Correlation between Constipation Symptoms and Abdominal CT Imaging: A Cross-Sectional Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Assessments of Clinical Characteristics
2.5. Scoring of Abdominal Symptoms and Fecal Properties
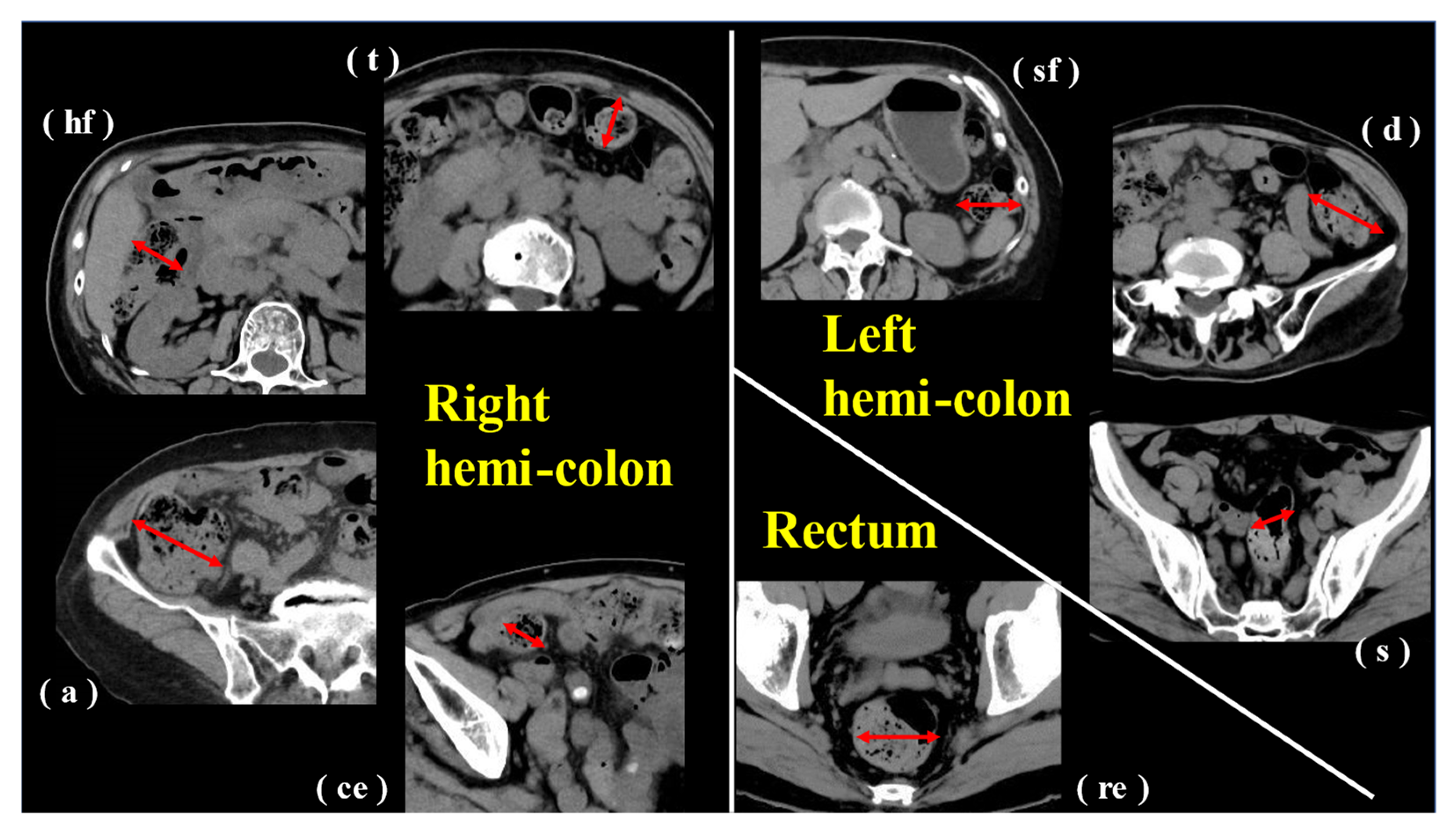
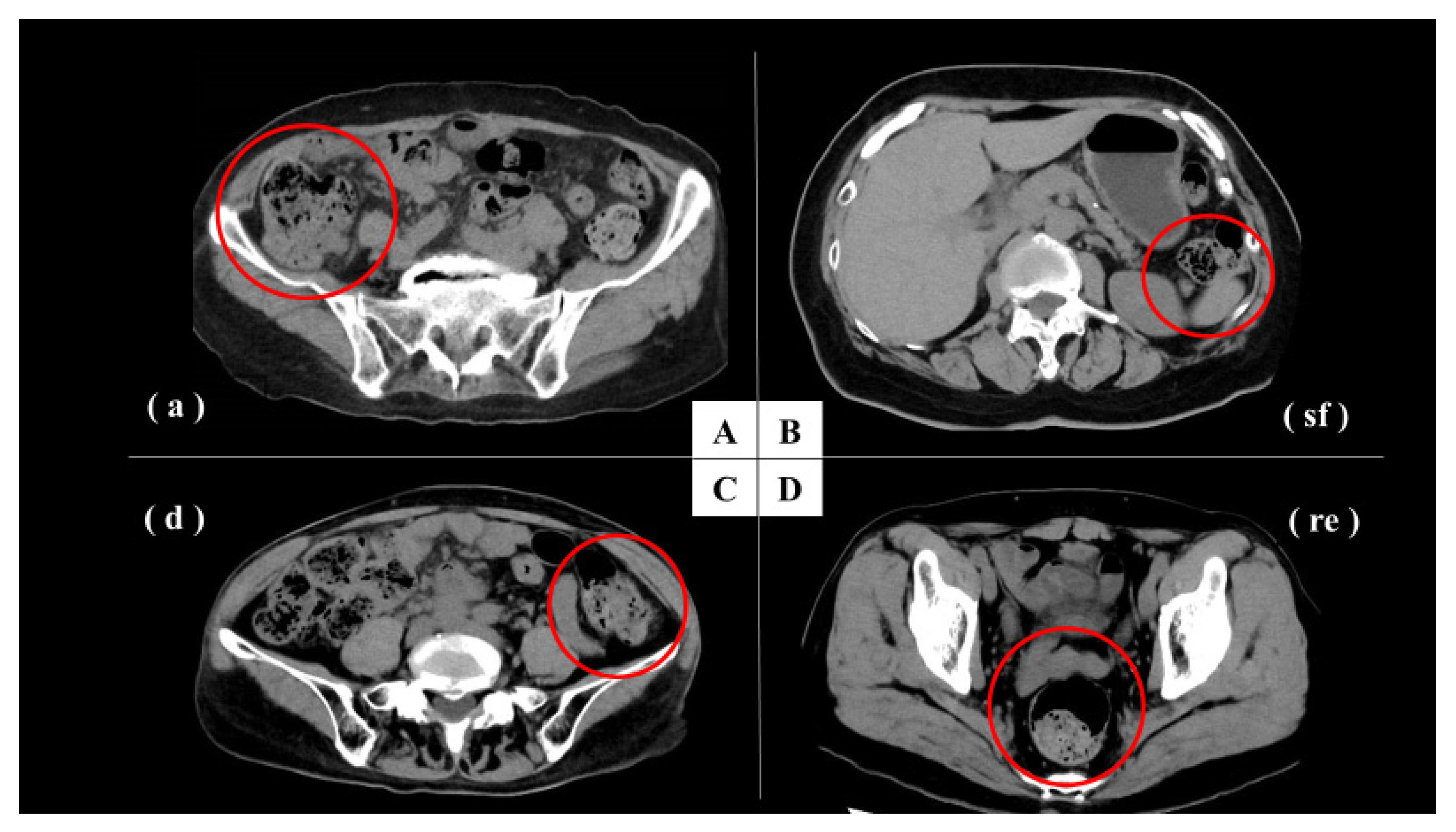
2.6. Image Evaluation and Analysis
2.7. Statistical Analysis
2.8. Ethical Considerations
3. Results
3.1. Study Participants’ Clinical Characteristics and Symptom Questionnaires
3.2. Image Evaluation and Analysis
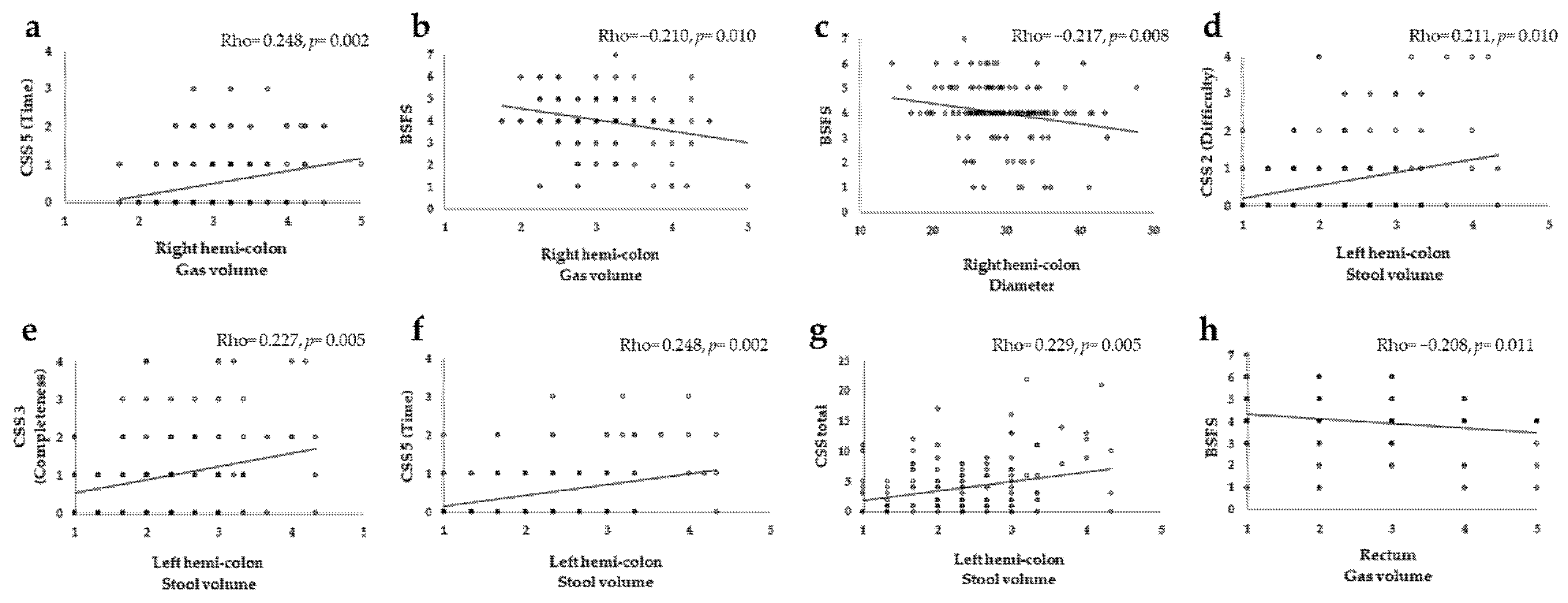
3.3. CT Image Evaluation and Correlation with Constipation Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bharucha, A.E.; Wald, A. Chronic Constipation. Mayo. Clin. Proc. 2019, 94, 2340–2357. [Google Scholar] [CrossRef] [PubMed]
- Wald, A.; Scarpignato, C.; Kamm, M.A.; Mueller-Lissner, S.; Helfrich, I.; Schuijt, C.; Bubeck, J.; Limoni, C.; Petrini, O. The burden of constipation on quality of life: Results of a multinational survey. Aliment. Pharmacol. Ther. 2007, 26, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Belsey, J.; Greenfield, S.; Candy, D.; Geraint, M. Systematic review: Impact of constipation on quality of life in adults and children. Aliment. Pharmacol. Ther. 2010, 31, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Honkura, K.; Tomata, Y.; Sugiyama, K.; Kaiho, Y.; Watanabe, T.; Zhang, S.; Sugawara, Y.; Tsuji, I. Defecation frequency and cardiovascular disease mortality in Japan: The Ohsaki cohort study. Atherosclerosis 2016, 246, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Krogh, K.; Chiarioni, G.; Whitehead, W. Management of chronic constipation in adults. United Eur. Gastroenterol. J. 2017, 5, 465–472. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Dorn, S.D.; Lembo, A.; Pressman, A. American Gastroenterological Association medical position statement on constipation. Gastroenterology 2013, 144, 211–217. [Google Scholar] [CrossRef]
