocriptine), LT4 + Hydrocortisone | LB at 40 WG by VD | Adrenal insufficiency Major decrease in size of pituitary tumor |
| Kita [66] | None | TSS (7 days after admission) | LB at 40 WG by CS | DI managed with 1-desamino-8-D-arginine vasopressin |
| Witek [67] | None | Conservative management with DA (bromocriptine) followed by TSS (at 20 WG due to visual field defects) | LB at 38 WG by CS | Complete recovery No tumor regrowth Normal pituitary function |
| Chegour [68] | DA (bromocriptine) | Conservative treatment with DA (cabergoline) | NA | Complete recovery: remission of symptoms and disappearance of the expansive process |
| Hayes [69] | DA (cabergoline) before pregnancy (discontinued when pregnancy was confirmed) | TSS | LB at term by VD | Resolution of symptoms Normal pituitary function Able to breastfeed for only 2 weeks No adenoma recurrence |
| Piantanida [70] | None | TSS (after delivery) 9 months after delivery cabergoline therapy was started | LB at 35 WG by urgent CS | Central hypothyroidism Total adenoma resection Hyperprolactinemia treated with cabergoline |
| Tandon [71] | DA (bromocriptine) before pregnancy | TSS | LB at 37 WG by CS | Transient DI postoperatively Improvement of visual symptoms |
| Bedford [72] | NA | NA | NA | NA |
| De Ycaza [73] | DA (bromocripine and cabergoline) before pregnancy (discontinued when pregnancy was confirmed) | Conservative treatment with glucocorticoid replacement and DA (cabergoline) until delivery | LB at term by VD | Tumor decreased in size after DA (cabergoline) treatment The patient had an uneventful second pregnancy |
| Grand’Maison [20] | Patient 1: None Patient 2: DA (cabergoline) before pregnancy (discontinued when pregnancy was confirmed) | Patient 1: Conservative management Patient 2: Conservative management and DA (cabergoline) | Patient 1: LB at 40 WG by VD Patient 2: LB at term by VD | # Patient 1: Normal pituitary function Patient 2: Normal pituitary function, diminished pituitary mass (9 × 9 mm), and uneventful second pregnancy |
| Watson [74] | Low-molecular-weight heparin | Conservative treatment with hydrocortisone 50 mg 6 times hourly Heparin postoperatively | LB at term by CS | Persistent hypocortisolism |
| Abraham [75] | None | TSS | NA | Transient postoperative DI Normal pituitary function |
| Annamalai [76] | DA (cabergoline) before pregnancy (discontinued when pregnancy was confirmed) | Conservative treatment with hydrocortisone and DA (cabergoline) | LB at 37 WG by CS | Resolution of PA Resolution of pituitary microadenoma Normal pituitary function |
| Galvão [77] | Patient 1: None Patient 2: None | Patient 1: Conservative management Patient 2: TSS during second trimester | Patient 1: LB Patient 2: LB | Patient 1: Pregnancy proceeded normally Patient 2: The patient developed DI and central hypothyroidism |
| Lambert [78] | NA | Both patients received conservative treatment | Patient 1: CS Patient 2: NA | Good outcome |
| O’Neal [79] | None | Conservative management with hydrocortisone and DA (bromocriptine) initially, followed by surgery | LB at term | DI postoperatively |
| Bachmeier [80] | None | TSS tumor resection PP | LB at 37 WG by CS | Resolution of symptoms Normal pituitary function postoperatively The patient was able to breastfeed |
| Jemel [81] | Patient 1: None Patient 2: DA (cabergoline) before pregnancy (discontinued when pregnancy was confirmed) Patient 3: None | Patient 1: Conservative management with hydrocortisone 100 mg 6 times hourly and DA in PP Patient 2: Conservative management initially, with hydrocortisone and DA, followed by TSS (3 days after admission) Patient 3: Hydrocortisone and TSS | Patient 1: LB at 37 WG Patient 2: LB at 37 WG Patient 3: LB at 38 WG | Patient 1: Regression of the pituitary mass Patient 2: NA Patient 3: Remission of symptoms |
| Barraud [82] | NA | Two patients underwent emergency pituitary surgery due to worsening of visual field defects The third patient underwent surgery after delivery | Patient 3: LB at 36 WG by CS | NA |
| Bichard [83] | NA | Conservative treatment with hydrocortisone, thyroxine, and desmopressin | LB at term by VD with forceps following induced labor | Clinically well and able to breastfeed Desmopressin and hydrocortisone requirements were reduced |
