Clinical Research Evidence Supporting Administration and Dosing Recommendations of Medicinal Cannabis as Analgesic in Cancer Patients
Abstract
1. Introduction
2. The Endocannabinoid System and Its Implication in Cancer Pain
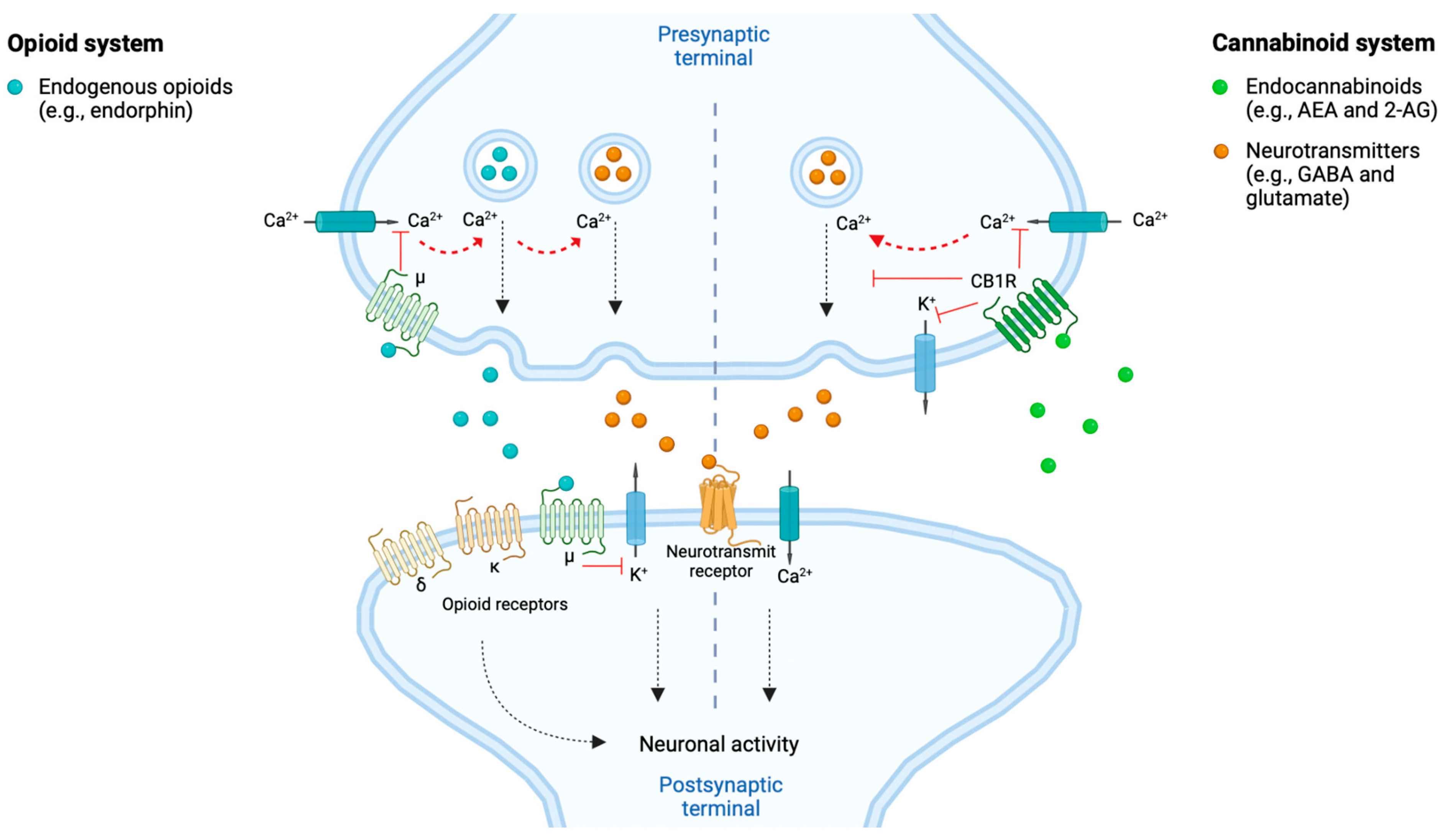
3. Cannabis sativa L. Compounds Possessing Analgesic Effects
4. Existing Clinical Research Evidence
4.1. Clinical Studies with Medicinal Cannabis (Full-Spectrum)
4.2. Clinical Studies of the Cannabis-Based Medication Sativex®
4.3. Administration and Dosing Guidance
4.4. Safety Profile and Opioid-Sparing Effects of Cannabinoids
5. Challenges and Barriers in Medicinal Cannabis Research
5.1. Regulatory Barriers
5.2. Several Methodological Challenges
5.2.1. Standardization of Materials
5.2.2. Placebo-Control
5.2.3. Clinical Data Evaluation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Schleider et al., 2018 [53] Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
| Aviram et al., 2020 [55] Short-term medical cannabis treatment regimens produced beneficial effects among palliative cancer patients | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
| Pawasarat et al., 2020 [56] The Efficacy of Medical Marijuana in the Treatment of Cancer-Related Pain | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
| Zarrabi et al., 2020 [57] Perception of Benefits and Harms of Medical Cannabis among Seriously Ill Patients in an Outpatient Palliative Care Practice | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
| Zylla et al., 2021 [52] A randomized trial of medical cannabis in patients with stage IV cancers to assess feasibility, dose requirements, impact on pain and opioid use, safety, and overall patient satisfaction | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
| Meghani et al., 2021 [61] Impact of Cannabis Use on Least Pain Scores Among African American and White Patients with Cancer Pain: A Moderation Analysis | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
| Schleider et al., 2022 [54] Adherence, Safety, and Effectiveness of Medical Cannabis and Epidemiological Characteristics of the Patient Population: A Prospective Study | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
| Aviram et al., 2022 [59] The Effectiveness and Safety of Medical Cannabis for Treating Cancer Related Symptoms in Oncology Patients | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
| Nimalan et al., 2022 [60] UK Medical Cannabis Registry palliative care patients cohort: initial experience and outcomes | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
| Sura et al., 2022 [58] Experience With Medical Marijuana for Cancer Patients in the Palliative Setting | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
Appendix B