- Lembo, A.; Camilleri, M. Chronic constipation. N. Engl. J. Med. 2003, 349, 1360–1368. [Google Scholar] [CrossRef]
- Park, S.Y.; Khemani, D.; Nelson, A.D.; Eckert, D.; Camilleri, M. Rectal Gas Volume Measured by Computerized Tomography Identifies Evacuation Disorders in Patients with Constipation. Clin. Gastroenterol. Hepatol. 2017, 15, 543–552.e4. [Google Scholar] [CrossRef]
- Inoh, Y.; Kanoshima, K.; Ohkuma, K.; Fuyuki, A.; Uchiyama, S.; Ohkubo, H.; Higurashi, T.; Iida, H.; Nonaka, T.; Fujita, K.; et al. Assessment of colonic contents in patients with chronic constipation using MRI. J. Clin. Biochem. Nutr. 2018, 62, 277–280. [Google Scholar] [CrossRef]
- Pritchard, S.E.; Marciani, L.; Garsed, K.C.; Hoad, C.; Thongborisute, W.; Roberts, E.; Gowland, P.; Spiller, R.C. Fasting and postprandial volumes of the undisturbed colon: Normal values and changes in diarrhea-predominant irritable bowel syndrome measured using serial MRI. Neurogastroenterol. Motil. 2014, 26, 124–130. [Google Scholar] [CrossRef]
- Mahadevan, V. Anatomy of the rectum and anal canal. Surgery 2020, 38, 7–11. [Google Scholar]
- Lam, C.; Chaddock, G.; Marciani, L.; Costigan, C.; Paul, J.; Cox, E.; Hoad, C.; Menys, A.; Pritchard, S.; Garsed, K.; et al. Colonic response to laxative ingestion as assessed by MRI differs in constipated irritable bowel syndrome compared to functional constipation. Neurogastroenterol. Motil. 2016, 28, 861–870. [Google Scholar] [CrossRef]
- Lam, C.; Chaddock, G.; Laurea, L.M.; Costigan, C.; Cox, E.; Hoad, C.; Pritchard, S.; Gowland, P.; Spiller, R. Distinct Abnormalities of Small Bowel and Regional Colonic Volumes in Subtypes of Irritable Bowel Syndrome Revealed by MRI. Am. J. Gastroenterol. 2017, 112, 346–355. [Google Scholar] [CrossRef]
