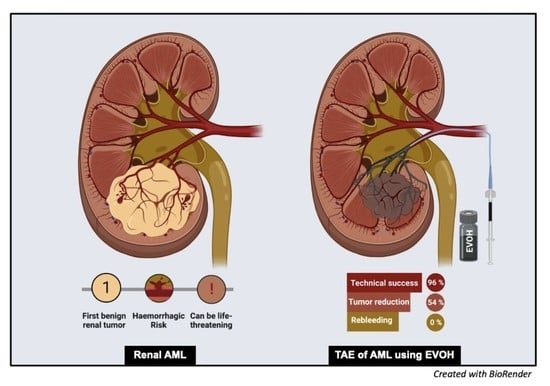
Safety, Efficacy and Mid-Term Outcome for Transarterial Embolization (TAE) of Renal Angiomyolipoma (AML) Using Ethylene Vinyl Alcohol Copolymer Liquid Embolic Agent (EVOH)
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients Demographics and Study Design
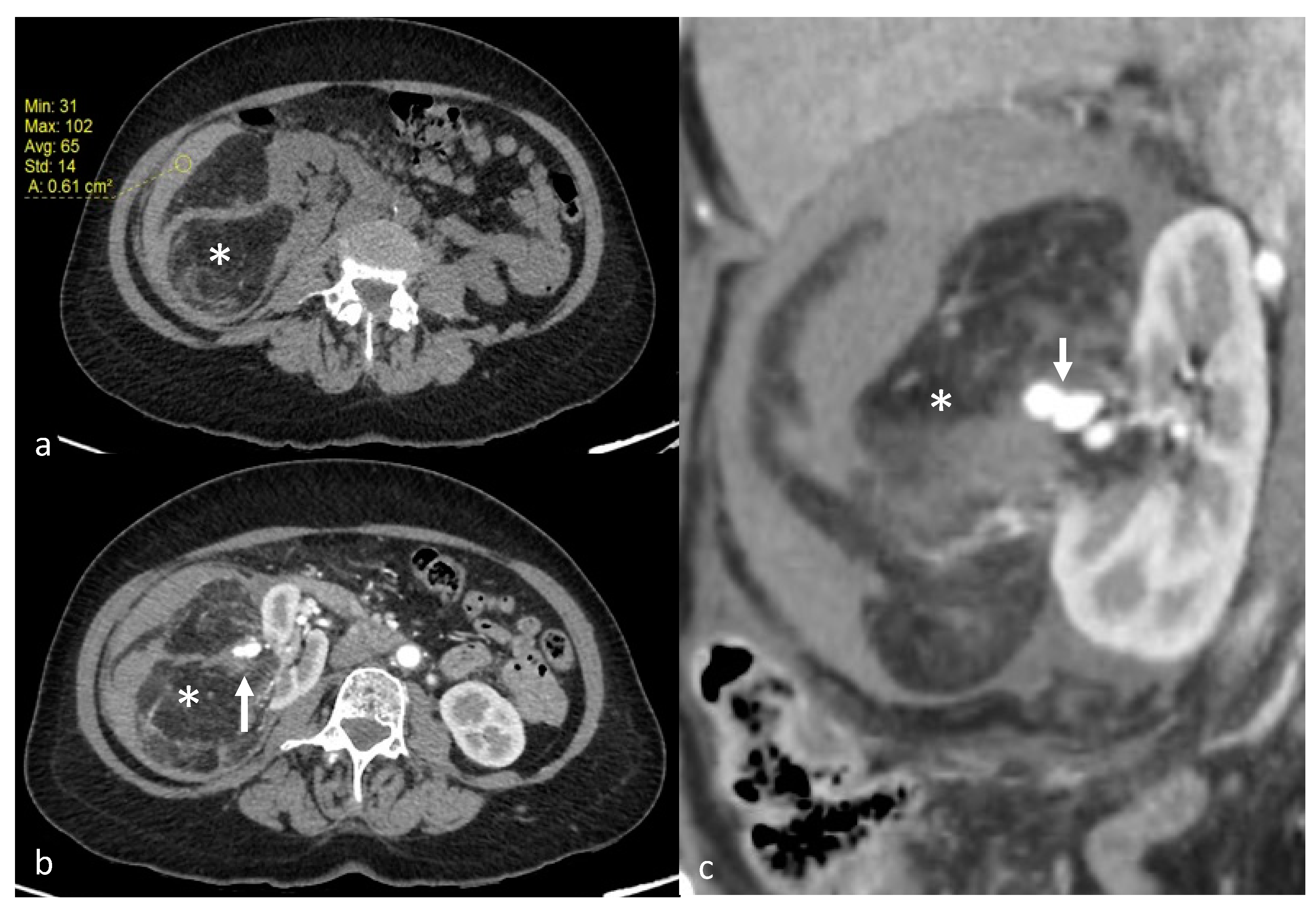
2.2. Diagnosis and Tumor Measurements
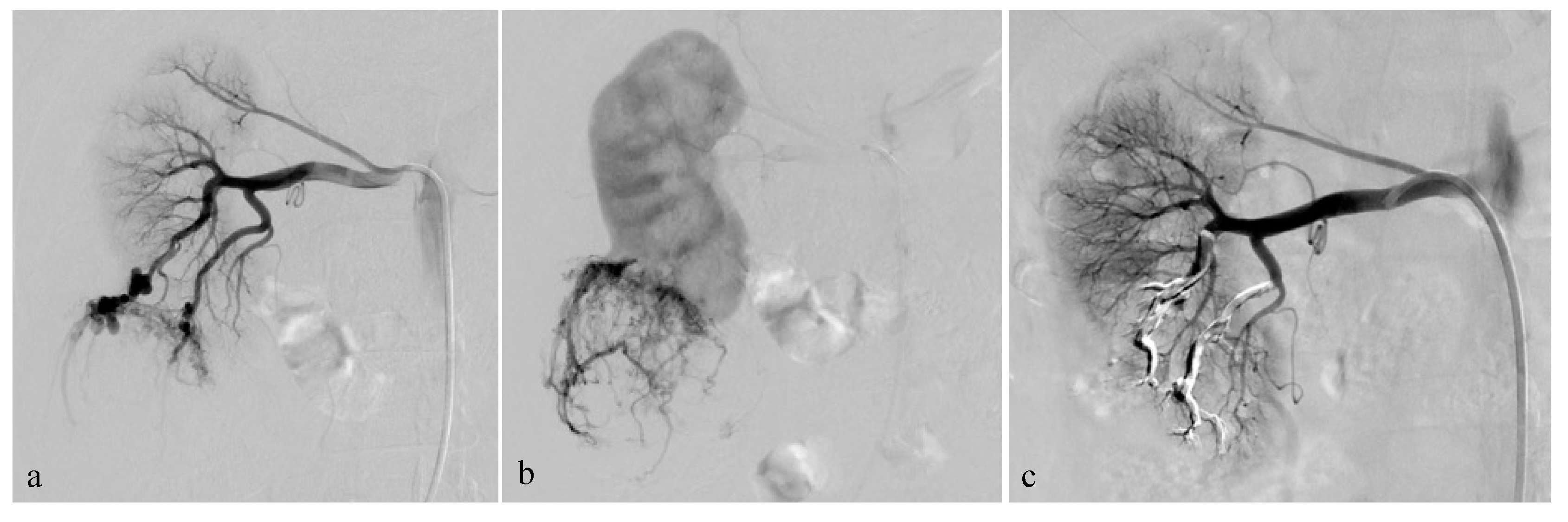
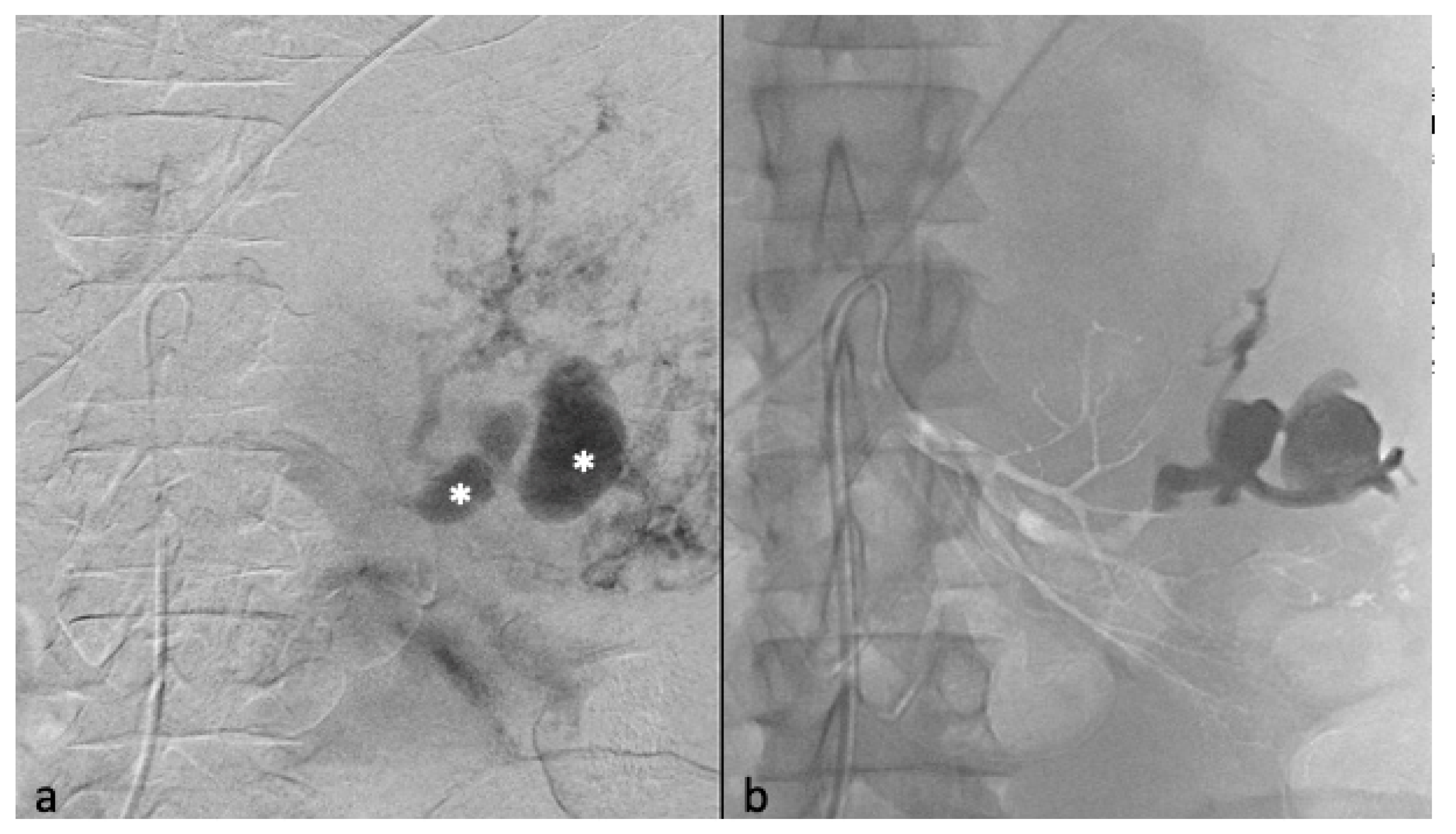
2.3. Embolization Technique
2.4. Postprocedure Management
2.5. Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Angiomyolipoma Characteristics
3.3. Selective Arterial Embolization and Volume Shrinkage
3.4. Other Outcomes
3.5. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fittschen, A.; Wendlik, I.; Oeztuerk, S.; Kratzer, W.; Akinli, A.S.; Haenle, M.M.; Graeter, T. Prevalence of sporadic renal angiomyolipoma: A retrospective analysis of 61,389 in- and out-patients. Abdom. Imaging 2014, 39, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Yamakado, K.; Tanaka, N.; Nakagawa, T.; Kobayashi, S.; Yanagawa, M.; Takeda, K. Renal Angiomyolipoma: Relationships between Tumor Size, Aneurysm Formation, and Rupture. Radiology 2002, 225, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Ramon, J.; Rimon, U.; Garniek, A.; Golan, G.; Bensaid, P.; Kitrey, N.D.; Nadu, A.; Dotan, Z.A. Renal angiomyolipoma: Long-term results following selective arterial embolization. Eur. Urol. 2009, 55, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pello, S.; Hora, M.; Kuusk, T.; Tahbaz, R.; Dabestani, S.; Abu-Ghanem, Y.; Albiges, L.; Giles, R.H.; Hofmann, F.; Kuczyk, M.A.; et al. Management of Sporadic Renal Angiomyolipomas: A Systematic Review of Available Evidence to Guide Recommendations from the European Association of Urology Renal Cell Carcinoma Guidelines Panel. Eur. Urol. Oncol. 2020, 3, 57–72. [Google Scholar] [CrossRef]
- Oesterling, J.E.; Fishman, E.K.; Goldman, S.M.; Marshall, F.F. The management of renal angiomyolipoma. J. Urol. 1986, 135, 1121–1124. [Google Scholar] [CrossRef]
