Effects of Chokeberries (Aronia spp.) on Cytoprotective and Cardiometabolic Markers and Semen Quality in 109 Mildly Hypercholesterolemic Danish Men: A Prospective, Double-Blinded, Randomized, Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
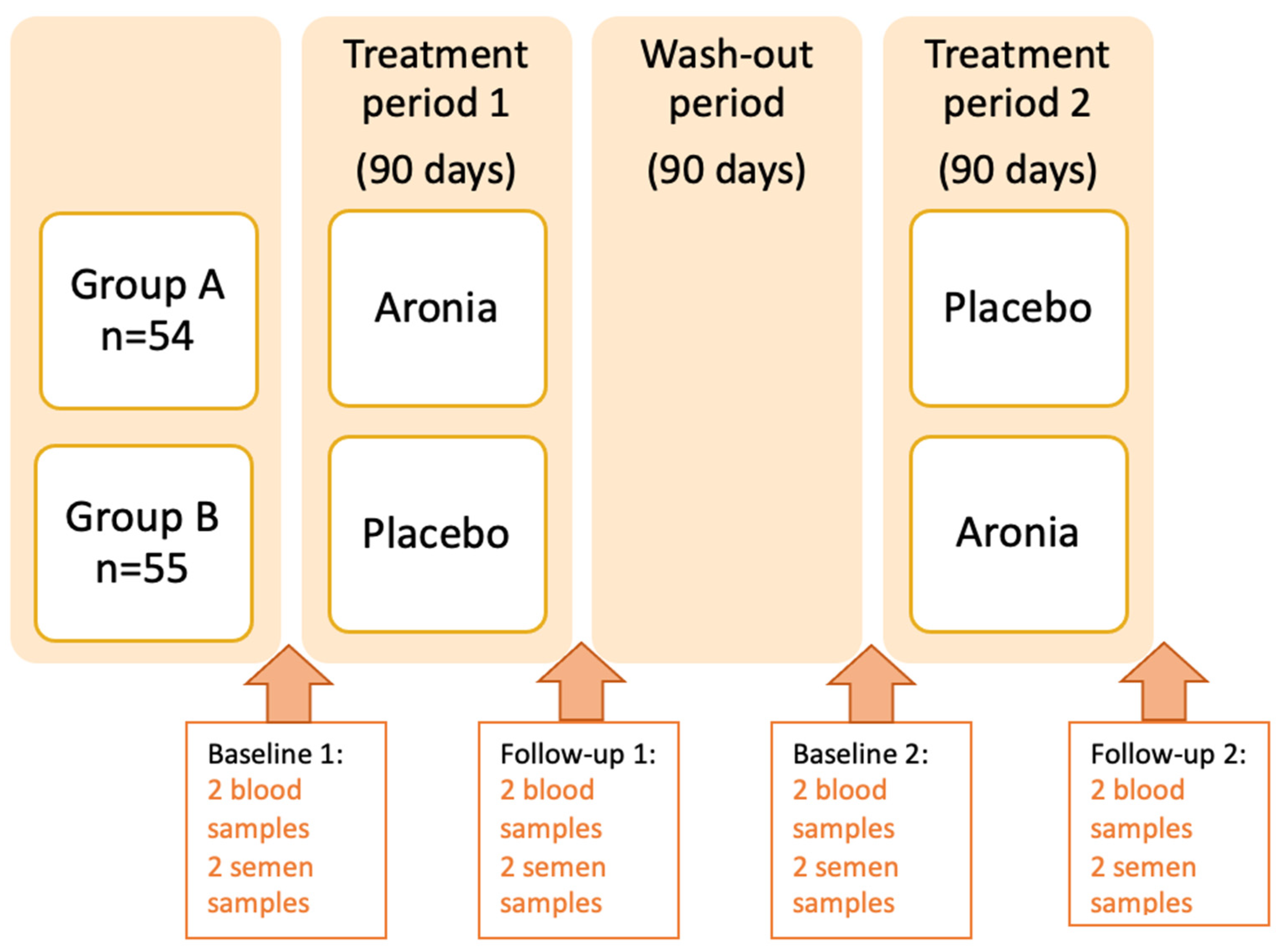
2.1. Study Design
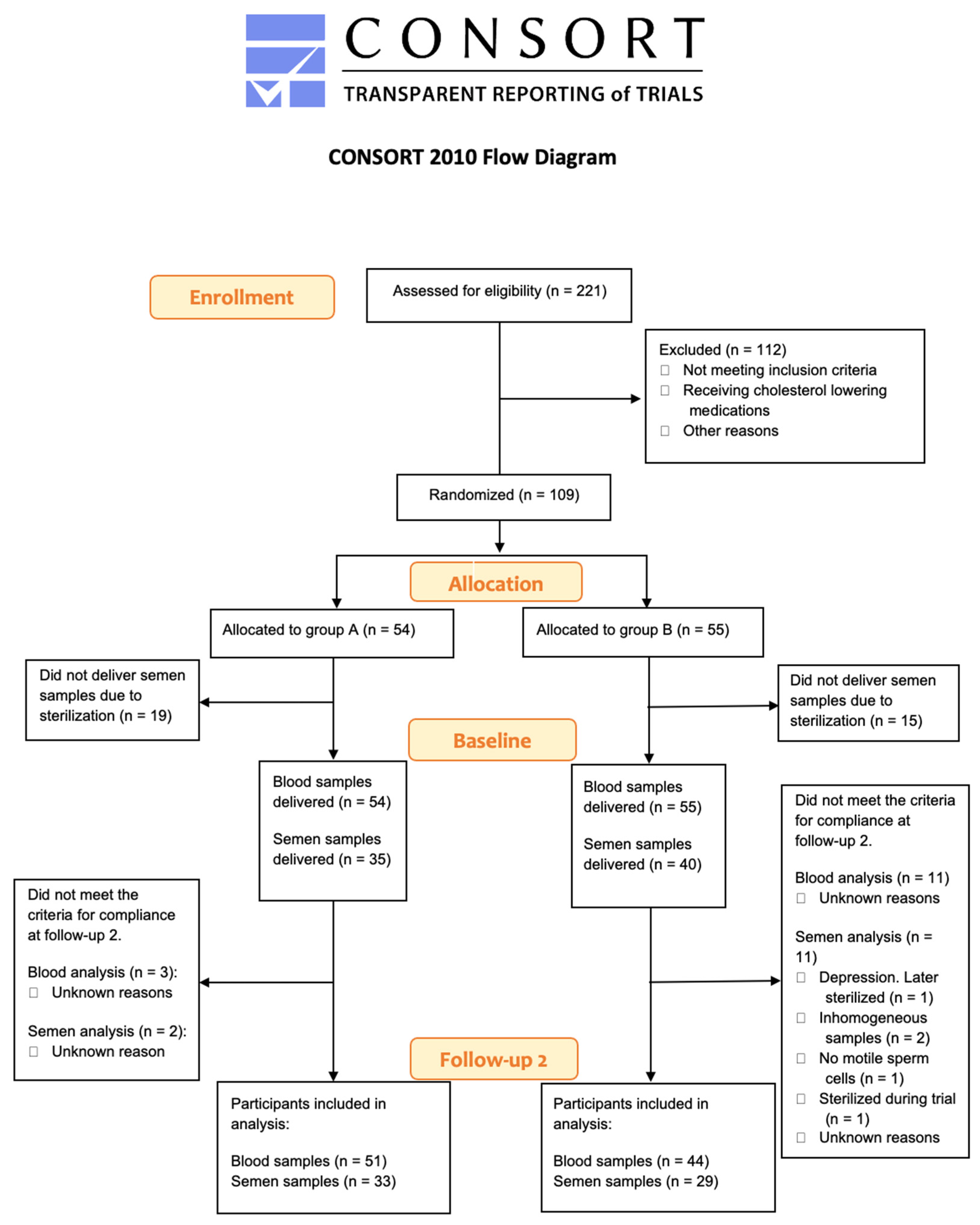
2.2. Recruitment and Randomization of the Study Population
2.3. Intervention
2.4. Clinical Tests and Questionnaires
2.5. Analytical Procedures, Outcomes and Statistical Methods
2.5.1. Blood Tests
2.5.2. SOD Determination
2.5.3. Glutathione Assay
2.5.4. Catalase Activity Assay
2.5.5. Isoprostanes
2.5.6. Insulin and Glucose
2.5.7. Handling of Semen Samples
2.5.8. Compliance
2.5.9. Primary and Secondary Outcomes
2.5.10. Statistical Analyses
Crossover Analyses
Subgroup Analyses
Test for Carry Over-Effect
3. Results
3.1. Participant Characteristics
3.2. Markers of Cytoprotection
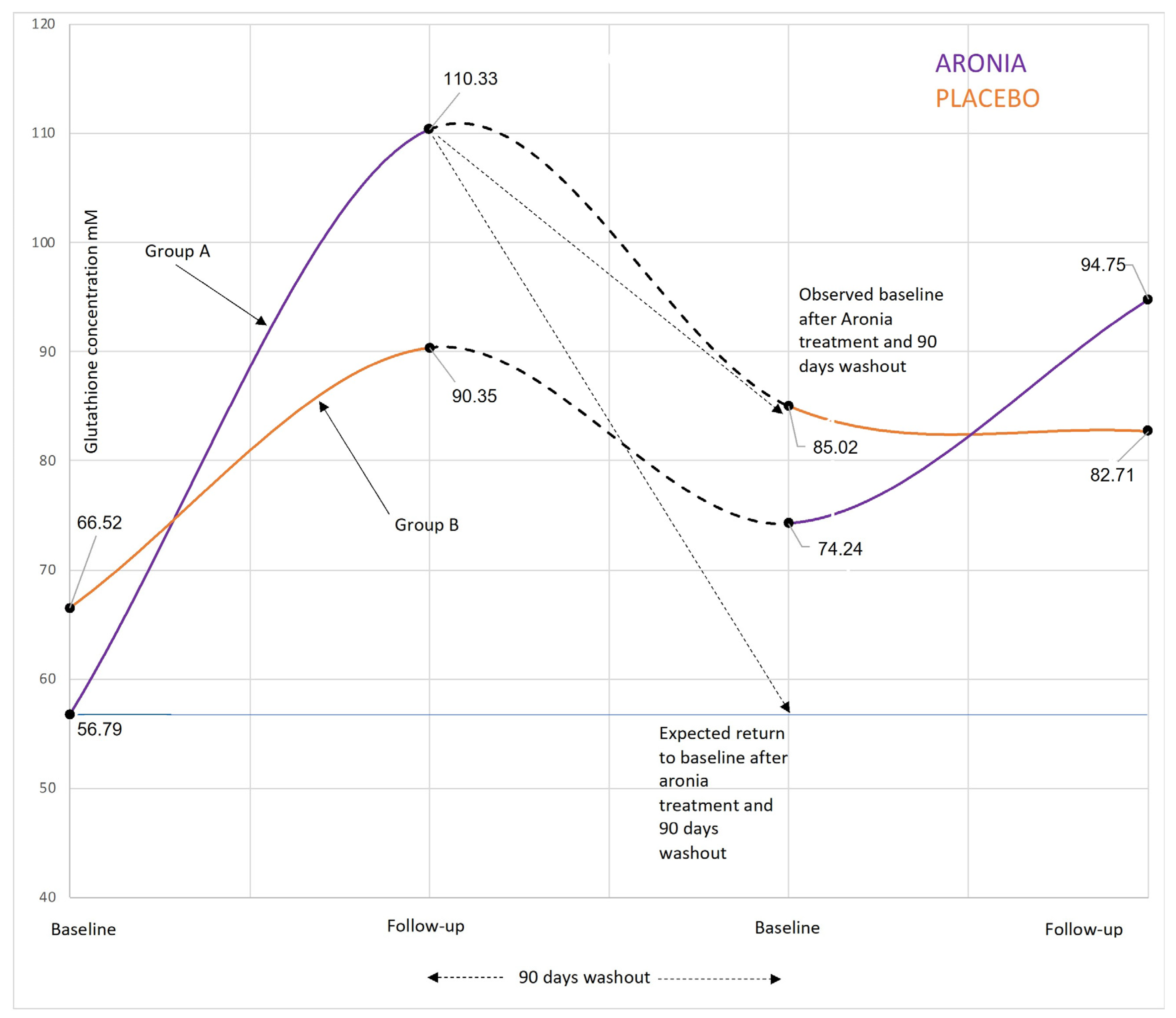
3.2.1. Glutathione
3.2.2. Isoprostanes
3.3. Sperm Quality
3.3.1. Sperm DNA Fragmentation
3.3.2. Percentage of Motile Sperm (PM)
3.4. Serum Lipids
3.4.1. Total Cholesterol
3.4.2. LDL-Cholesterol
3.4.3. HDL-Cholesterol and Triglycerides (TG)
3.5. Side Effects
4. Discussion
4.1. Effects of Aronia on Cytoprotective Targets
4.2. Glutathione
4.3. DNA Fragmentation and Percentage of Motile Sperm
4.4. Significant Cytoprotective Effects of Aronia among Coffee Drinkers
4.5. Effects of Aronia on Blood Lipid Levels
4.6. Side Effects
4.7. Strengths
4.8. Limitations
5. Conclusions
6. Registration
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CAT | Catalase |
| CMS | Concentration of motile sperm |
| CPMS | Concentration of progressive motile sperm |
| CRP | C-reactive protein |
| CVD | Cardiovascular disease |
| DFI | DNA Fragmentation Index |
| GLU | Glutathione |
| HbA1c | Hemoglobin A1c |
| HDL-C | High-density lipoprotein cholesterol |
| Hs CRP | High-sensitivity C-reactive protein |
| LDL-C | Low-density lipoprotein cholesterol |
| OS | Oxidative stress |
| PM | Percentage of motile sperm |
| RCT | Randomized controlled trial |
| ROS | Reactive oxidative species |
| SOD | Superoxide dismutase |
| TC | Total cholesterol |
| TG | Triglycerides |
| TMSC | Total Motile Sperm Count |
| TPMSC | Total Progressive Motile Sperm Count |
References
- Milic, P.; Jeremic, J.; Zivkovic, V.; Srejovic, I.; Jeremic, N.; Bradic, J.; Turnic, T.N.; Milosavljevic, I.; Bolevich, S.; Bolevich, S.; et al. Effects of different dietary regimes alone or in combination with standardized Aronia melanocarpa extract supplementation on lipid and fatty acids profiles in rats. Mol. Cell. Biochem. 2019, 461, 141–150. [Google Scholar] [CrossRef]
