Glycoprotein and Lipoprotein Profiles Assessed by 1H-NMR and Its Relation to Ascending Aortic Dilatation in Bicuspid Aortic Valve Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Plasma Preparation
2.3. 1H-NMR Lipid and Glycoprotein Profile Evaluation
2.4. Statistical Analysis
3. Results
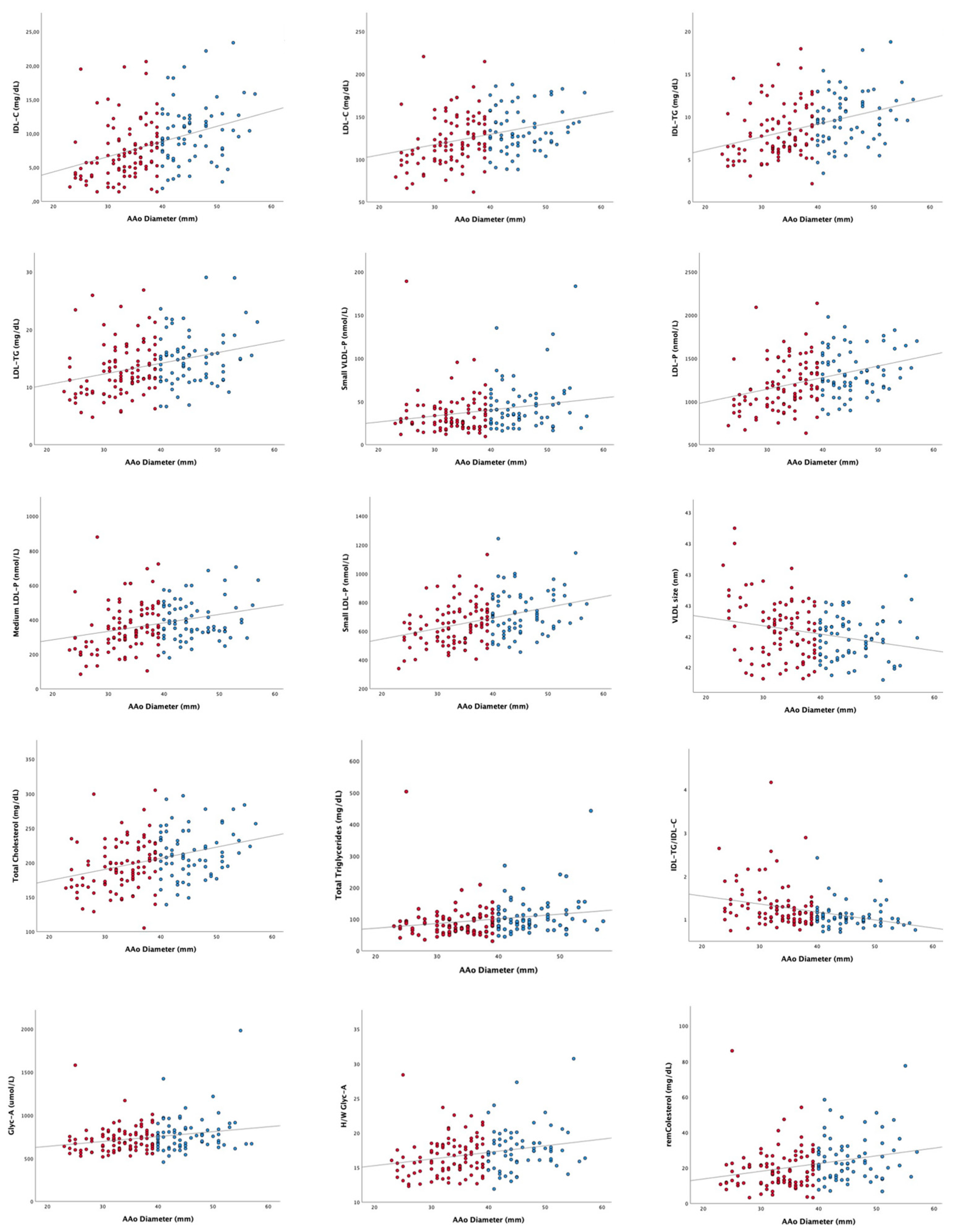
3.1. Evaluation of the Relation of Lipoprotein and Glycoprotein Profiles to AAoD in BAV Disease: Transversal Study
3.1.1. Baseline Clinical Characteristics
3.1.2. Lipoprotein and Glycoprotein Profiles Assessed by 1H-NMR: Nondilated Vs. Dilated
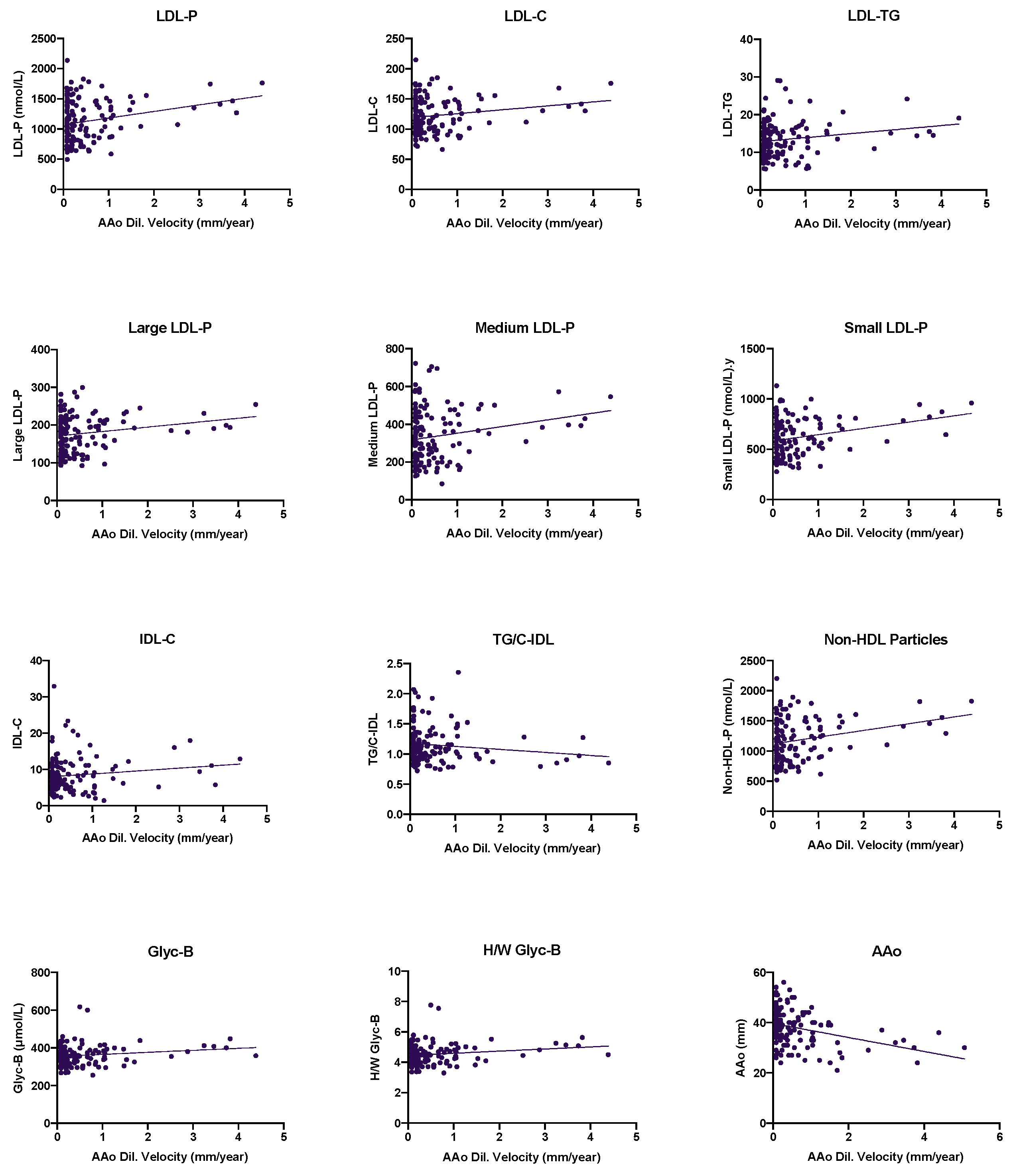
3.2. Effect of the Lipoprotein and Glycoprotein Profiles in the Progression of Ascending Aorta Diameter in BAV Disease: Longitudinal Analysis
3.2.1. Ascending Aorta Diameter Progression in BAV Patients: A Long-Term Follow-Up
3.2.2. Evaluation of the Lipoprotein and Glycoprotein Profiles’ Influence in AAoD Progression in BAV Aortopathy: A Longitudinal Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, S.; Siu, S.C. Aortic Dilatation in Patients with Bicuspid Aortic Valve. N. Engl. J. Med. 2014, 370, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Alegret, J.M.; Palomares, R.; Duran, I.; Vernis, J.M.; Palazón, Ó. Effect of Age on Valvular Dysfunction and Aortic Dilatation in Patients With a Bicuspid Aortic Valve. Rev. Esp. Cardiol. 2006, 59, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.E.; Kaplan, S. The Incidence of Congenital Heart Disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Adams, R.J.; Berry, J.D.; Brown, T.M.; Carnethon, M.R.; Dai, S.; de Simone, G.; Ford, E.S.; et al. Heart Disease and Stroke Statistics—2011 Update. Circulation 2011, 123, e18–e209. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.J.; Lee, S.; Jang, J.Y.; Kwon, O.; Bae, J.S.; Lee, J.H.; Kim, D.-H.; Jung, S.-H.; Song, J.-M.; Kang, D.-H.; et al. Performance of a Simplified Dichotomous Phenotypic Classification of Bicuspid Aortic Valve to Predict Type of Valvulopathy and Combined Aortopathy. J. Am. Soc. Echocardiogr. 2017, 30, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Michelena, H.I.; Khanna, A.D.; Mahoney, D.; Margaryan, E.; Topilsky, Y.; Suri, R.M.; Eidem, B.; Edwards, W.D.; Sundt, T.M.; Enriquez-Sarano, M. Incidence of Aortic Complications in Patients with Bicuspid Aortic Valves. JAMA 2011, 306, 1104–1112. [Google Scholar] [CrossRef]