| Johnson et al., 2010 [41] Multicenter, double-blind, randomized, placebo-controlled, parallel group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
| Portenoy et al., 2012 [63] Nabiximols for opioid-treated cancer patients with poorly controlled chronic pain: A randomized, placebo-controlled, graded-doses trial | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
| Johnson et al., 2013 [62] An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
| Lynch et al., 2014 [66] A double-blind, placebo-controlled, crossover pilot trial with extension using an oral mucosal cannabinoid extract for treatment of chemotherapy-induced neuropathic pain | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
| Fallon et al., 2017 (study 1) [64] Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: two double-blind, randomized, placebo-controlled phase 3 studies | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
| Fallon et al., 2017 (study 2) [64] Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: two double-blind, randomized, placebo-controlled phase 3 studies | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
| Lichtman et al., 2018 [65] Results of a double-blind, randomized, placebo-controlled study of nabiximols oromucosal spray as an adjunctive therapy in advanced cancer patients with chronic uncontrolled pain | |
| Location |
|
| Aim of study |
|
| Study design |
|
| Drug, administration, and dosing |
|
| Primary outcomes in pain |
|
| Side effects/ adverse effects |
|
| Additional notes |
|
References
- Chung, M.; Kim, H.K.; Abdi, S. Update on Cannabis and Cannabinoids for Cancer Pain. Curr. Opin. Anaesthesiol. 2020, 33, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Cyr, C.; Davis, M.P.; Schecter, D.; Daeninck, P. Cannabis and Cannabinoid-Based Medicines in Cancer Care: A Comprehensive Guide to Medical Management; Springer Nature: Cham, Switzerland, 2022; pp. 2–145. [Google Scholar] [CrossRef]
- Kvamme, S.L.; Pedersen, M.M.; Alagem-Iversen, S.; Thylstrup, B. Beyond the high: Mapping patterns of use and motives for use of cannabis as medicine. Nord. Stud. Alcohol Drugs 2021, 38, 270–292. [Google Scholar] [CrossRef] [PubMed]
- Swift, W.; Gates, P.; Dillon, P. Survey of Australians using cannabis for medical purposes. Harm Reduct. J. 2005, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Hawley, P.; Gobbo, M.; Afghari, N. The impact of legalization of access to recreational Cannabis on Canadian medical users with Cancer. BMC Health Serv. Res. 2020, 20, 977. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Good Agricultural and Collection Practices (GACP) for Medicinal Plants. Available online: https://www.who.int/publications/i/item/9241546271 (accessed on 30 September 2022).