- Dufour, C.; Villa-Rodriguez, J.A.; Furger, C.; Lessard-Lord, J.; Gironde, C.; Rigal, M.; Badr, A.; Desjardins, Y.; Guyonnet, D. Cellular Antioxidant Effect of an Aronia Extract and Its Polyphenolic Fractions Enriched in Proanthocyanidins, Phenolic Acids, and Anthocyanins. Antioxidants 2022, 11, 1561. [Google Scholar] [CrossRef]
- Christensen, L.P. Chapter 13—The Role of Direct and Indirect Polyphenolic Antioxidants in Protection Against Oxidative Stress. In Polyphenols: Mechanisms of Action in Human Health and Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 147–179. [Google Scholar]
- Kardum, N.; Milovanović, B.; Šavikin, K.; Zdunić, G.; Mutavdžin, S.; Gligorijević, T.; Spasić, S. Beneficial Effects of Polyphenol-Rich Chokeberry Juice Consumption on Blood Pressure Level and Lipid Status in Hypertensive Subjects. J. Med. Food 2015, 18, 1231–1238. [Google Scholar] [CrossRef]
- Kim, Y.S.; Young, M.R.; Bobe, G.; Colburn, N.H.; Milner, J.A. Bioactive Food Components, Inflammatory Targets, and Cancer Prevention. Cancer Prev. Res. 2009, 2, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Broncel, M.; Kozirog, M.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Chojnowska-Jezierska, J. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. J. Pharmacol. Exp. Ther. 2010, 16, CR28–CR34. [Google Scholar]
- Zheng, W.; Wang, S.Y. Oxygen Radical Absorbing Capacity of Phenolics in Blueberries, Cranberries, Chokeberries, and Lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, C.; Li, G.; Chrubasik, S. The clinical effectiveness of chokeberry: A systematic review. Phytother. Res. 2010, 24, 1107–1114. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Xu, H.; Zhu, F.; Li, Z.; Lu, H.; Zhang, J.; Yang, Z.; Liu, Y. The Protective Effect of Anthocyanins Extracted from Aronia Melanocarpa Berry in Renal Ischemia-Reperfusion Injury in Mice. Mediat. Inflamm. 2021, 2021, 372893. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Soja, J.; Gancarz, M.; Wojtunik-Kulesza, K.; Markut-Miotła, E.; Oniszczuk, A. The Efficacy of Black Chokeberry Fruits against Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 6541. [Google Scholar] [CrossRef]
- Cikiriz, N.; Milosavljevic, M.I.; Jakovljevic, B.; Bolevich, S.; Jeremic, M.J.; Turnic, T.R.N.; Mitrovic, M.; Srejovic, I.M.; Bolevich, S.; Jakovljevic, V. The influences of chokeberry extract supplementation on redox status and body composition in handball players during competition phase. Can. J. Physiol. Pharmacol. 2021, 99, 42–47. [Google Scholar] [CrossRef]