| Chan [84] | Acute COVID-19 infection | Initial conservative management with corticosteroids (dexamethasone) TSS: 2 days after delivery | LB at 39 WG by VD under epidural anesthesia | Central hypothyroidism and hypogonadism Possible persistence of secondary adrenal insufficiency (the patient did not undergo cortisol stimulation test) |
| Oguz [85] | DA (cabergoline) before pregnancy (discontinued when pregnancy was confirmed) | TSS (at 22 WG) | LB at 37 WG by CS | ## Full recovery Hypothyroidism No residual tumor |
| Geissler [86] | gestational DM during both pregnancies | In both pregnancies: conservative steroid treatment | First presentation: LB at 36th week by CS Second presentation: LB at 34th week by CS | Complete recovery Decreased pituitary size on MRI after the first pregnancy Normal pituitary function Lack of milk production |
| Kanneganti [87] | None | Conservative treatment with hydrocortisone | LB at term by CS | NA |
| Kato [88] | Patient 1: DA treatment (terguride) before pregnancy (discontinued when pregnancy was confirmed) | Initial conservative treatment with hydrocortisone, followed by elective TSS after delivery in all three cases | Patient 1: LB at 36th week by CS Patient 2: LB at 34th week by CS Patient 3: LB at 37th week by CS | Complete recovery in all three cases |
| Khaldi [89] | gestational DM DA (cabergoline) before pregnancy | Conservative management with DA (bromocriptine) and hydrocortisone | LB at 28 WG by premature VD due to premature rupture of membranes Twins died on the 7th day of life | Adrenal insufficiency and central hypothyroidism Decrease in tumor size |
| Kuhn [90] | ### | #### | Patient 1: LB at term by CS Patient 2: LB by VD Patient 3: LB by VD Patient 4: LB at 38th week by CS Patient 5: LB at term by VD | Patient 1: Resolution of symptoms Patient 2: DI and hyperprolactinemia Patient 3: Resolution of headache, improvement of vision, and corticotropic deficiency Patient 4: Resolution of symptoms and able to breastfeed Patient 5: unable to breastfeed |
| Ye [91] | None | Conservative treatment with hydrocortisone and levothyroxine | LB at 38 + 1 WG by CS | ##### Lack of lactation after delivery Regression of pituitary tumor Remission of symptoms |
| Sedai [92] | None | Initially conservative treatment for eclampsia (initial diagnosis) Craniotomy—tumor resection and hematoma evacuation | Maternal exitus | Exitus on the 2nd postoperative day (Initially misdiagnosed as eclampsia) |
| Parameter | Outcome |
|---|---|
| reviewed period | 2012–2022 |
| number of original studies | 35 |
| number of observational studies | 7 |
| case series | 4 |
| case reports | 28 |
| total number of patients with PA | 49 |
| PA in pregnancy/postpartum ratio | 43/6 |
| PA in pregnancy: age ranges | 21–41 years |
| mean age at PA diagnostic in pregnancy | 27.76 years |
| presentation during third trimester | 21/43 |
| average week of gestation | 26.38 |
| cesarean section | 19/30 |
| pre-pregnancy medication | dopamine agonists 15/43 terguride (1/43) |
| conservative approach | 29/43 |
| trans-sphenoidal surgery | 22/43 (10/22 neurosurgery as initial approach) |
| number of patients with pituitary tumor not diagnosed before surgery | 18/43 |
| type of pituitary tumors | prolactinomas (26/43) |
| PA in postpartum: mean age at diagnosis | 33 years |
| rate of PA in postpartum after second pregnancy | 50% |
| timing (after delivery) of PA | 5 min–12 days |
| rate of persistent hypopituitarism after PA in postpartum | 50% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe, A.-M.; Trandafir, A.-I.; Stanciu, M.; Popa, F.L.; Nistor, C.; Carsote, M. Challenges of Pituitary Apoplexy in Pregnancy. J. Clin. Med. 2023, 12, 3416. https://doi.org/10.3390/jcm12103416
Gheorghe A-M, Trandafir A-I, Stanciu M, Popa FL, Nistor C, Carsote M. Challenges of Pituitary Apoplexy in Pregnancy. Journal of Clinical Medicine. 2023; 12(10):3416. https://doi.org/10.3390/jcm12103416
Chicago/Turabian StyleGheorghe, Ana-Maria, Alexandra-Ioana Trandafir, Mihaela Stanciu, Florina Ligia Popa, Claudiu Nistor, and Mara Carsote. 2023. "Challenges of Pituitary Apoplexy in Pregnancy" Journal of Clinical Medicine 12, no. 10: 3416. https://doi.org/10.3390/jcm12103416
APA StyleGheorghe, A.-M., Trandafir, A.-I., Stanciu, M., Popa, F. L., Nistor, C., & Carsote, M. (2023). Challenges of Pituitary Apoplexy in Pregnancy. Journal of Clinical Medicine, 12(10), 3416. https://doi.org/10.3390/jcm12103416