- Biner, S.; Rafique, A.M.; Ray, I.; Cuk, O.; Siegel, R.J.; Tolstrup, K. Aortopathy Is Prevalent in Relatives of Bicuspid Aortic Valve Patients. J. Am. Coll. Cardiol. 2009, 53, 2288–2295. [Google Scholar] [CrossRef]
- Fedak, P.W.M.; Verma, S.; David, T.E.; Leask, R.L.; Weisel, R.D.; Butany, J. Clinical and Pathophysiological Implications of a Bicuspid Aortic Valve. Circulation 2002, 106, 900–904. [Google Scholar] [CrossRef]
- Milan, A.; Tosello, F.; Naso, D.; Avenatti, E.; Leone, D.; Magnino, C.; Veglio, F. Ascending Aortic Dilatation, Arterial Stiffness and Cardiac Organ Damage in Essential Hypertension. J. Hypertens. 2013, 31, 109–116. [Google Scholar] [CrossRef]
- Guala, A.; Rodriguez-Palomares, J.; Dux-Santoy, L.; Teixido-Tura, G.; Maldonado, G.; Galian, L.; Huguet, M.; Valente, F.; Gutiérrez, L.; González-Alujas, T.; et al. Influence of Aortic Dilation on the Regional Aortic Stiffness of Bicuspid Aortic Valve Assessed by 4-Dimensional Flow Cardiac Magnetic Resonance: Comparison with Marfan Syndrome and Degenerative Aortic Aneurysm. JACC Cardiovasc. Imaging 2019, 12, 1020–1029. [Google Scholar] [CrossRef]
- Martínez-Micaelo, N.; Beltrán-Debón, R.; Baiges, I.; Faiges, M.; Alegret, J.M. Specific Circulating MicroRNA Signature of Bicuspid Aortic Valve Disease. J. Transl. Med. 2017, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Micaelo, N.; Beltrán-Debón, R.; Aragonés, G.; Faiges, M.; Alegret, J.M. MicroRNAs Clustered within the 14q32 Locus Are Associated with Endothelial Damage and Microparticle Secretion in Bicuspid Aortic Valve Disease. Front. Physiol. 2017, 8, 648. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Dentamaro, I.; Galian, L.; Calvo, F.; Alegret, J.M.; Sanchez, V.; Citro, R.; Moreo, A.; Chirillo, F.; Colonna, P.; et al. Predictors of Ascending Aorta Enlargement and Valvular Dysfunction Progression in Patients with Bicuspid Aortic Valve. J. Clin. Med. 2021, 10, 5264. [Google Scholar] [CrossRef]
- Billaud, M.; Phillippi, J.A.; Kotlarczyk, M.P.; Hill, J.C.; Ellis, B.W.; St Croix, C.M.; Cantu-Medéllin, N.; Kelley, E.E.; Gleason, T.G. Elevated Oxidative Stress in the Aortic Media of Patients with Bicuspid Aortic Valve. J. Thorac. Cardiovasc. Surg. 2017, 154, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Poggio, P.; Songia, P.; Moschetta, D.; Valerio, V.; Myasoedova, V.; Perrucci, G.L.; Pompilio, G. MiRNA Profiling Revealed Enhanced Susceptibility to Oxidative Stress of Endothelial Cells from Bicuspid Aortic Valve. J. Mol. Cell. Cardiol. 2019, 131, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Antequera-González, B.; Martínez-Micaelo, N.; Alegret, J.M. Bicuspid Aortic Valve and Endothelial Dysfunction: Current Evidence and Potential Therapeutic Targets. Front. Physiol. 2020, 11, 1015. [Google Scholar] [CrossRef]