- Pei, R.; Liu, J.; Martin, D.A.; Valdez, J.C.; Jeffety, J.; Barrett-Wilt, G.A.; Liu, Z.; Bolling, B.W. Aronia Berry Supplementation Mitigates Inflammation in T Cell Transfer-Induced Colitis by Decreasing Oxidative Stress. Nutrients 2019, 11, 1316. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Zheng, Y.; Liu, W.; Ding, C. Aronia melanocarpa polysaccharide ameliorates inflammation and aging in mice by modulating the AMPK/SIRT1/NF-κB signaling pathway and gut microbiota. Sci. Rep. 2021, 11, 20558. [Google Scholar] [CrossRef] [PubMed]
- Bakuradze, T.; Meiser, P.; Galan, J.; Richling, E. DNA Protection by an Aronia Juice-Based Food Supplement. Antioxidants 2021, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Pawłowicz, P.; Stachowiak, G.; Bielak, A.; Wilczyński, J. Administration of natural anthocyanins derived from chokeberry (Aronia melanocarpa) extract in the treatment of oligospermia in males with enhanced autoantibodies to oxidized low density lipoproteins (oLAB). The impact on fructose levels. Ginekol. Polska 2001, 72, 848–853. [Google Scholar]
- Christiansen, C.B.; Mellbye, F.B.; Hermansen, K.; Jeppesen, P.B.; Gregersen, S. Effects of Aronia melanocarpa on Cardiometabolic Diseases: A Systematic Review of Quasi-Design Studies and Randomized Controlled Trials. Rev. Diabet. Stud. 2022, 18, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, J.; Clark, C.; Varkaneh, H.K.; Lakiang, T.; Vasanthan, L.; Onyeche, V.; Mousavi, S.; Zhang, Y. The effect of Aronia consumption on lipid profile, blood pressure, and biomarkers of inflammation: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Imai, M.; Handa, S.; Harada, N.; Yamaji, R.; Sakamoto, T.; Ishida, T.; Inui, H.; Nakagaki, T.; Nakano, Y. Aronia juice supplementation inhibits lipid accumulation in both normal and obesity model mice. Pharmanutrition 2020, 14, 100223. [Google Scholar] [CrossRef]
- Tasic, N.; Jakovljevic, V.L.J.; Mitrovic, M.; Djindjic, B.; Tasic, D.; Dragisic, D.; Citakovic, Z.; Kovacevic, Z.; Radoman, K.; Zivkovic, V.; et al. Black chokeberry Aronia melanocarpa extract reduces blood pressure, glycemia and lipid profile in patients with metabolic syndrome: A prospective controlled trial. Mol. Cell. Biochem. 2021, 476, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Hires, C.; Baker, C.; Keenan, L.; Bush, M. Daily supplementation with aronia melanocarpa (chokeberry) reduces blood pressure and cholesterol: A meta analysis of controlled clinical trials. J. Diet. Suppl. 2021, 18, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Updat. 