- European Commission. Eudralex—Volume 4—Good Manufacturing Practice (GMP) Guidelines; Annex 4 and 7. Available online: https://health.ec.europa.eu/medicinal-products/eudralex/eudralex-volume-4_en (accessed on 30 September 2022).
- Danish Medicines Agency (DKMA). Medicinal Cannabis Pilot Programme. Available online: https://laegemiddelstyrelsen.dk/en/special/medicinal-cannabis-/medicinal-cannabis-pilot-programme/ (accessed on 30 September 2022).
- FDA News. Health Canada Approves Sativex for Cancer Pain. Available online: https://www.fdanews.com/articles/96888-health-canada-approves-sativex-for-cancer-pain (accessed on 1 October 2022).
- Electronic Medicines Compendium (EMC). Sativex Oromucosal Spray. Available online: https://www.medicines.org.uk/emc/product/602/smpc#gref (accessed on 3 October 2022).
- The National Center for Advancing Translational Sciences (NCATS). Nabiximols. Available online: https://drugs.ncats.io/drug/K4H93P747O#publications (accessed on 3 October 2022).
- Danish Medicines Agency (DKMA). Rules Applicable to Active Substances (APIs). Available online: https://laegemiddelstyrelsen.dk/en/licensing/company-authorisations-and-registrations/registration-as-manufacturer,-importer-and-distributor-of-active-substances-apis/rules-for-active-substances/ (accessed on 3 October 2022).
- The National Academics of Sciences, Engineering and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. 2017. Available online: https://nap.nationalacademies.org/catalog/24625/the-health-effects-of-cannabis-and-cannabinoids-the-current-state (accessed on 24 November 2022).
- Nielsen, S.W.; Ruhlmann, C.H.; Eckhoff, L.; Brønnum, D.; Herrstedt, J. Cannabis use among Danish patients with cancer: A cross-sectional survey of sociodemographic traits, quality of life, and patient experiences. Support. Care Cancer 2022, 30, 1181–1190. [Google Scholar] [CrossRef]
- Raghunathan, N.J.; Brens, J.; Vemuri, S.; Li, Q.S.; Mao, J.J.; Korenstein, D. In the weeds: A retrospective study of patient interest in and experience with cannabis at a cancer center. Support. Care Cancer 2022, 30, 7491–7497. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, H.; Wang, Q. Prevalence of chronic pain and high-impact chronic pain in cancer survivors in the United States. JAMA Oncol. 2019, 5, 1224–1226. [Google Scholar] [CrossRef]
- Wiffen, P.J.; Wee, B.; Derry, S.; Bell, R.F.; Moore, R.A. Opioids for cancer pain—An overview of Cochrane reviews. Cochrane Database Syst. Rev. 2017, 7, CD012592. [Google Scholar] [CrossRef]
- Meng, H.; Dai, T.; Hanlon, J.G.; Downar, J.; Alibhai, A.M.H.; Clarke, H. Cannabis and cannabinoids in cancer pain management. Curr. Opin. Support. Palliat. Care 2020, 14, 87–93. [Google Scholar] [CrossRef]
- Brown, D.; Watson, M.; Schloss, J. Pharmacological evidence of medicinal cannabis in oncology: A systematic review. Support. Care Cancer 2019, 27, 3195–3207. [Google Scholar] [CrossRef]
- Alexander, S.P.H. Barriers to the wider adoption of medicinal Cannabis. British Journal of Pain. SAGE J. 2020, 14, 122–132. [Google Scholar] [CrossRef]
- McTaggart-Cowan, H.; Bentley, C.; Raymakers, A.; Metcalfe, R.; Hawley, P.; Peacock, S. Understanding cancer survivors’ reasons to medicate with cannabis: A qualitative study based on the theory of planned behavior. Cancer Med. 2020, 10, 396–404. [Google Scholar] [CrossRef]