- Prigent, F.-V.; Guillen, K.; Comby, P.-O.; Pellegrinelli, J.; Falvo, N.; Midulla, M.; Majbri, N.; Chevallier, O.; Loffroy, R. Selective Arterial Embolization of Renal Angiomyolipomas with a N-Butyl Cyanoacrylate-Lipiodol Mixture: Efficacy, Safety, Short- and Mid-Term Outcomes. J. Clin. Med. 2021, 10, 4062. [Google Scholar] [CrossRef]
- Minervini, A.; Giubilei, G.; Masieri, L.; Lanzi, F.; Serni, S.; Carini, M. Simple enucleation for the treatment of renal angiomyolipoma. BJU Int. 2007, 99, 887–891. [Google Scholar] [CrossRef]
- Boorjian, S.A.; Frank, I.; Inman, B.; Lohse, C.M.; Cheville, J.C.; Leibovich, B.C.; Blute, M.L. The role of partial nephrectomy for the management of sporadic renal angiomyolipoma. Urology 2007, 70, 1064–1068. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.-Z.; Ma, X.; Zheng, T.; Li, L.-C.; Ye, Z.-Q. Retroperitoneal laparoscopic nephron-sparing surgery for renal tumors: Report of 32 cases. Urology 2005, 65, 1080–1084; discussion 1084–1085. [Google Scholar] [CrossRef]
- Stein, R.J.; White, W.M.; Goel, R.K.; Irwin, B.H.; Haber, G.P.; Kaouk, J.H. Robotic laparoendoscopic single-site surgery using GelPort as the access platform. Eur. Urol. 2010, 57, 132–136. [Google Scholar] [CrossRef]
- Prevoo, W.; van den Bosch, M.A.A.J.; Horenblas, S. Radiofrequency ablation for treatment of sporadic angiomyolipoma. Urology 2008, 72, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Byrd, G.F.; Lawatsch, E.J.; Mesrobian, H.-G.; Begun, F.; Langenstroer, P. Laparoscopic cryoablation of renal angiomyolipoma. J. Urol. 2006, 176 Pt 1, 1512–1516; discussion 1516. [Google Scholar] [CrossRef] [PubMed]
- Bissler, J.J.; Kingswood, J.C. Renal angiomyolipomata. Kidney Int. 2004, 66, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Yu, S.; Yip, S.; Lee, P. The efficacy, safety and durability of selective renal arterial embolization in treating symptomatic and asymptomatic renal angiomyolipoma. Urology 2011, 77, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, J.D.; Fritzsche, P.; Hadley, H.L. Management of Hemorrhage Secondary to Renal Angiomyolipoma with Selective Arterial Embolization. J. Urol. 1977, 117, 122–123. [Google Scholar] [CrossRef]