- Martínez-Micaelo, N.; Ligero, C.; Antequera-González, B.; Junza, A.; Yanes, O.; Alegret, J.M. Plasma Metabolomic Profiling Associates Bicuspid Aortic Valve Disease and Ascending Aortic Dilation with a Decrease in Antioxidant Capacity. J. Clin. Med. 2020, 9, 2215. [Google Scholar] [CrossRef]
- Wild, J.B.; Stather, P.W.; Sylvius, N.; Choke, E.; Sayers, R.D.; Bown, M.J. Low Density Lipoprotein Receptor Related Protein 1 and Abdominal Aortic Aneurysms. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2012, 44, 127–132. [Google Scholar] [CrossRef]
- Kubota, Y.; Folsom, A.R.; Ballantyne, C.M.; Tang, W. Lipoprotein(a) and Abdominal Aortic Aneurysm Risk: The Atherosclerosis Risk in Communities Study. Atherosclerosis 2018, 268, 63–67. [Google Scholar] [CrossRef]
- Alegret, J.M.; Masana, L.; Martinez-Micaelo, N.; Heras, M.; Beltrán-Debón, R. LDL Cholesterol and Apolipoprotein B Are Associated with Ascending Aorta Dilatation in Bicuspid Aortic Valve Patients. QJM 2015, 108, 795–801. [Google Scholar] [CrossRef]
- Obel, L.M.; Diederichsen, A.C.; Steffensen, F.H.; Frost, L.; Lambrechtsen, J.; Busk, M.; Urbonaviciene, G.; Egstrup, K.; Karon, M.; Rasmussen, L.M.; et al. Population-Based Risk Factors for Ascending, Arch, Descending, and Abdominal Aortic Dilations for 60–74-Year-Old Individuals. J. Am. Coll. Cardiol. 2021, 78, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Mallol, R.; Amigó, N.; Rodríguez, M.A.; Heras, M.; Vinaixa, M.; Plana, N.; Rock, E.; Ribalta, J.; Yanes, O.; Masana, L.; et al. Liposcale: A Novel Advanced Lipoprotein Test Based on 2D Diffusion-Ordered 1H NMR Spectroscopy. J. Lipid Res. 2015, 56, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Fuertes-Martín, R.; Taverner, D.; Vallvé, J.-C.; Paredes, S.; Masana, L.; Correig Blanchar, X.; Amigó Grau, N. Characterization of 1H NMR Plasma Glycoproteins as a New Strategy To Identify Inflammatory Patterns in Rheumatoid Arthritis. J. Proteome Res. 2018, 17, 3730–3739. [Google Scholar] [CrossRef] [PubMed]
- Jeyarajah, E.J.; Cromwell, W.C.; Otvos, J.D. Lipoprotein Particle Analysis by Nuclear Magnetic Resonance Spectroscopy. Clin. Lab. Med. 2006, 26, 847–870. [Google Scholar] [CrossRef] [PubMed]