- Buchwald, D.; Winter, K.A.I.; Brønnum, D.; Melgaard, D.; Leutscher, P. Perception of Patients with Cancer Enquiring About Adjuvant Therapy with Cannabis Medicine for Palliation of Symptoms: An Interview Study among Danish Health Care Professionals. Palliat. Med. Rep. 2022, 3, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, D.; Brønnum, D.; Melgaard, D.; Leutscher, P. Living with a Hope of Survival Is Challenged by a Lack of Clinical Evidence: An Interview Study among Cancer Patients Using Cannabis-Based Medicine. J. Palliat. Med. 2019, 23, 1090–1093. [Google Scholar] [CrossRef] [PubMed]
- Gorzo, A.; Havasi, A.; Spînu, S.; Oprea, A.; Burz, C.; Sur, D. Practical Considerations for the Use of Cannabis in Cancer Pain Management -What a Medical Oncologist Should Know. J. Clin. Med. 2022, 11, 5036. [Google Scholar] [CrossRef] [PubMed]
- Petrocellis, L.D.; Marzo, V.D. An introduction to the endocannabinoid system: From the early to the latest concepts. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 1–15. [Google Scholar] [CrossRef]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef]
- Salami, S.A.; Martinelli, F.; Giovino, A.; Bachari, A.; Arad, N.; Mantri, N. It Is Our Turn to Get Cannabis High: Put Cannabinoids in Food and Health Baskets. Molecules 2020, 25, 4036. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Maldonado, R.; Banos, J.E.; Cabanero, D. The endocannabinoid system and neuropathic pain. Pain 2016, 157, 23–32. [Google Scholar] [CrossRef]
- Maayah, Z.H.; Takahara, S.; Ferdaoussi, M.; Dyck, J.R.B. The molecular mechanisms that underpin the biological benefits of fullspectrum cannabis extract in the treatment of neuropathic pain and inflammation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165771. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Clinical Endocannabinoid Deficiency Reconsidered: Current Research Supports the Theory in Migraine, Fibromyalgia, Irritable Bowel, and Other Treatment-Resistant Syndromes. Cannabis Cannabinoid Res. 2016, 1, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.S.; Lourenco, D.M.; Paulo, S.L. Cannabinoid Actions on Neural Stem Cells: Implications for Pathophysiology. Molecules 2019, 24, 1350. [Google Scholar] [CrossRef]
- Stemmer, K.; Pfluger, P.T.; Tschop, M.H. CNS-targeting pharmacological interventions for the metabolic syndrome. J. Clin. Investig. 2019, 129, 4058–4071. [Google Scholar] [CrossRef] [PubMed]
- Nahtigal, I.; Blake, A.; Florentinus-Mefailoski, A.; Hashemi, H.; Friedberg, J. The pharmacological properties of Cannabis. J. Pain Manag. 2016, 9, 481–491. [Google Scholar]
- Nielsen, S.; Picco, L.; Murnion, B.; Winters, B.; Matheson, J.; Graham, M.; Campbell, G.; Parvaresh, L.; Khor, K.; Betz-Stablein, B.; et al. Opioid-sparing effect of cannabinoids for analgesia: An updated systematic review and meta-analysis of preclinical and clinical studies. Neuropsychopharmacology 2022, 47, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Hojo, M.; Sudo, Y.; Ando, Y.; Minami, K.; Takada, M.; Matsubara, T.; Taniyama, K.; Sumikawa, K.; Uezono, Y. mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: Electrophysiological and FRET assay analysis. J. Pharm. Sci. 2008, 108, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Babalonis, S.; Walsh, S.L. Therapeutic potential of opioid/cannabinoid combinations in humans: Review of the evidence. Eur. Neuropsychopharmacol. 2020, 36, 206–216. [Google Scholar] [CrossRef]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- McPartland, J.M.; Russo, E.B. Non-phytocannabinoid constituents of cannabis and herbal synergy. In Handbook of Cannabis; Oxford Univeristy Press: Oxford, UK, 2014; Chapter 15; pp. 280–295. [Google Scholar]
- Johnson, J.R.; Burnell-Nugent, M.; Lossignol, D.; Ganae-Motan, E.D.; Potts, R.; Fallon, M. Multicenter, double-blind, randomized, placebo-controlled, parallel group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J. Pain Symptom Manag. 2010, 39, 167–179. [Google Scholar] [CrossRef]