- Villalta, J.D.; Sorensen, M.D.; Durack, J.C.; Kerlan, R.K.; Stoller, M.L. Selective arterial embolization of angiomyolipomas: A comparison of smaller and larger embolic agents. J. Urol. 2011, 186, 921–927. [Google Scholar] [CrossRef]
- Piacentino, F.; Fontana, F.; Curti, M.; Macchi, E.; Coppola, A.; Ossola, C.; Giorgianni, A.; Marra, P.; Mosconi, C.; Ierardi, A.M.; et al. Non-Adhesive Liquid Embolic Agents in Extra-Cranial District: State of the Art and Review of the Literature. J. Clin. Med. 2021, 10, 4841. [Google Scholar] [CrossRef]
- Bommart, S.; Bourdin, A.; Giroux, M.F.; Klein, F.; Micheau, A.; Bares, V.M.; Kovacsik, H. Transarterial ethylene vinyl alcohol copolymer visualization and penetration after embolization of life-threatening hemoptysis: Technical and clinical outcomes. Cardiovasc. Intervent. Radiol. 2012, 35, 668–675. [Google Scholar] [CrossRef]
- Taki, W.; Yonekawa, Y.; Iwata, H.; Uno, A.; Yamashita, K.; Amemiya, H. A new liquid material for embolization of arteriovenous malformations. AJNR Am. J. Neuroradiol. 1990, 11, 163–168. [Google Scholar]
- Urbano, J.; Manuel Cabrera, J.; Franco, A.; Alonso-Burgos, A. Selective arterial embolization with ethylene-vinyl alcohol copolymer for control of massive lower gastrointestinal bleeding: Feasibility and initial experience. J. Vasc. Interv. Radiol. JVIR 2014, 25, 839–846. [Google Scholar] [CrossRef]
- Lim, R.S.; Flood, T.A.; McInnes, M.D.F.; Lavallee, L.T.; Schieda, N. Renal angiomyolipoma without visible fat: Can we make the diagnosis using CT and MRI? Eur. Radiol. 2018, 28, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Mayr, N.A.; Taoka, T.; Yuh, W.T.C.; Denning, L.M.; Zhen, W.K.; Paulino, A.C.; Gaston, R.C.; Sorosky, J.I.; Meeks, S.L.; Walker, J.L.; et al. Method and timing of tumor volume measurement for outcome prediction in cervical cancer using magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Angle, J.F.; Siddiqi, N.H.; Wallace, M.J.; Kundu, S.; Stokes, L.; Wojak, J.C.; Cardella, J.F. Quality Improvement Guidelines for Percutaneous Transcatheter Embolization. J. Vasc. Interv. Radiol. 