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Garrido, N.; Meseguer, M.; Alvarez, J.; Simón, C.; Pellicer, A.; Remohí, J. Relationship among standard semen parameters, glutathione peroxidase/glutathione reductase activity, and mRNA expression and reduced glutathione content in ejaculated spermatozoa from fertile and infertile men. Fertil. Steril. 2004, 82 (Suppl. 3), 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the Cardiovascular System in Health and in Aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef] [PubMed]
- Fedder, J. Nonsperm Cells in Human Semen: With Special Reference to Seminal Leukocytes and their Possible Influence on Fertility. Arch. Androl. 1996, 36, 41–65. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.-H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Koter, M.; Franiak, I.; Strychalska, K.; Broncel, M.; Chojnowska-Jezierska, J. Damage to the structure of erythrocyte plasma membranes in patients with type-2 hypercholesterolemia. Int. J. Biochem. Cell Biol. 2004, 36, 205–215. [Google Scholar] [CrossRef]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Park, E.; Mori, Y.; Haber, C.; Han, P.; Uchida, T.; Stavar, L.; Oprescu, A.; Koulajian, K.; Ivovic, A.; et al. FFA-induced hepatic insulin resistance in vivo is mediated by PKCδ, NADPH oxidase, and oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E34–E46. [Google Scholar] [CrossRef]
- John Aitken, R.; Clarkson, J.S.; Fishel, S. Generation of Reactive Oxygen Species, Lipid Peroxidation, and Human Sperm Function. Biol. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef]
- Takeshima, T.; Usui, K.; Mori, K.; Asai, T.; Yasuda, K.; Kuroda, S.; Yumura, Y. Oxidative stress and male infertility. Reprod. Med. Biol. 2020, 20, 41–52. [Google Scholar] [CrossRef]
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Azad, M.B.; Gibson, S.B. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009, 16, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, M.B.; Imam, S.N.; Dada, R. Sperm DNA integrity assays: Diagnostic and prognostic challenges and implications in management of infertility. J. Assist. Reprod. Genet. 2011, 28, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.F.; Cabezas, M.C. Dyslipidemia in Obesity: Mechanisms and Potential Targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef]
- National Heart, Lung and Blood Institute. Cardiovascular Disease Is on the Rise, but We Know How to Curb It. We’ve Done It before. 2021. Available online: https://www.nhlbi.nih.gov/news/2021/cardiovascular-disease-rise-we-know-how-curb-it-weve-done-it (accessed on 9 September 2022).