- Pertwee, R.G. GPR55: A new member of the cannabinoid receptor clan? Br. J. Pharmacol. 2007, 152, 984–986. [Google Scholar] [CrossRef] [PubMed]
- Grotsch, K.; Fokin, V.V. Between Science and Big Business: Tapping Mary Jane’s Uncharted Potential. ACS Cent. Sci. 2022, 8, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Christino, L.; Bisogno, T.; Marzo, V.D. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2020, 16, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain”, No Gain. Front. Plant Sci. 2018, 9, 969. [Google Scholar] [CrossRef]
- Worth, T. Unpicking the entourage effect. Nature 2019, 527, 12–13. [Google Scholar] [CrossRef]
- McPartland, J.M.; Russo, E.B. Non-phytocannabinoid constituents of cannabis and herbal synergy. In Handbook of Cannabis; Oxford Univeristy Press: Oxford, UK, 2014; Section 15.3; pp. 283–284. [Google Scholar]
- McPartland, J.M.; Russo, E.B. Non-phytocannabinoid constituents of cannabis and herbal synergy. In Handbook of Cannabis; Oxford Univeristy Press: Oxford, UK, 2014; Section 15.5; pp. 286–288. [Google Scholar]
- Andre, C.M.; Hausman, J.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Russo, E.; Guy, G.W. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med. Hypotheses 2006, 66, 234–246. [Google Scholar] [CrossRef]
- Zylla, D.M.; Eklund, J.; Gilmore, G.; Gavenda, A.; Guggisberg, J.; VazquezBenitez, G.; Pawloski, P.A.; Arneson, T.; Richter, S.; Birnbaum, A.K.; et al. A randomized trial of medical cannabis in patients with stage IV cancers to assess feasibility, dose requirements, impact on pain and opioid use, safety, and overall patient satisfaction. Support. Care Cancer 2021, 29, 7471–7478. [Google Scholar] [CrossRef]
- Schleider, L.B.; Mechoulam, R.; Lederman, V.; Hilou, M.; Lencovsky, O.; Betzalel, O.; Shbiro, L.; Novack, V. Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur. J. Intern. Med. 2018, 49, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Schleider, L.B.; Mechoulam, R.; Sikorin, I.; Naftali, T.; Novack, V. Adherence, Safety, and Effectiveness of Medical Cannabis and Epidemiological Characteristics of the Patient Population: A Prospective Study. Front. Med. 2022, 9, 96. [Google Scholar] [CrossRef]
- Aviram, J.; Lewitus, G.M.; Vysotski, Y.; Uribayev, A.; Procaccia, S.; Cohen, I.; Leibovici, A.; Abo-Amna, M.; Akria, L.; Goncharov, D.; et al. Short-Term Medical Cannabis Treatment Regimens Produced Beneficial Effects among Palliative Cancer Patients. Pharmaceuticals 2020, 13, 435. [Google Scholar] [CrossRef] [PubMed]
- Pawasarat, I.M.; Schultz, E.M.; Frisby, J.C.; Mehta, S.; Angelo, M.A.; Hardy, S.S.; Kim, T.W.B. The Efficacy of Medical Marijuana in the Treatment of Cancer-Related Pain. J. Palliat. Med. 2020, 23, 809–816. [Google Scholar] [CrossRef]
- Zarrabi, A.J.; Welsh, J.W.; Sniecinski, R.; Curseen, K.; Gillespie, T.; Baer, W.; McKenzie-Brown, A.M.; Singh, V. Perception of Benefits and Harms of Medical Cannabis among Seriously Ill Patients in an Outpatient Palliative Care Practice. J. Palliat. Med. 2020, 23, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Sura, K.T.; Kohman, L.; Huang, D.; Pasniciuc, S.V. Experience With Medical Marijuana for Cancer Patients in the Palliative Setting. Cureus 2022, 14, e26406. [Google Scholar] [CrossRef] [PubMed]
- Avriam, J.; Lewitus, G.M.; Vysotski, Y.; Amna, M.A.; Ouryvaev, A.; Procaccia, S.; Cohen, I.; Leibovici, A.; Akria, L.; Goncharov, D.; et al. The Effectiveness and Safety of Medical Cannabis for Treating Cancer Related Symptoms in Oncology Patients. Front. Pain Res. 2022, 3, 861037. [Google Scholar] [CrossRef]