2010, 21, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.; Sabharwal, T.; Ahmad, F.; Dourado, R.; Adam, A. Onyx Embolization of Sporadic Angiomyolipoma. Cardiovasc. Intervent. Radiol. 2009, 32, 1291–1295. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Hsu, H.-H.; Chen, Y.-C.; Huang, C.-C.; Wong, Y.-C.; Wang, L.-J.; Chuang, C.-K.; Yang, C.-W. Embolization of renal angiomyolipomas: Short-term and long-term outcomes, complications, and tumor shrinkage. Cardiovasc. Intervent. Radiol. 2009, 32, 1171–1178. [Google Scholar] [CrossRef]
- Navuluri, R.; Kang, L.; Patel, J.; Van Ha, T. Acute lower gastrointestinal bleeding. Semin. Interv. Radiol. 2012, 29, 178–186. [Google Scholar] [CrossRef]
- Thulasidasan, N.; Sriskandakumar, S.; Ilyas, S.; Sabharwal, T. Renal Angiomyolipoma: Mid- to Long-Term Results Following Embolization with Onyx. Cardiovasc. Intervent. Radiol. 2016, 39, 1759–1764. [Google Scholar] [CrossRef]
- Ouzaid, I.; Autorino, R.; Fatica, R.; Herts, B.R.; McLennan, G.; Remer, E.M.; Haber, G.-P. Active surveillance for renal angiomyolipoma: Outcomes and factors predictive of delayed intervention. BJU Int. 2014, 114, 412–417. [Google Scholar] [CrossRef]
- Nelson, C.P.; Sanda, M.G. Contemporary diagnosis and management of renal angiomyolipoma. J. Urol. 2002, 168 Pt 1, 1315–1325. [Google Scholar] [CrossRef]
- Rimon, U.; Duvdevani, M.; Garniek, A.; Golan, G.; Bensaid, P.; Ramon, J.; Morag, B. Large renal angiomyolipomas: Digital subtraction angiographic grading and presentation with bleeding. Clin. Radiol. 2006, 61, 520–526. [Google Scholar] [CrossRef]