- Fuertes-Martín, R.; Moncayo, S.; Insenser, M.; Martínez-García, M.Á.; Luque-Ramírez, M.; Grau, N.A.; Blanchar, X.C.; Escobar-Morreale, H.F. Glycoprotein A and B Height-to-Width Ratios as Obesity-Independent Novel Biomarkers of Low-Grade Chronic Inflammation in Women with Polycystic Ovary Syndrome (PCOS). J. Proteome Res. 2019, 18, 4038–4045. [Google Scholar] [CrossRef] [PubMed]
- Fuertes-Martín, R.; Correig, X.; Vallvé, J.-C.; Amigó, N. Title: Human Serum/Plasma Glycoprotein Analysis by 1H-NMR, an Emerging Method of Inflammatory Assessment. J. Clin. Med. 2020, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Gittenberger-de Groot, A.C.; von der Thusen, J.; Wisse, L.J.; Bartelings, M.M.; DeRuiter, M.C.; Klautz, R.J.M.; Poelmann, R.E. The Development of the Ascending Aortic Wall in Tricuspid and Bicuspid Aortic Valve: A Process from Maturation to Degeneration. J. Clin. Med. 2020, 9, 908. [Google Scholar] [CrossRef]
- Rodríguez-Palomares, J.F.; Dux-Santoy, L.; Guala, A.; Kale, R.; Maldonado, G.; Teixidó-Turà, G.; Galian, L.; Huguet, M.; Valente, F.; Gutiérrez, L.; et al. Aortic Flow Patterns and Wall Shear Stress Maps by 4D-Flow Cardiovascular Magnetic Resonance in the Assessment of Aortic Dilatation in Bicuspid Aortic Valve Disease. J. Cardiovasc. Magn. Reson. 2018, 20, 28. [Google Scholar] [CrossRef]
- Andreassi, M.G.; Della Corte, A. Genetics of Bicuspid Aortic Valve Aortopathy. Curr. Opin. Cardiol. 2016, 31, 585–592. [Google Scholar] [CrossRef]
- Castañer, O.; Pintó, X.; Subirana, I.; Amor, A.J.; Ros, E.; Hernáez, Á.; Martínez-González, M.Á.; Corella, D.; Salas-Salvadó, J.; Estruch, R.; et al. Remnant Cholesterol, Not LDL Cholesterol, Is Associated With Incident Cardiovascular Disease. J. Am. Coll. Cardiol. 2020, 76, 2712–2724. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, A.B.; Frikke-Schmidt, R.; West, A.S.; Grande, P.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Genetically Elevated Non-Fasting Triglycerides and Calculated Remnant Cholesterol as Causal Risk Factors for Myocardial Infarction. Eur. Heart J. 2013, 34, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Triglycerides, Remnant Cholesterol and Atherosclerotic Cardiovascular Disease. Available online: https://www.acc.org/latest-in-cardiology/articles/2019/02/07/09/47/http%3a%2f%2fwww.acc.org%2flatest-in-cardiology%2farticles%2f2019%2f02%2f07%2f09%2f47%2ftriglycerides-remnant-cholesterol-and-atherosclerotic-cv-disease (accessed on 30 September 2021).