- Jørgensen, N.; Joensen, U.N.; Jensen, T.K.; Jensen, M.B.; Almstrup, K.; Olesen, I.A.; Juul, A.; Andersson, A.-M.; Carlsen, E.; Petersen, J.H.; et al. Human semen quality in the new millennium: A prospective cross-sectional population-based study of 4867 men. BMJ Open 2012, 2, e000990. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Karlsen, A.; Retterstøl, L.; Laake, P.; Paur, I.; Bøhn, S.; Sandvik, L.; Blomhoff, R. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef]
- Kowalczyk, E.; Fijałkowski, P.; Kura, M.; Krzesiński, P.; Błaszczyk, J.; Kowalski, J.; Smigielski, J.; Rutkowski, M.; Kopff, M. The influence of anthocyanins from Aronia melanocarpa on selected parameters of oxidative stress and microelements contents in men with hypercholesterolemia. Pol. Merkur. Lekarski. 2005, 19, 651–653. [Google Scholar]
- Shih, P.-H.; Yeh, C.-T.; Yen, G.-C. Anthocyanins Induce the Activation of Phase II Enzymes through the Antioxidant Response Element Pathway against Oxidative Stress-Induced Apoptosis. J. Agric. Food Chem. 2007, 55, 9427–9435. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen Radical Absorbing Capacity of Anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Christensen, L.P.; Christensen, K.B. The Role of Direct and Indirect Polyphenolic Antioxidants in Protection Against Oxidative Stress. In Polyphenols in Human Health and Disease; Academic Press: San Diego, CA, USA, 2014; Chapter 23; pp. 289–309. [Google Scholar]
- Taheri, R.; Connolly, B.A.; Brand, M.H.; Bolling, B.W. Underutilized Chokeberry (Aronia melanocarpa, Aronia arbutifolia, Aronia prunifolia) Accessions Are Rich Sources of Anthocyanins, Flavonoids, Hydroxycinnamic Acids, and Proanthocyanidins. J. Agric. Food Chem. 2013, 61, 8581–8588. [Google Scholar] [CrossRef] [PubMed]
- Brand, M. Aronia: Native Shrubs With Untapped Potential. Arnoldia 2009, 67, 14–25. [Google Scholar]
- Amann, R.P. The cycle of the seminiferous epithelium in humans: A need to revisit? J. Androl. 2008, 29, 469–487. [Google Scholar] [CrossRef]
- CONSORT. CONSORT 2010 Flow Diagram. 2010. Available online: http://www.consort-statement.org/consort-statement/flow-diagram (accessed on 16 September 2022).
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Bassuk, S.S.; Rifai, N.; Ridker, P.M. High-sensitivity C-reactive protein: Clinical importance. Curr. Probl. Cardiol. 2004, 29, 439–493. [Google Scholar] [CrossRef]
- Thérond, P.; Auger, J.; Legrand, A.; Jouannet, P. Alpha-Tocopherol in human spermatozoa and seminal plasma: Relationships with motility, antioxidant enzymes and leukocytes. Mol. Hum. Reprod. 1996, 2, 739–744. [Google Scholar] [CrossRef]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef]
- Montuschi, P.; Barnes, P.J.; Roberts, L.J., 2nd. Isoprostanes: Markers and mediators of oxidative stress. FASEB J. 2004, 18, 1791–1800. [Google Scholar] [CrossRef]
- Efsa Panel on Dietetic Products; Allergies, N.; Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.; Mangelsdorf, I.; et al. Guidance for the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health. EFSA J. 2018, 16, e05136. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Evenson, D.P.; Larson, K.L.; Jost, L.K. Sperm Chromatin Structure Assay: Its Clinical Use for Detecting Sperm DNA Fragmentation in Male Infertility and Comparisons With Other Techniques. J. Androl. 2002, 23, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Senn, S.S. Cross-Over Trials in Clinical Research, 2nd ed.; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Committee for Proprietary Medicinal Products (CPMP): Points to consider on adjustment for baseline covariates. Stat. Med. 2004, 23, 701–709. [CrossRef] [PubMed]
- Patienthåndbogen. BMI—Kropsmasseindeks. 2022. Available online: https://www.sundhed.dk/borger/patienthaandbogen/hormoner-og-stofskifte/undersoegelser/bmi-kropsmasseindeks/ (accessed on 16 September 2022).