- Seyam, R.M.; Bissada, N.K.; Kattan, S.A.; Mokhtar, A.A.; Aslam, M.; Fahmy, W.E.; Mourad, W.A.; Binmahfouz, A.A.; Alzahrani, H.M.; Hanash, K.A. Changing Trends in Presentation, Diagnosis and Management of Renal Angiomyolipoma: Comparison of Sporadic and Tuberous Sclerosis Complex-associated Forms. Urology 2008, 72, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Hocquelet, A.; Cornelis, F.; Le Bras, Y.; Meyer, M.; Tricaud, E.; Lasserre, A.S.; Ferrière, J.M.; Robert, G.; Grenier, N. Long-term results of preventive embolization of renal angiomyolipomas: Evaluation of predictive factors of volume decrease. Eur. Radiol. 2014, 24, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, X.; Guan, H.; Wang, J.; Tong, X.; Yang, M.; Zou, Y. Renal function, complications, and outcomes of a reduction in tumor size after transarterial embolization for renal angiomyolipomas: A meta-analysis. J. Int. Med. Res. 2019, 47, 1417–1428. [Google Scholar] [CrossRef]
- Urbano, J.; Paul, L.; Cabrera, M.; Alonso-Burgos, A.; Gómez, D. Elective and Emergency Renal Angiomyolipoma Embolization with Ethylene Vinyl Alcohol Copolymer: Feasibility and Initial Experience. J. Vasc. Interv. Radiol. 2017, 28, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Kuusk, T.; Biancari, F.; Lane, B.; Tobert, C.; Campbell, S.; Rimon, U.; D’Andrea, V.; Mehik, A.; Vaarala, M.H. Treatment of renal angiomyolipoma: Pooled analysis of individual patient data. BMC Urol. 2015, 15, 123. [Google Scholar] [CrossRef]
- Kothary, N.; Soulen, M.C.; Clark, T.W.I.; Wein, A.J.; Shlansky-Goldberg, R.D.; Crino, P.B.; Stavropoulos, S.W. Renal angiomyolipoma: Long-term results after arterial embolization. J. Vasc. Interv. Radiol. JVIR 2005, 16, 45–50. [Google Scholar] [CrossRef]
- Northrup, H.; Aronow, M.E.; Bebin, E.M.; Bissler, J.; Darling, T.N.; de Vries, P.J.; Frost, M.D.; Fuchs, Z.; Gosnell, E.S.; Gupta, N.; et al. Updated International Tuberous Sclerosis Complex Diagnostic Criteria and Surveillance and Management Recommendations. Pediatr. Neurol. 2021, 123, 50–66. [Google Scholar] [CrossRef]
- Patatas, K.; Robinson, G.J.; Ettles, D.F.; Lakshminarayan, R. Patterns of renal angiomyolipoma regression post embolisation on medium- to long-term follow-up. Br. J. Radiol. 2013, 86, 20120633. [Google Scholar] [CrossRef]