- Miller, Y.I.; Choi, S.-H.; Fang, L.; Tsimikas, S. Lipoprotein Modification and Macrophage Uptake: Role of Pathologic Cholesterol Transport in Atherogenesis. In Cholesterol Binding and Cholesterol Transport Proteins: Structure and Function in Health and Disease; Harris, J.R., Ed.; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2010; pp. 229–251. ISBN 978-90-481-8622-8. [Google Scholar]
- Raposeiras-Roubin, S.; Rosselló, X.; Oliva, B.; Fernández-Friera, L.; Mendiguren, J.M.; Andrés, V.; Bueno, H.; Sanz, J.; Martínez de Vega, V.; Abu-Assi, E.; et al. Triglycerides and Residual Atherosclerotic Risk. J. Am. Coll. Cardiol. 2021, 77, 3031–3041. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.C.; Holmes, M.V.; Burgess, S.; Asselbergs, F.W.; Jones, G.T.; Baas, A.F.; van ’t Hof, F.N.; de Bakker, P.I.W.; Blankensteijn, J.D.; Powell, J.T.; et al. Genetic Association of Lipids and Lipid Drug Targets With Abdominal Aortic Aneurysm: A Meta-Analysis. JAMA Cardiol. 2018, 3, 26–33. [Google Scholar] [CrossRef]
- Carr, S.S.; Hooper, A.J.; Sullivan, D.R.; Burnett, J.R. Non-HDL-Cholesterol and Apolipoprotein B Compared with LDL-Cholesterol in Atherosclerotic Cardiovascular Disease Risk Assessment. Pathology 2019, 51, 148–154. [Google Scholar] [CrossRef]
- Johannesen, C.D.L.; Mortensen, M.B.; Langsted, A.; Nordestgaard, B.G. Apolipoprotein B and Non-HDL Cholesterol Better Reflect Residual Risk Than LDL Cholesterol in Statin-Treated Patients. J. Am. Coll. Cardiol. 2021, 77, 1439–1450. [Google Scholar] [CrossRef]
- Aggarwal, D.J.; Kathariya, M.G.; Verma, D.P.K. LDL-C, NON-HDL-C and APO-B for Cardiovascular Risk Assessment: Looking for the Ideal Marker. Indian Heart J. 2021, 73, 544–548. [Google Scholar] [CrossRef]
- Mora, S.; Otvos, J.D.; Rifai, N.; Rosenson, R.S.; Buring, J.E.; Ridker, P.M. Lipoprotein Particle Profiles by Nuclear Magnetic Resonance Compared With Standard Lipids and Apolipoproteins in Predicting Incident Cardiovascular Disease in Women. Circulation 2009, 119, 931–939. [Google Scholar] [CrossRef]
| NonDIL (n: 83) | DIL (n: 69) | ||
|---|---|---|---|
| Mean (SD) or n (%) | Mean (SD) or n (%) | p Value | |
| Sex (male %) | 61 (73.5%) | 54 (78.3%) | 0.430 *** |
| Age (years) | 41.7 (14.6) | 54.8 (17.3) | <0.001 *** |
| Body mass index (kg/m2) | 24.60 (4.01) | 27.53 (4.23) | <0.001 *** |
| Smoking (%) | 34 (41.0%) | 26 (37.7%) | 0.792 |
| NYHA Scale (≥II) | 1 (1.2%) | 2 (2.9%) | 0.887 |
| Hypertension | 19 (22.9%) | 36 (52.2%) | 0.001 ** |