- Bendezú, R.A.; Mego, M.; Monclus, E.; Merino, X.; Accarino, A.; Malagelada, J.R.; Navazo, I.; Azpiroz, F. Colonic content: Effect of diet, meals, and defecation. Neurogastroenterol. Motil. 2017, 29, e12930. [Google Scholar] [CrossRef] [PubMed]
- Keuzenkamp-Jansen, C.W.; Fijnvandraat, C.J.; Kneepkens, C.M.; Douwes, A.C. Diagnostic dilemmas and results of treatment for chronic constipation. Arch. Dis. Child. 1996, 75, 36–41. [Google Scholar] [CrossRef][Green Version]
- Sharif, H.; Hoad, C.; Abrehart, N.; Gowland, P.; Spiller, R.; Kirkham, S.; Loganathan, S.; Papadopoulos, M.; Benninga, M.; Devadason, D.; et al. Colonic Volume Changes in Paediatric Constipation Compared to Normal Values Measured Using MRI. Diagnostics 2021, 11, 974. [Google Scholar] [CrossRef] [PubMed]
- Agachan, F.; Chen, T.; Pfeifer, J.; Reissman, P.; Wexner, S.D. A constipation scoring system to simplify evaluation and management of constipated patients. Dis. Colon. Rectum. 1996, 39, 681–685. [Google Scholar] [CrossRef]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef]
- Cannon, W.B. The Movements of the Intestines studied by Means of the Röntgen Rays. J. Med. Res. 1902, 7, 72–75. [Google Scholar] [CrossRef]
- Krevsky, B.; Malmud, L.S.; D’Ercole, F.; Maurer, A.H.; Fisher, R.S. Colonic Transit Scintigraphy. Gastroenterology 1986, 91, 1102–1112. [Google Scholar] [CrossRef]
- Rao, S.S.; Mudipalli, R.S.; Stessman, M.; Zimmerman, B. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (Anismus). Neurogastroenterol. Motil. 2004, 16, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Roth, H.P.; Fein, S.B.; Sturman, M.F. The mechanisms responsible for the urge to defecate. Gastroenterology 1957, 32, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, H.; Yoshihara, T.; Misawa, N.; Ashikari, K.; Fuyuki, A.; Matsuura, T.; Higurashi, T.; Imajo, K.; Hosono, K.; Yoneda, M.; et al. Relationship between Stool Form and Quality of Life in Patients with Chronic Constipation: An Internet Questionnaire Survey. Digestion 2021, 102, 147–154. [Google Scholar] [CrossRef]