- Venturini, M.; Della Corte, A.; Lanza, C.; Fontana, F.; Chiesa, R.; De Cobelli, F. Embolization of 2 Coexisting Intraparenchymal Renal Artery Aneurysms with an Ethylene Vinyl Alcohol Copolymer Agent (Squid) and Coils. Cardiovasc. Intervent. Radiol. 2020, 43, 942–944. [Google Scholar] [CrossRef]
- Venturini, M.; Lanza, C.; Marra, P.; Colarieti, A.; Panzeri, M.; Augello, L.; Gusmini, S.; Salvioni, M.; De Cobelli, F.; Del Maschio, A. Transcatheter embolization with Squid, combined with other embolic agents or alone, in different abdominal diseases: A single-center experience in 30 patients. CVIR Endovasc. 2019, 2, 8. [Google Scholar] [CrossRef]
- Lucatelli, P.; Corona, M.; Teodoli, L.; Nardis, P.; Cannavale, A.; Rocco, B.; Trobiani, C.; Cipollari, S.; Zilahi De Gyurgyokai, S.; Bezzi, M.; et al. Use of Phil Embolic Agent for Bleeding in Non-Neurological Interventions. J. Clin. Med. 2021, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Giurazza, F.; Cionfoli, N.; Paladini, A.; Vallone, M.; Corvino, F.; Teodoli, L.; Moramarco, L.; Quaretti, P.; Catalano, C.; Niola, R.; et al. PHIL® (precipitating hydrophobic injectable liquid): Retrospective multicenter experience on 178 patients in peripheral embolizations. Radiol. Med. 2022, 127, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Vollherbst, D.F.; Chapot, R.; Bendszus, M.; Möhlenbruch, M.A. Glue, Onyx, Squid or PHIL? Liquid Embolic Agents for the Embolization of Cerebral Arteriovenous Malformations and Dural Arteriovenous Fistulas. Clin. Neuroradiol. 2022, 32, 25–38. [Google Scholar] [CrossRef] [PubMed]

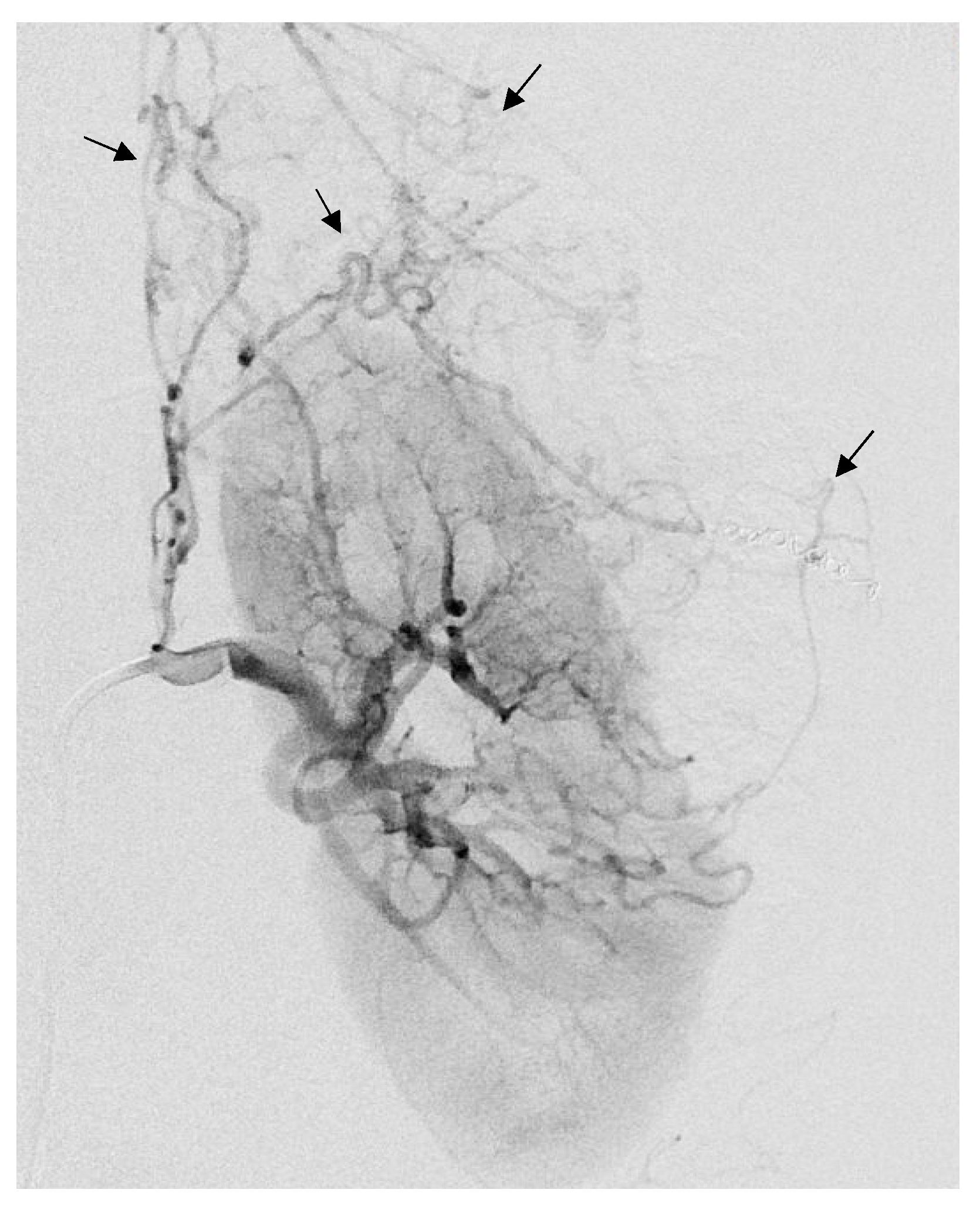
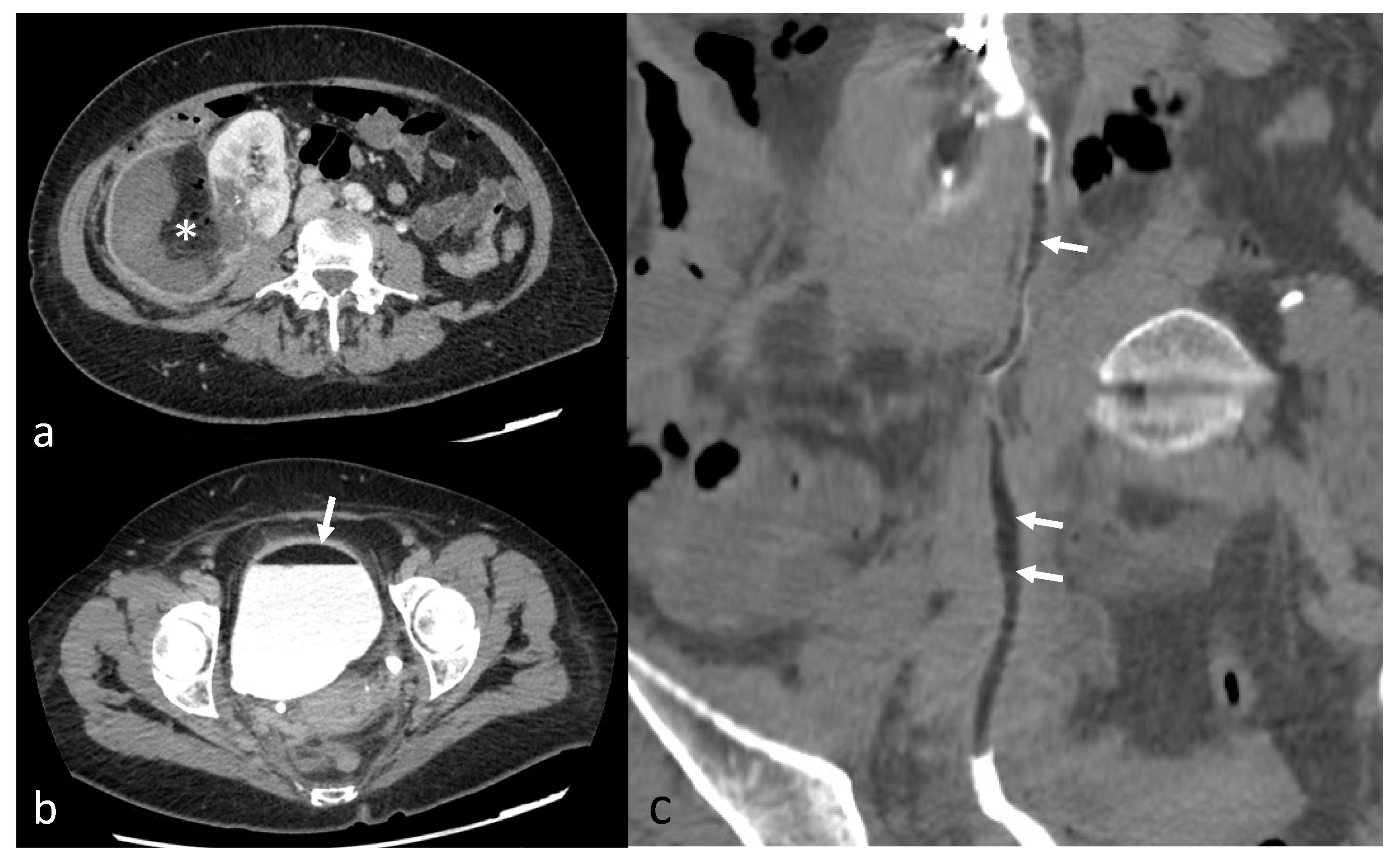
| Variable | Value |
|---|---|
| N. of patients | 24 |
| N. of embolized AML | 25 |
| N. of embolization | 29 |
| Females/Males, n (%) | 21 (87.5)/3 (12.5) |
| Age (years), mean ± SD | 53.9 (±16.2) |
| tuberous sclerosis complex n (%) | 1 (4) |
| Clinical Presentation, n (%) | |