| Type 2 diabetes | 3 (3.6%) | 10 (14.5%) | 0.024* |
| Peripheral artery disease | 2 (2.4%) | 3 (4.3%) | 0.517 |
| Stroke | 1 (1.2%) | 2 (2.9%) | 0.465 |
| Coronary artery disease | 1 (1.2%) | 3 (4.3%) | 0.231 |
| Aortic regurgitation (≥2) | 34 (41.0%) | 30 (43.5%) | 0.986 |
| LVEDD (mm) | 51.6 (6.1) | 52 (5.8) | 0.692 |
| LVESD (mm) | 31.2 (7.2) | 33.2 (6.3) | 0.078 |
| Aortic root (mm) | 33.9 (5.1) | 39.9 (5.7) | <0.001 *** |
| Ascending aorta (mm) | 33.7 (4.1) | 45.4 (4.6) | <0.001 *** |
| AV gradient (mean) (mmHg) | 13.1 (13.5) | 22 (19) | 0.001 ** |
| Statins | 9 (10.8%) | 10 (14.5%) | 0.501 |
| BAV morphology (typical) | 47 (56.6%) | 45 (65.2%) | 0.243 |
| NonDIL (n:83) | DIL (n:69) | ||
|---|---|---|---|
| Mean (SD) | Mean (SD) | p Value | |
| VLDL-C (mg/dL) | 12.08 (8.96) | 15.48 (10.39) | 0.032 * |
| IDL-C (mg/dL) | 7.76 (4.20) | 10.16 (4.35) | 0.001 ** |
| LDL-C (mg/dL) | 125.17 (30.14) | 135.8 (24.69) | 0.02 * |
| HDL-C (mg/dL) | 55.08 (10.35) | 54.37 (9.06) | 0.657 |
| VLDL-TG (mg/dL) | 59.48 (50.63) | 76.72 (55.01) | 0.046 * |
| IDL-TG (mg/dL) | 8.44 (3.02) | 10.07 (2.90) | >0.001 *** |
| LDL-TG (mg/dL) | 13.55 (4.48) | 15.12 (4.52) | 0.033 * |
| HDL-TG (mg/dL) | 9.61 (3.70) | 10.84 (3.86) | 0.046 * |
| VLDL-P (nmol/L) | 41.33 (30.35) | 53.36 (35.37) | 0.026 * |
| Large VLDL-P (nmol/L) | 1.07 (0.75) | 1.28 (0.74) | 0.074 |
| Medium VLDL-P (nmol/L) | 5.08 (5.97) | 6.36 (5.66) | 0.180 |
| Small VLDL-P (nmol/L) | 35.18 (24.25) | 45.71 (29.45) | 0.017 * |
| LDL-P (nmol/L) | 1223.88 (291.67) | 1348.36 (253.78) | 0.006 ** |
| Large LDL-P (nmol/L) | 198.54 (37.91) | 208 (33.88) | 0.110 |
| Medium LDL-P (nmol/L) | 370.51 (139.25) | 406.07 (111.42) | 0.089 |
| Small LDL-P (nmol/L) | 654.83 (142.33) | 734.29 (154.03) | 0.001 ** |
| HDL-P (µmol/L) | 26.26 (5.01) | 26.59 (3.87) | 0.649 |
| Large HDL-P (µmol/L) | 0.26 (0.04) | 0.27 (0.04) | 0.140 |
| Medium HDL-P (µmol/L) | 9.44 (1.74) | 9.35 (1.78) | 0.754 |
| Small HDL-P (µmol/L) | 16.55 (3.85) | 16.97 (2.78) | 0.455 |
| VLDL-Z (nm) | 42.3 (0.24) | 42.24 (0.17) | 0.080 |
| LDL-Z (nm) | 21.13 (0.24) | 21.07 (0.25) | 0.110 |
| HDL-Z (nm) | 8.28 (0.08) | 8.26 (0.06) | 0.173 |
| TOTAL-C (mg/dL) | 200.1 (35.32) | 215.81 (33.76) | 0.006 ** |
| TOTAL-TGs (mg/dL) | 91.08 (55.95) | 112.75 (60.14) | 0.023 * |
| VLDL-TG/VLDL-C | 5.38 (1.84) | 5.22 (1.15) | 0.526 |
| IDL-TG/IDL-C | 1.23 (0.40) | 1.08 (0.27) | 0.006 ** |
| LDL-TG/LDL-C | 0.11 (0.03) | 0.11 (0.02) | 0.671 |