- Delgado-Aros, S.; Locke, G.R., III; Camilleri, M.; Talley, N.J.; Fett, S.; Zinsmeister, A.R.; Melton, L.J., III. Obesity is associated with increased risk of gastrointestinal symptoms: A population-based study. Am. J. Gastroenterol. 2004, 99, 1801–1806. [Google Scholar] [CrossRef]
- Manabe, N.; Wong, B.S.; Camilleri, M.; Burton, D.; Mckinzie, S.; Zinsmeister, A.R. Lower functional gastrointestinal disorders: Evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol. Motil. 2010, 22, 293-e82. [Google Scholar] [CrossRef]
- Asaoka, D.; Takeda, T.; Inami, Y.; Abe, D.; Shimada, Y.; Matsumoto, K.; Ueyama, H.; Matsumoto, K.; Komori, H.; Akazawa, Y.; et al. The Association between Frailty and Abdominal Symptoms: A Hospital-based Cross-sectional Study. Intern. Med. 2020, 59, 1677–1685. [Google Scholar] [CrossRef]
- Asaoka, D.; Takeda, T.; Inami, Y.; Abe, D.; Shimada, Y.; Matsumoto, K.; Ueyama, H.; Matsumoto, K.; Komori, H.; Akazawa, Y.; et al. Association between the severity of constipation and sarcopenia in elderly adults: A single-center university hospital-based, cross-sectional study. Biomed. Rep. 2021, 14, 2. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Wald, A.; Enck, P.; Rao, S. Functional Anorectal Disorders. Gastroenterology 2016, 130, 1510–1518. [Google Scholar] [CrossRef]
| Characteristics | Value |
|---|---|
| Sex (male:female) | 54:95 |
| Age (y), mean ± SD (range) | 72.1 ± 10.4 (38–89) |
| BMI | 22.6 ± 3.5 |
| PPI/PCAB | Non-users: 109, Users: 40 |
| Laxative | Non-users: 129, Users: 20 |
| Questionnaires, median (IQR) | |
| CSS | 2.0 (1–6) |
| BSFS | 4.0 (4–5) |
| Anatomic Location | CT Findings | ||
|---|---|---|---|
| Stool Volume | Gas Volume | Diameter (mm) | |
| Right hemi-colon | 3.2 ± 0.6 | 3.1 ± 0.7 | 29.3 ± 5.6 |
| Cecum | 3.6 ± 0.8 | 3.2 ± 0.7 | 35.8 ± 9.3 |