| Symptomatic | 8 (32) |
| Emergency | 4 (16) |
| Incidental discovery | 13 (52) |
| Therapeutic indication | |
| Size a | 23 (92) |
| Bleeding | 4 (16) |
| Aneurysm b | 5 (20) |
| Pre-therapeutic imaging: CT/MRI, n (%) | 15 (60)/10 (40) |
| Post-therapeutic imaging: CT/MRI, n (%) | 6 (24)/19 (76) |
| Side of AML: left/right, n (%) | 14 (56)/11 (44) |
| Location: Exophytic/Intraparenchymatous, n (%) | 24 (96)/1(4) |
| Previous embolic agents, n (%) | 3 (12) |
| Variable | Value | |
|---|---|---|
| Mean (±SD) | Median (Range) | |
| N. of arterial pedicles per AML | 1.4 (±0.8) | 1 (1–4) |
| AML before TAE | ||
| Size, cm | 70.9 (±38.0) | 54 (41–203) |
| Volume, cm3 | 201.1 (±504.1) | 41.47 (13.2–2539.8) |
| Radiologic follow-up, days | 448.6 (±473.8) | 248 (2–1735) |
| AML after TAE | ||
| Size, cm | 53.1 (±34.1) | 48 (21–169) |
| Volume, cm3 | 93.4 (±253.1) | 24.92 (2.4–1223.1) |
| Tumor size reduction | ||
| cm | 17.78 (±21.1) | 12.4 (−2–98.5) |
| % | 24.38 (±20.3) | 18 (−1.4–77.9) |
| Tumor volume reduction | ||
| cm3 | 107.7 (±267.3) | 25.3 (−14.3–1315.8) |
| % | 53.6 (±28.3) | 51.8 (−3.2–99.1) |
| Volume reduction < 5% | 2 (8) | |
| Size increasing after TAE, n (%) | 1 (4) | |
| Onyx (EVOH) | ||
| Type used: 18/34, n (%) | 22 (88)/3 (12) | |
| Quantity per TAE, mL | 1.2 (±2.3) | 0.5 (0.1–11) |
| Total quantity used per patient | 1.4 (±2.4) | 0.6 (0.2–11) |
| Serum creatinine level | ||
| Before TAE, μmol/L | 70.5 (±24.2) | 63 (49–161) |
| After TAE, μmol/L | 68.3 (±17) | 64 (49–122) |
| Technical success rate, n (%) | 24 (96) | |
| After one embolization | 23 (92) | |
| After two embolizations | 1 (4) | |
| N. of TAE per AML | 1.2 (±0.4) | 1 (1–2) |
| Need for re-embolization, n (%) | 4 (16) | |
| Renal surgery after TAE, n (%) | 2 (8) | |
| Minor complications after TAE, n (%) | 3 (12) | |
| Non-targeted embolization | 1 (4) | |
| Femoral pseudoaneurysm | 1 (4) | |
| Post-embolization syndrome | 1 (4) | |
| Major complications after TAE, n (%) | 3 (8) | |
| Abcess with lipuria | 1 (4) | |