| HDL-TG/HDL-C | 0.18 (0.07) | 0.20 (0.07) | 0.024 * |
| remCholesterol (mg/dL) | 19.84 (11.91) | 25.64 (13.15) | 0.005 ** |
| NonDIL (n:83) | DIL (n:69) | ||
|---|---|---|---|
| Mean (SD) | Mean (SD) | p Value | |
| GlycB (µmol/L) | 364.99 (52.77) | 371.58 (55.55) | 0.455 0.123 0.031 * 0.470 0.068 |
| GlycF (µmol/L) | 236.35 (78.75) | 257.74 (91.11) | |
| GlycA (µmol/L) | 723.67 (154.61) | 789.09 (214.47) | |
| H/W GlycB | 4.59 (0.67) | 4.67 (0.70) | |
| H/W GlycA | 16.87 (2.77) | 17.74 (3.08) |
| 95% CI of OR | ||||
|---|---|---|---|---|
| OR | Inf. | Sup. | p Value | |
| Age (year) | 1.041 | 1.017 | 1.065 | 0.001 ** |
| Remnant cholesterol (by mg/dL) | 1.042 | 1.007 | 1.079 | 0.019 * |
| Ascending Aorta Dilation Velocity (mm/Year) | ||
|---|---|---|
| Pearson/Spearman Correlation | p Value | |
| Sex (male %) | 0.044 | 0.622 |
| Age (years) | 0.062 | 0.491 |
| Body mass index (kg/m2) | 0.008 | 0.933 |
| Smoking (%) | 0.024 | 0.804 |
| NYHA scale (≥II) | −0.004 | 0.974 |
| Hypertension | −0.036 | 0.699 |
| Type 2 diabetes | −0.155 | 0.166 |
| Peripheral artery disease | 0.103 | 0.367 |
| Stroke | 0.103 | 0.368 |
| Coronary artery disease | −0.077 | 0.504 |
| Aortic regurgitation (≥II) | 0.071 | 0.537 |
| LVEDD (mm) | 0.207 | 0.060 |
| LVESD (mm) | 0.121 | 0.290 |
| Aortic root (mm) | −0.146 | 0.106 |
| Ascending aorta (mm) | −0.369 ** | <0.001 |
| AV gradient (mean) (mmHg) | −0.027 | 0.765 |
| Statins | −0.057 | 0.538 |
| BAV morphology (typical) | −0.025 | 0.835 |
| Ascending Aorta Dilation Velocity (mm/Year) | ||
|---|---|---|
| Pearson/Spearman Correlation | p Value | |
| TOTAL-C (mg/dL) | 0.166 | 0.071 |
| TOTAL-TG (mg/dL) | 0.095 | 0.302 |
| TG/C-VLDL | 0.035 | 0.705 |
| TG/C-IDL | −0.182 * | 0.047 |
| TG/C-LDL | 0.107 | 0.248 |
| TG/C-HDL | 0.031 | 0.740 |
| Small VLDL % | 0.156 | 0.090 |
| Small LDL % | 0.022 | 0.812 |
| Small HDL % | 0.142 | 0.123 |
| VLDL-C (mg/dL) | 0.088 | 0.341 |
| IDL-C (mg/dL) | 0.184 * | 0.045 |
| LDL-C (mg/dL) | 0.199 * | 0.030 |
| HDL-C (mg/dL) | −0.131 | 0.155 |
| VLDL-TG (mg/dL) | 0.077 | 0.403 |
| IDL-TG (mg/dL) | 0.165 | 0.073 |
| LDL-TG (mg/dL) | 0.216 * | 0.018 |
| HDL-TG (mg/dL) | −0.026 | 0.776 |
| VLDL-P (nmol/L) | 0.092 | 0.321 |
| Large VLDL-P (nmol/L) | 0.072 | 0.436 |
| Medium VLDL-P (nmol/L) | 0.038 | 0.685 |
| Small VLDL-P (nmol/L) | 0.101 | 0.274 |
| LDL-P (nmol/L) | 0.269 ** | 0.003 |
| Large LDL-P (nmol/L) | 0.216 * | 0.018 |
| Medium LDL-P (nmol/L) | 0.226 * | 0.014 |