| Ascending colon | 3.3 ± 0.8 | 3.1 ± 0.7 | 32.6 ± 8.1 |
| Hepatic flexure | 3.1 ± 0.8 | 3.0 ± 0.8 | 26.8 ± 7.4 |
| Transverse colon | 2.8 ± 0.9 | 3.0 ± 1.8 | 22.7 ± 6.6 |
| Left hemi-colon | 2.4 ± 1.2 | 2.3 ± 0.6 | 19.3 ± 5.3 |
| Splenic flexure | 2.5 ± 0.9 | 2.7 ± 0.9 | 22.2 ± 7.0 |
| Descending colon | 2.3 ± 2.1 | 2.1 ± 0.8 | 18.2 ± 6.6 |
| Sigmoid colon | 2.3 ± 1.0 | 2.2 ± 0.9 | 17.4 ± 6.1 |
| Rectum | 2.1 ± 0.9 | 2.5 ± 1.2 | 27.1 ± 11.0 |
| CSS1 (Frequency) | CSS2 (Difficulty) | CSS3 (Completeness) | CSS4 (Pain) | CSS5 (Time) | CSS6 (Assistance) | CSS7 (Failure) | CSS8 (History) | CSS Total | BSFS | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Right hemi-colon | Stool volume | 0.041 (0.618) | 0.150 (0.067) | 0.011 (0.893) | −0.027 (0.746) | 0.150 (0.068) | −0.099 (0.229) | 0.034 (0.678) | 0.039 (0.636) | 0.047 (0.567) | −0.138 (0.094) |
| Gas volume | 0.120 (0.145) | 0.142 (0.084) | 0.073 (0.376) | 0.098 (0.235) | 0.248 * (0.002) | −0.116 (0.160) | 0.073 (0.381) | 0.055 (0.507) | 0.122 (0.139) | −0.210 * (0.010) | |
| Diameter | −0.074 (0.368) | 0.012 (0.885) | 0.016 (0.844) | −0.027 (0.742) | 0.062 (0.449) | −0.112 (0.175) | 0.019 (0.818) | −0.066 (0.423) | −0.056 (0.497) | −0.217 * (0.008) | |
| Left hemi-colon | Stool volume | 0.142 (0.008) | 0.211 * (0.010) | 0.227 * (0.005) | −0.012 (0.885) | 0.248 * (0.002) | 0.058 (0.483) | 0.036 (0.483) | 0.154 (0.060) | 0.229 * (0.005) | −0.106 (0.198) |
| Gas volume | 0.169 (0.039) | 0.146 (0.075) | 0.102 (0.217) | −0.009 (0.914) | 0.101 (0.219) | 0.081 (0.326) | 0.057 (0.491) | 0.043 (0.598) | 0.149 (0.070) | 0.050 (0.543) | |
| Diameter | 0.078 (0.347) | 0.169 (0.039) | 0.172 (0.036) | 0.061 (0.457) | 0.078 (0.344) | 0.036 (0.660) | 0.112 (0.176) | 0.089 (0.278) | 0.189 (0.021) | −0.099 (0.231) | |
| Rectum | Stool volume | 0.100 (0.226) | 0.025 (0.762) | 0.047 (0.572) | −0.052 (0.531) | 0.006 (0.941) | −0.170 (0.038) | −0.105 (0.205) | 0.055 (0.503) | 0.078 (0.344) | −0.116 (0.159) |