- Nimalan, D.; Kawka, M.; Erridge, S.; Ergisi, M.; Harris, M.; Salazar, O.; Ali, R.; Loupasaki, K.; Holvey, C.; Coomber, R.; et al. UK Medical Cannabis Registry palliative care patients cohort: Initial experience and outcomes. Letter to the editor. J. Cannabis Res. 2022, 4, 3. [Google Scholar] [CrossRef]
- Meghani, S.H.; Quinn, R.; Ashare, R.; Levoy, K.; Worster, B.; Baylor, M.; Chittams, J.; Cheatle, M. Impact of Cannabis Use on Least Pain Scores Among African American and White Patients with Cancer Pain: A Moderation Analysis. J. Pain Res. 2021, 14, 3493–3502. [Google Scholar] [CrossRef]
- Johnson, J.J.; Lossignol, D.; Burnell-Nugent, M.; Fallon, M.T. An Open-Label Extension Study to Investigate the Long-Term Safety and Tolerability of THC/CBD Oromucosal Spray and Oromucosal THC Spray in Patients With Terminal Cancer-Related Pain Refractory to Strong Opioid Analgesics. J. Pain Symptom Manag. 2013, 46, 207–218. [Google Scholar] [CrossRef]
- Portenoy, R.K.; Ganae-Motan, E.D.; Allende, S.; Yanagihara, R.; Shaiova, L.; Weinstein, S.; McQuade, R.; Wright, S.; Fallon, M.T. Nabiximols for Opioid-Treated Cancer Patients With Poorly-Controlled Chronic Pain: A Randomized, Placebo-Controlled, Graded-Dose Trial. J. Pain 2012, 13, 438–499. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.T.; Lux, E.A.; McQuade, R.; Rossetti, S.; Sanchez, R.; Sun, W.; Wright, S.; Lichtman, A.; Kornyeyeva, E. Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: Two double-blind, randomized, placebo-controlled phase 3 studies. Br. Pain Soc. 2017, 11, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, A.H.; Lux, E.A.; McQuade, R.; Rossetti, S.; Sanchez, R.; Sun, W.; Wright, S.; Kornyeyeva, E.; Fallon, M.T. Results of a Double-Blind, Randomized, Placebo-Controlled Study of Nabiximols Oromucosal Spray as an Adjunctive Therapy in Advanced Cancer Patients with Chronic Uncontrolled Pain. J. Pain Symptom Manag. 2018, 55, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.E.; Cesar-Rittenberg, P.; Hohmann, A.G. A Double-Blind, Placebo-Controlled, Crossover Pilot Trial With Extension Using an Oral Mucosal Cannabinoid Extract for Treatment of Chemotherapy-Induced Neuropathic Pain. J. Pain Symptom Manag. 2014, 47, 166–173. [Google Scholar] [CrossRef]
- Boland, E.G.; Bennett, M.I.; Allgar, V.; Boland, J.W. Cannabinoids for adult cancer-related pain: Systematic review and meta-analysis. BMJ Support. Palliat. Care 2020, 10, 14–24. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Tateo, S. State of the evidence: Cannabinoids and cancer pain -A systematic review. J. Am. Assoc. Nurse Pract. 2017, 29, 94–103. [Google Scholar] [CrossRef]
- Hauser, W.; Welsch, P.; Klose, P.; Radbruch, L.; Fitzcharles, A. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain. Der Schmerz 2019, 33, 424–436. [Google Scholar] [CrossRef]
- Blake, A.; Wan, B.A.; Malek, L.; DeAngelis, C.; Diaz, P.; Lao, N.; Chow, E.; O´Hearn, S. A selective review of medical cannabis in cancer pain management. Ann. Palliat. Med. 2017, 6, 215–222. [Google Scholar] [CrossRef]
- Bhaskar, A.; Bell, A.; Boivin, M.; Briques, W.; Brown, M.; Clarke, H.; Cyr, C.; Eisenberg, E.; de Oliveira Silva, R.F.; Frohlich, E.; et al. Consensus recommendations on dosing and administration of medical cannabis to treat chronic pain: Results of a modified Delphi process. J. Cannabis Res. 2021, 3, 22. [Google Scholar] [CrossRef]