- Hammoud, A.O.; Gibson, M.; Peterson, C.M.; Meikle, A.W.; Carrell, D.T. Impact of male obesity on infertility: A critical review of the current literature. Fertil. Steril. 2008, 90, 897–904. [Google Scholar] [CrossRef]
- Evenson, D.P.; Djira, G.; Kasperson, K.; Christianson, J. Relationships between the age of 25,445 men attending infertility clinics and sperm chromatin structure assay (SCSA®) defined sperm DNA and chromatin integrity. Fertil. Steril. 2020, 114, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Surhone, L.M.; Susan, M.T.T.; Marseken, F. Analysis of 2x2 Cross-Over Designs Using T-Tests, NCSS Statistical Software; John Wiley & Sons Ltd.: Chichester, UK, 2010. [Google Scholar]
- Skoczyñska, A.; Jêdrychowska, I.; Porêba, R.; Affelska-Jercha, A.; Turczyn, B.; Wojakowska, A.; Andrzejak, R. Influence of chokeberry juice on arterial blood pressure and lipid parameters in men with mild hypercholesterolemia. Pharmacol. Rep. 2007, 59, 177–182. [Google Scholar]
- Naruszewicz, M.; Łaniewska, I.; Millo, B.; Dłużniewski, M. Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (MI). Atherosclerosis 2007, 194, e179–e184. [Google Scholar] [CrossRef]
- Duchnowicz, P.; Nowicka, A.; Koter-Michalak, M.; Broncel, M. In vivo influence of extract from Aronia melanocarpa on the erythrocyte membranes in patients with hypercholesterolemia. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, CR569–CR574. [Google Scholar] [CrossRef]
- Pilaczynska-Szczesniak, L.; Skarpanska-Steinborn, A.; Deskur, E.; Basta, P.; Horoszkiewicz-Hassan, M. The Influence of Chokeberry Juice Supplementation on the Reduction of Oxidative Stress Resulting from an Incremental Rowing Ergometer Exercise. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 48–58. [Google Scholar] [CrossRef]
- Skarpańska-Stejnborn, A.; Basta, P.; Sadowska, J.; Pilaczyńska-Szczeńniak, L. Effect of supplementation with chokeberry juice on the inflammatory status and markers of iron metabolism in rowers. J. Int. Soc. Sports Nutr. 2014, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Vance, T.; Kim, B.; Gil Lee, S.G.; Caceres, C.; Wang, Y.; Hubert, P.A.; Lee, J.-Y.; Chun, O.K.; Bolling, B.W. Aronia berry polyphenol consumption reduces plasma total and low-density lipoprotein cholesterol in former smokers without lowering biomarkers of inflammation and oxidative stress: A randomized controlled trial. Nutr. Res. 2016, 37, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.H.; Wilcox, A.J.; Skjaerven, R.; Baird, D.D. Men’s body mass index and infertility. Hum. Reprod. 2007, 22, 2488–2493. [Google Scholar] [CrossRef]
- Sallmén, M.; Sandler, D.P.; Hoppin, J.A.; Blair, A.; Baird, D.D. Reduced Fertility Among Overweight and Obese Men. Epidemiology 2006, 17, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Kort, H.I.; Massey, J.B.; Elsner, C.W.; Mitchell-Leef, D.; Shapiro, D.B.; Witt, M.A.; Roudebush, W.E. Impact of Body Mass Index Values on Sperm Quantity and Quality. J. Androl. 2006, 27, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.K.; Andersson, A.M.; Jørgensen, N.; Andersen, A.G.; Carlsen, E.; Petersen, J.H.; Skakkebaek, N.E. Body mass index in relation to semen quality and reproductive hormones among 1558 Danish men. Fertil. Steril. 2004, 82, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.A.; Tirado, E.; Garcia, D.; Datta, V.; Sakkas, D. DNA fragmentation of sperm: A radical examination of the contribution of oxidative stress and age in 16 945 semen samples. Hum. Reprod. 2020, 35, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Fedder, M.D.K.; Jakobsen, H.B.; Giversen, I.; Christensen, L.P.; Parner, E.T.; Fedder, J. An Extract of Pomegranate Fruit and Galangal Rhizome Increases the Numbers of Motile Sperm: A Prospective, Randomised, Controlled, Double-Blinded Trial. PLoS ONE 2014, 9, e108532. [Google Scholar] [CrossRef]
- Silberstein, T.; Har-Vardi, I.; Harlev, A.; Friger, M.; Hamou, B.; Barac, T.; Levitas, E.; Saphier, O. Antioxidants and Polyphenols: Concentrations and Relation to Male Infertility and Treatment Success. Oxidative Med. Cell. Longev. 2016, 2016, 9140925. [Google Scholar] [CrossRef]