| Gas volume | 0.032 (0.701) | 0.042 (0.614) | 0.007 (0.933) | −0.119 (0.148) | 0.062 (0.456) | −0.158 (0.055) | −0.033 (0.693) | −0.032 (0.696) | −0.035 (0.672) | −0.208 * (0.011) | |
| Diameter | 0.019 (0.822) | 0.066 (0.421) | −0.078 (0.344) | −0.113 (0.168) | 0.054 (0.515) | −0.100 (0.225) | −0.025 (0.760) | 0.011 (0.893) | 0.055 (0.502) | −0.139 (0.090) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haraikawa, M.; Takeda, T.; Oki, S.; Hojo, M.; Asaoka, D.; Iwano, T.; Uchida, R.; Utsunomiya, H.; Susuki, N.; Abe, D.; et al. Correlation between Constipation Symptoms and Abdominal CT Imaging: A Cross-Sectional Pilot Study. J. Clin. Med. 2023, 12, 341. https://doi.org/10.3390/jcm12010341
Haraikawa M, Takeda T, Oki S, Hojo M, Asaoka D, Iwano T, Uchida R, Utsunomiya H, Susuki N, Abe D, et al. Correlation between Constipation Symptoms and Abdominal CT Imaging: A Cross-Sectional Pilot Study. Journal of Clinical Medicine. 2023; 12(1):341. https://doi.org/10.3390/jcm12010341
Chicago/Turabian StyleHaraikawa, Mayuko, Tsutomu Takeda, Shotaro Oki, Mariko Hojo, Daisuke Asaoka, Tomoyo Iwano, Ryouta Uchida, Hisanori Utsunomiya, Nobuyuki Susuki, Daiki Abe, and et al. 2023. "Correlation between Constipation Symptoms and Abdominal CT Imaging: A Cross-Sectional Pilot Study" Journal of Clinical Medicine 12, no. 1: 341. https://doi.org/10.3390/jcm12010341
APA StyleHaraikawa, M., Takeda, T., Oki, S., Hojo, M., Asaoka, D., Iwano, T., Uchida, R., Utsunomiya, H., Susuki, N., Abe, D., Ikeda, A., Akazawa, Y., Ueda, K., Ueyama, H., Shibuya, T., Nojiri, S., Nagasawa, H., Suzuki, M., Kuwatsuru, R., & Nagahara, A. (2023). Correlation between Constipation Symptoms and Abdominal CT Imaging: A Cross-Sectional Pilot Study. Journal of Clinical Medicine, 12(1), 341. https://doi.org/10.3390/jcm12010341