- MacCallum, C.A.; Russo, E.B. Practical considerations in medical cannabis administration and dosing. Eur. J. Intern. Med. 2018, 49, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Byars, T.; Theisen, E.; Bolton, D.L. Using Cannabis to Treat Cancer-Related Pain. In Seminars in Oncology Nursing; WB Saunders: Philadelphia, PA, USA, 2019; Volume 35, pp. 300–309. [Google Scholar] [CrossRef]
- Herkenham, M.; Lynn, A.B.; Little, M.D.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R.; Rice, K.C. Cannabinoid receptors localization in brain. Proc. Natl. Acad. Sci. USA 1990, 87, 1932–1936. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Miroshnychenko, A.; Shergill, Y.; Ashoorion, V.; Rehman, Y.; Couban, R.J.; Buckley, D.N.; Thabane, L.; Bhandari, M.; Guyatt, G.H.; et al. Opioid-sparing effects of medical cannabis or cannabinoids for chronic pain: A systematic review and meta-analysis of randomised and observational studies. BMJ Open. 2021, 11, e047717. [Google Scholar] [CrossRef] [PubMed]
- Bachhuber, M.A.; Saloner, B.; Cunningham, C.O.; Barry, C.L. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern. Med. 2014, 174, 1668–1673. [Google Scholar] [CrossRef]
- Pritchett, C.E.; Flynn, H.; Wang, Y.; Polston, J.E. Medical Cannabis Patients Report Improvements in Health Functioning and Reductions in Opiate Use. Subst. Use Misuse 2022, 57, 1883–1892. [Google Scholar] [CrossRef]
- Bolognini, D.; Ross, R.A. Medical Cannabis vs. Synthetic Cannabinoids: What Does the Future Hold? Am. Soc. Clin. Pharmacol. Ther. 2015, 97, 568–570. [Google Scholar] [CrossRef]
- Banerjee, R.; Erridge, S.; Salazar, O.; Mangal, N.; Couch, D.; Pacchetti, B.; Sodergen, M.H. Real World Evidence in Medical Cannabis Research. Ther. Innov. Regul. Sci. 2022, 56, 8–14. [Google Scholar] [CrossRef]
- Brown, J.D.; Goodin, A.J. Evidence in Context: High Risk of Bias in Medical Cannabis and Cannabinoid Clinical Trials Dictates the Need for Cautious Interpretation. Med. Cannabis Cannabinoids 2021, 4, 63–66. [Google Scholar] [CrossRef]
- Bonn-Miller, M.O.; ElSohly, M.A.; Loflin, M.J.E.; Chandra, S.; Vandrey, R. Cannabis and cannabinoid drug development: Evaluating botanical versus single molecule approaches. Int. Rev. Psychiatry 2018, 30, 277–284. [Google Scholar] [CrossRef]
- Hussain, T.; Jeena, G.; Pitakbut, T.; Vasilev, N.; Kayser, O. Cannabis sativa research trends, challenges, and new-age perspectives. iScience 2021, 24, 103391. [Google Scholar] [CrossRef]
- Owens, B. The treasure chest. Nature 2015, 525, s6–s8. [Google Scholar] [CrossRef] [PubMed]
 receptors (CB1Rs) are primarily expressed within brain regions and the nervous system, especially in the central nervous system (CNS), and to a lesser extent at other sites of the body.
receptors (CB1Rs) are primarily expressed within brain regions and the nervous system, especially in the central nervous system (CNS), and to a lesser extent at other sites of the body.  receptors (CB2Rs) are primarily expressed within cells related to the immune system and peripheral body tissues [27,28]. Created with BioRender.com.
receptors (CB2Rs) are primarily expressed within cells related to the immune system and peripheral body tissues [27,28]. Created with BioRender.com.
 receptors (CB1Rs) are primarily expressed within brain regions and the nervous system, especially in the central nervous system (CNS), and to a lesser extent at other sites of the body.
receptors (CB1Rs) are primarily expressed within brain regions and the nervous system, especially in the central nervous system (CNS), and to a lesser extent at other sites of the body.  receptors (CB2Rs) are primarily expressed within cells related to the immune system and peripheral body tissues [27,28]. Created with BioRender.com.