- Salehi, P.; Shahrokhi, S.Z.; Kamran, T.; Ajami, A.; Taghiyar, S.; Deemeh, M.R.; Student, T.K. Effect of antioxidant therapy on the sperm DNA integrity improvement; a longitudinal cohort study. Int. J. Reprod. Biomed. (IJRM) 2019, 17, 99. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Simoni, M. Sperm DNA fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management–meta-analyses. Reprod. Biomed. Online 2018, 37, 315–326. [Google Scholar] [CrossRef]
- Rex, A.; Wu, C.; Aagaard, J.; Fedder, J. DNA Fragmentation in Human Spermatozoa and Pregnancy Rates after Intrauterine Insemination. Should the DFI Threshold Be Lowered? J. Clin. Med. 2021, 10, 1310. [Google Scholar] [CrossRef]
- Ricci, E.; Viganò, P.; Cipriani, S.; Somigliana, E.; Chiaffarino, F.; Bulfoni, A.; Parazzini, F. Coffee and caffeine intake and male infertility: A systematic review. Nutr. J. 2017, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Morisco, F.; Verde, V.; Ritieni, A.; Alezio, A.; Caporaso, N.; Fogliano, V. Moderate coffee consumption increases plasma glutathione but not homocysteine in healthy subjects. Aliment. Pharmacol. Ther. 2003, 17, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Sakaguchi, H.; Higuchi, O.; Suzuki, T.; Chiji, H. Intestinal absorption of black chokeberry cyanidin 3-glycosides is promoted by capsaicin and capsiate in a rat ligated small intestinal loop model. Food Chem. 2019, 277, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-K.; Choi, M.S.; Yoo, H.H.; Kim, D.-H. The Intake of Coffee Increases the Absorption of Aspirin in Mice by Modifying Gut Microbiome. Pharmaceutics 2022, 14, 746. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified Anthocyanin Supplementation Reduces Dyslipidemia, Enhances Antioxidant Capacity, and Prevents Insulin Resistance in Diabetic Patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ling, W.; Guo, H.; Song, F.; Ye, Q.; Zou, T.; Li, D.; Zhang, Y.; Li, G.; Xiao, Y.; et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Kunz, C.; Herrmann, J.; Borsch, C.H.; Abel, G.; Fröhling, B.; Dietrich, H.; Rudloff, S. Anthocyanins from fruit juices improve the antioxidant status of healthy young female volunteers without affecting anti-inflammatory parameters: Results from the randomised, double-blind, placebo-controlled, cross-over ANTHONIA (ANTHOcyanins in Nutrition Investigation Alliance) study. Br. J. Nutr. 2014, 112, 925–936. [Google Scholar] [CrossRef]
- Bakuradze, T.; Tausend, A.; Galan, J.; Groh, I.A.M.; Berry, D.; Tur, J.A.; Marko, D.; Richling, E. Antioxidative activity and health benefits of anthocyanin-rich fruit juice in healthy volunteers. Free. Radic. Res. 2019, 53, 1045–1055. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; Du Plessis, S.S. Effect of Oxidative Stress on Male Reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kefer, J.C.; Agarwal, A.; Sabanegh, E. Role of antioxidants in the treatment of male infertility. Int. J. Urol. 2009, 16, 449–457. [Google Scholar] [CrossRef] [PubMed]
| Group A (n = 54) | Group B (n = 55) | p Value [95% CI] | |
|---|---|---|---|
| Age (years) | 47.7 ± 9.7 | 51.9 ± 9.3 | 0.03 [−7.9; −0.4] |
| BMI (kg/m2) | 27.2 ± 3.2 | 27.3 ± 3.5 | 0.87 [−1.4; 1.2] |
| Smoking, n (%) | 3 (6.1) | 5 (9.3) | |
| Baseline total cholesterol (mM) | 5.5 ± 0.8 | 5.6 ± 0.9 | 0.73 [−0.4; 0.3] |
| Baseline blood pressure (systolic) (mmHg) | 133.4 ± 14.4 | 131.9 ± 15.4 | 0.59 [−4.1; 7.2] |
| Baseline blood pressure (diastolic) (mmHg) | 87.0 ± 10.8 | 85.3 ± 11.0 | 0.41 [−2.4; 5.9] |
| Fasting blood glucose (mM) | 5.8 ± 0.4 | 6.0 ± 1.2 | 0.32 [−0.5; 0.2] |
| Hs CRP (mg/L) | 2.3 ± 2.3 | 2.3 ± 3.0 | 1.00 [−1.0; 1.0] |
| Glutathione (nM) | 56.8 ± 69.9 | 64.5 ± 83.4 | 0.60 [−36.9; 21.5] |
| DFI (%) | 19.6 ± 14.0 | 19.7 ± 12.2 | 0.98 [−6.3; 6.1] |
| TPMSC (mio.) | 108.9 ± 106.3 | 85.5 ± 87.7 | 0.31 [−22.4; 69.2] |
| Analysis of All Participants Incl. Subgroups | Glutathione (mM) | DNA Fragmentation (%) | Motile Sperm (%) (PM) | ||||
|---|---|---|---|---|---|---|---|
| Treatment Order | Aronia Then Placebo | Placebo Then Aronia | Aronia Then Placebo | Placebo Then Aronia | Aronia Then Placebo | Placebo Then Aronia | |
| N | 51 | 42 | 33 | 27 | 33 | 29 | |
| Treatment effect [CI] | +19.8 [−7.6;47.1] | −1.2 (−3.3;0.8) | +2.7 | ||||
| Crossover analysis 1 | 0.15 | 0.230 | 0.067 | ||||
| Crossover analysis 2, including baseline data | 0.0095 ** | ||||||