| Small LDL-P (nmol/L) | 0.283 ** | 0.002 |
| HDL-P (μmol/L) | −0.091 | 0.327 |
| Large HDL-P (μmol/L) | −0.053 | 0.565 |
| Medium HDL-P (μmol/L) | −0.161 | 0.081 |
| Small HDL-P (μmol/L) | −0.027 | 0.770 |
| VLDL-Z (nm) | −0.176 | 0.055 |
| LDL-Z (nm) | −0.101 | 0.272 |
| HDL-Z (nm) | −0.134 | 0.145 |
| Non-HDL-P (nmol/L) remCholesterol (mg/dL) | 0.270 ** 0.080 | 0.003 0.351 |
| Ascending Aorta Dilation Velocity (mm/Year) | ||
|---|---|---|
| Pearson/Spearman Correlation | p Value | |
| Glyc-B (μmol/L) | 0.182 * | 0.048 |
| Glyc-F (μmol/L) | 0.091 | 0.326 |
| Glyc-A (μmol/L) | 0.135 | 0.144 |
| H/W Glyc-B | 0.189 * | 0.039 |
| H/W Glyc-A | 0.146 | 0.113 |
| 95% C.I | ||||
|---|---|---|---|---|
| ß | Inf. | Sup. | p Value | |
| AAo (by mm) | −0.356 | −0.073 | −0.027 | <0.001 *** |
| Non-HDL-P (by nmol/L) | 0.285 | <0.001 | 0.001 | 0.001 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antequera-González, B.; Faiges, M.; Martínez-Micaelo, N.; Galian-Gay, L.; Ligero, C.; Ferré-Vallverdú, M.; Masana, L.; Amigó, N.; Evangelista, A.; Alegret, J.M. Glycoprotein and Lipoprotein Profiles Assessed by 1H-NMR and Its Relation to Ascending Aortic Dilatation in Bicuspid Aortic Valve Disease. J. Clin. Med. 2023, 12, 332. https://doi.org/10.3390/jcm12010332
Antequera-González B, Faiges M, Martínez-Micaelo N, Galian-Gay L, Ligero C, Ferré-Vallverdú M, Masana L, Amigó N, Evangelista A, Alegret JM. Glycoprotein and Lipoprotein Profiles Assessed by 1H-NMR and Its Relation to Ascending Aortic Dilatation in Bicuspid Aortic Valve Disease. Journal of Clinical Medicine. 2023; 12(1):332. https://doi.org/10.3390/jcm12010332
Chicago/Turabian StyleAntequera-González, Borja, Marta Faiges, Neus Martínez-Micaelo, Laura Galian-Gay, Carmen Ligero, María Ferré-Vallverdú, Lluís Masana, Núria Amigó, Arturo Evangelista, and Josep M. Alegret. 2023. "Glycoprotein and Lipoprotein Profiles Assessed by 1H-NMR and Its Relation to Ascending Aortic Dilatation in Bicuspid Aortic Valve Disease" Journal of Clinical Medicine 12, no. 1: 332. https://doi.org/10.3390/jcm12010332
APA StyleAntequera-González, B., Faiges, M., Martínez-Micaelo, N., Galian-Gay, L., Ligero, C., Ferré-Vallverdú, M., Masana, L., Amigó, N., Evangelista, A., & Alegret, J. M. (2023). Glycoprotein and Lipoprotein Profiles Assessed by 1H-NMR and Its Relation to Ascending Aortic Dilatation in Bicuspid Aortic Valve Disease. Journal of Clinical Medicine, 12(1), 332. https://doi.org/10.3390/jcm12010332