receptors (CB2Rs) are primarily expressed within cells related to the immune system and peripheral body tissues [27,28]. Created with BioRender.com.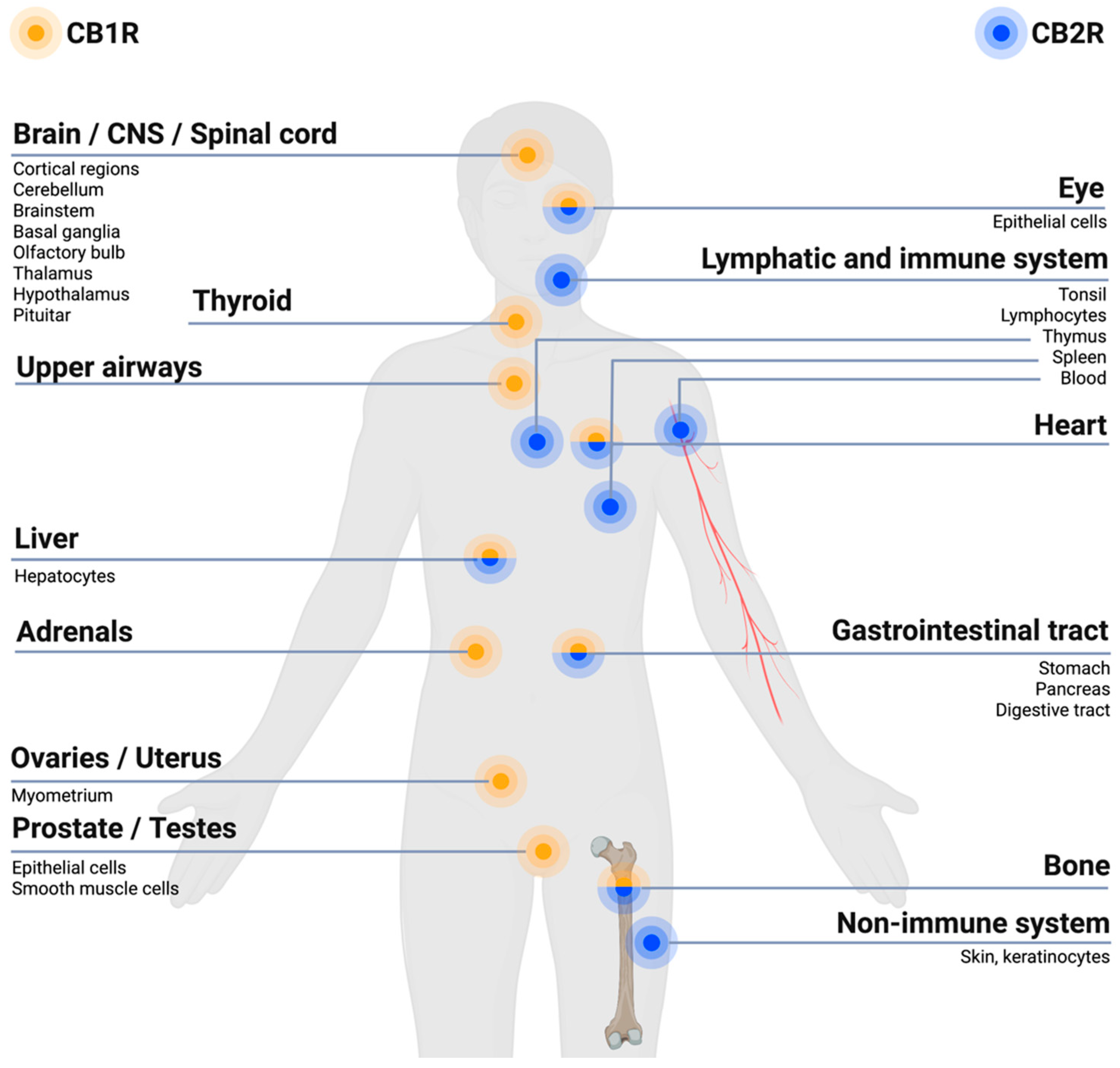
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christensen, C.; Allesø, M.; Rose, M.; Cornett, C. Clinical Research Evidence Supporting Administration and Dosing Recommendations of Medicinal Cannabis as Analgesic in Cancer Patients. J. Clin. Med. 2023, 12, 307. https://doi.org/10.3390/jcm12010307
Christensen C, Allesø M, Rose M, Cornett C. Clinical Research Evidence Supporting Administration and Dosing Recommendations of Medicinal Cannabis as Analgesic in Cancer Patients. Journal of Clinical Medicine. 2023; 12(1):307. https://doi.org/10.3390/jcm12010307
Chicago/Turabian StyleChristensen, Catalina, Morten Allesø, Martin Rose, and Claus Cornett. 2023. "Clinical Research Evidence Supporting Administration and Dosing Recommendations of Medicinal Cannabis as Analgesic in Cancer Patients" Journal of Clinical Medicine 12, no. 1: 307. https://doi.org/10.3390/jcm12010307
APA StyleChristensen, C., Allesø, M., Rose, M., & Cornett, C. (2023). Clinical Research Evidence Supporting Administration and Dosing Recommendations of Medicinal Cannabis as Analgesic in Cancer Patients. Journal of Clinical Medicine, 12(1), 307. https://doi.org/10.3390/jcm12010307