| Arterial Dissection | 1 (4) | |
| Healthcare associated pyelonephritis | 1 (4) | |
| Anesthesia: General/Hypnosedation, n (%) | 20 (80)/5 (20) | |
| Rebleeding after TAE, n (%) | 0 | |
| Recurrence, n (%) | 1 (4) | |
| Tumor regrowth | 1 (4) | |
| Dosimetry per TAE: Gy.cm2, n (%) | 55.1 (± 52.1) | 31.0 (6.9–215.2) |
| Contrast agent used per TAE: mL, n (%) | 53.5 (±25.4) | 46 (10–100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolland, R.; Loubet, A.; Bommart, S.; Monnin-Bares, V.; Zarqane, H.; Vanoverschelde, J.; Herman, F.; Molinari, N.; Kovacsik, H. Safety, Efficacy and Mid-Term Outcome for Transarterial Embolization (TAE) of Renal Angiomyolipoma (AML) Using Ethylene Vinyl Alcohol Copolymer Liquid Embolic Agent (EVOH). J. Clin. Med. 2023, 12, 3385. https://doi.org/10.3390/jcm12103385
Rolland R, Loubet A, Bommart S, Monnin-Bares V, Zarqane H, Vanoverschelde J, Herman F, Molinari N, Kovacsik H. Safety, Efficacy and Mid-Term Outcome for Transarterial Embolization (TAE) of Renal Angiomyolipoma (AML) Using Ethylene Vinyl Alcohol Copolymer Liquid Embolic Agent (EVOH). Journal of Clinical Medicine. 2023; 12(10):3385. https://doi.org/10.3390/jcm12103385
Chicago/Turabian StyleRolland, Rémi, Antoine Loubet, Sébastien Bommart, Valérie Monnin-Bares, Hamid Zarqane, Juliette Vanoverschelde, Fanchon Herman, Nicolas Molinari, and Hélène Kovacsik. 2023. "Safety, Efficacy and Mid-Term Outcome for Transarterial Embolization (TAE) of Renal Angiomyolipoma (AML) Using Ethylene Vinyl Alcohol Copolymer Liquid Embolic Agent (EVOH)" Journal of Clinical Medicine 12, no. 10: 3385. https://doi.org/10.3390/jcm12103385
APA StyleRolland, R., Loubet, A., Bommart, S., Monnin-Bares, V., Zarqane, H., Vanoverschelde, J., Herman, F., Molinari, N., & Kovacsik, H. (2023). Safety, Efficacy and Mid-Term Outcome for Transarterial Embolization (TAE) of Renal Angiomyolipoma (AML) Using Ethylene Vinyl Alcohol Copolymer Liquid Embolic Agent (EVOH). Journal of Clinical Medicine, 12(10), 3385. https://doi.org/10.3390/jcm12103385