| Analysis of subgroups | |||||||
| Age > 40 | Effect | −2.4 | +5.4 | ||||
| p-value | 0.032 * | 0.004 ** | |||||
| n | 41 | 43 | |||||
| BMI > 25 | Effect | −2.0 | +3.6 | ||||
| p-value | 0.037 * | 0.052 | |||||
| n | 42 | 43 | |||||
| Glutathione < 42 mM at baseline 1 | Effect | +41.3 | |||||
| p-value | 0.038 * | ||||||
| n | 54 | ||||||
| No D vitamin supplementation | Effect | 0.043 * | |||||
| p-value | −1.8 | ||||||
| n | 51 | ||||||
| No fish oil supplementation | Effect | −2.1 | |||||
| p-value | 0.031 * | ||||||
| n | 49 | ||||||
| High fruit and veggie intake | Effect | −5.6 | |||||
| p-value | 0.031 | ||||||
| n | 13 | ||||||
| Low daily sugar intake | Effect | −2.7 | |||||
| p-value | 0.032 * | ||||||
| n | 37 | ||||||
| Sitting down less than 6 h/day | Effect | −2.3 | |||||
| p-value | 0.046 * | ||||||
| n | 40 | ||||||
| Low alcohol intake | Effect | −1.2 | +4.6 | ||||
| p-value | 0.037 * | 0.01 * | |||||
| n | 48 | 50 | |||||
| No smoking | Effect | −1.9 | +3.4 | ||||
| p-value | 0.051 | 0.036 * | |||||
| n | 53 | 54 | |||||
| High coffee intake | Effect | +32.6 | −3.2 | +5.7 | |||
| p-value | 0.045 * | 0.01 * | 0.006 ** | ||||
| n | 60 | 37 | 39 | ||||
| No magnesium supplementation | Effect | +3.6 | |||||
| p-value | 0.024 * | ||||||
| n | 50 | ||||||
| No calcium supplementation | Effect | +3.6 | |||||
| p-value | 0.024 * | ||||||
| n | 50 | ||||||
| Analysis of All Participants Incl. Subgroups | Total Cholesterol (mM) | LDL-Cholesterol (mM) | HDL-Cholesterol (mM) | Triglyceride (mM) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment order | Aronia then Placebo | Placebo then Aronia | Aronia then Placebo | Placebo then Aronia | Aronia then Placebo | Placebo then Aronia | Aronia then Placebo | Placebo then Aronia | |
| n | 51 | 43 | 51 | 43 | 51 | 43 | 51 | 43 | |
| Treatment effect (CI) | −0.1 (−0.2; 0.1) | −0.1 (−0.2; 0.1) | −0.0 (−0.1; 0.0) | −0.0 (−0.1; 0.1) | |||||
| Cross over analysis p-value | 0.16 | 0.43 | 0.12 | 0.78 | |||||
| Analysis of subgroups | |||||||||
| Age < 40 years | p-value | 0.046 * | |||||||
| Effect (CI) | −0.4 (−0.9; 0.0) | ||||||||
| n | 15 | ||||||||
| Low daily coffee intake | p-value | 0.052 | 0.033 * | ||||||
| Effect (CI) | −0.2 (−0.5; −0.0) | −0.3 (−0.6; 0.0) | |||||||
| n | 28 | 28 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sangild, J.; Faldborg, A.; Schousboe, C.; Fedder, M.D.K.; Christensen, L.P.; Lausdahl, A.K.; Arnspang, E.C.; Gregersen, S.; Jakobsen, H.B.; Knudsen, U.B.; et al. Effects of Chokeberries (Aronia spp.) on Cytoprotective and Cardiometabolic Markers and Semen Quality in 109 Mildly Hypercholesterolemic Danish Men: A Prospective, Double-Blinded, Randomized, Crossover Trial. J. Clin. Med. 2023, 12, 373. https://doi.org/10.3390/jcm12010373
Sangild J, Faldborg A, Schousboe C, Fedder MDK, Christensen LP, Lausdahl AK, Arnspang EC, Gregersen S, Jakobsen HB, Knudsen UB, et al. Effects of Chokeberries (Aronia spp.) on Cytoprotective and Cardiometabolic Markers and Semen Quality in 109 Mildly Hypercholesterolemic Danish Men: A Prospective, Double-Blinded, Randomized, Crossover Trial. Journal of Clinical Medicine. 2023; 12(1):373. https://doi.org/10.3390/jcm12010373
Chicago/Turabian StyleSangild, Julie, Anne Faldborg, Cecilie Schousboe, Maja Døvling Kaspersen Fedder, Lars Porskjær Christensen, Astrid Komal Lausdahl, Eva Christensen Arnspang, Søren Gregersen, Henrik Byrial Jakobsen, Ulla Breth Knudsen, and et al. 2023. "Effects of Chokeberries (Aronia spp.) on Cytoprotective and Cardiometabolic Markers and Semen Quality in 109 Mildly Hypercholesterolemic Danish Men: A Prospective, Double-Blinded, Randomized, Crossover Trial" Journal of Clinical Medicine 12, no. 1: 373. https://doi.org/10.3390/jcm12010373
APA StyleSangild, J., Faldborg, A., Schousboe, C., Fedder, M. D. K., Christensen, L. P., Lausdahl, A. K., Arnspang, E. C., Gregersen, S., Jakobsen, H. B., Knudsen, U. B., & Fedder, J. (2023). Effects of Chokeberries (Aronia spp.) on Cytoprotective and Cardiometabolic Markers and Semen Quality in 109 Mildly Hypercholesterolemic Danish Men: A Prospective, Double-Blinded, Randomized, Crossover Trial. Journal of Clinical Medicine, 12(1), 373. https://doi.org/10.3390/jcm12010373







