Temporal Trends in Mortality Associated with Comorbid Type 2 Diabetes and Schizophrenia: The Fremantle Diabetes Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Baseline Assessment
2.3. Ascertainment of Schizophrenia, Comorbidities and All-Cause Mortality
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
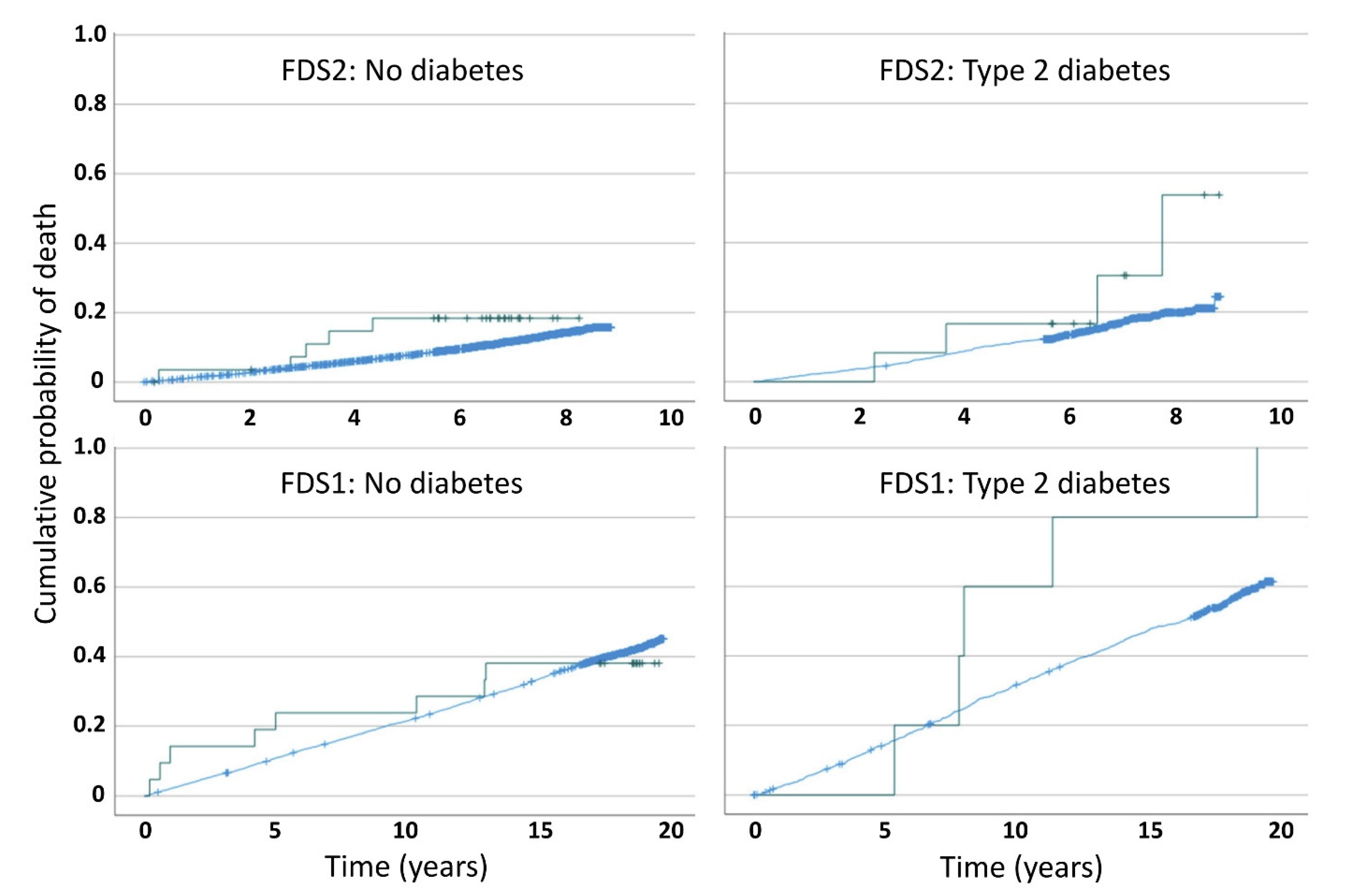
3.2. Mortality by Type 2 Diabetes and Schizophrenia Status, and Study Phase
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, M.; Druss, B. The epidemiology of diabetes in psychotic disorders. Lancet Psychiatry 2015, 2, 431–451. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.; Burge, M.R. Diabetes mellitus associated with atypical anti-psychotic medications. Diabetes Technol. Ther. 2003, 5, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.I.; Shuldiner, A.R. Rethinking the genetic basis for comorbidity of schizophrenia and type 2 diabetes. Schizophr. Res. 2010, 123, 234–243. [Google Scholar] [CrossRef]
- Stubbs, B.; Vancampfort, D.; De Hert, M.; Mitchell, A.J. The prevalence and predictors of type two diabetes mellitus in people with schizophrenia: A systematic review and comparative meta-analysis. Acta Psychiatr. Scand. 2015, 132, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Hjorthoj, C.; Sturup, A.E.; McGrath, J.J.; Nordentoft, M. Years of potential life lost and life expectancy in schizophrenia: A systematic review and meta-analysis. Lancet Psychiatry 2017, 4, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Chant, D.; McGrath, J. A systematic review of mortality in schizophrenia: Is the differential mortality gap worsening over time? Arch. Gen. Psychiatry 2007, 64, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.E.; Banner, J.; Jensen, S.E. Cardiovascular disease in patients with severe mental illness. Nat. Rev. Cardiol. 2021, 18, 136–145. [Google Scholar] [CrossRef]
- Sagud, M.; Mihaljevic Peles, A.; Pivac, N. Smoking in schizophrenia: Recent findings about an old problem. Curr. Opin. Psychiatry 2019, 32, 402–408. [Google Scholar] [CrossRef]
- Davis, W.A.; Starkstein, S.E.; Bruce, D.G.; Davis, T.M.E. The interactive effects of type 2 diabetes mellitus and schizophrenia on all-cause mortality: The fremantle diabetes study. J. Diabetes Complicat. 2015, 29, 1320–1322. [Google Scholar] [CrossRef]
- Toender, A.; Vestergaard, M.; Munk-Olsen, T.; Larsen, J.T.; Kristensen, J.K.; Laursen, T.M. Risk of diabetic complications and subsequent mortality among individuals with schizophrenia and diabetes—A population-based register study. Schizophr. Res. 2020, 218, 99–106. [Google Scholar] [CrossRef]
- Momen, N.C.; Plana-Ripoll, O.; Agerbo, E.; Christensen, M.K.; Iburg, K.M.; Laursen, T.M.; Mortensen, P.B.; Pedersen, C.B.; Prior, A.; Weye, N.; et al. Mortality associated with mental disorders and comorbid general medical conditions. JAMA Psychiatry 2022, 79, 444–453. [Google Scholar] [CrossRef]
- Mai, Q.; Holman, C.D.; Sanfilippo, F.M.; Emery, J.D.; Preen, D.B. Mental illness related disparities in diabetes prevalence, quality of care and outcomes: A population-based longitudinal study. BMC Med. 2011, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Lai, M.S.; Gau, S.S. Complications and mortality in patients with schizophrenia and diabetes: Population-based cohort study. Br. J. Psychiatry 2015, 207, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Phelan, M.; Stradins, L.; Morrison, S. Physical health of people with severe mental illness. BMJ 2001, 322, 443–444. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Patel, V.; Saxena, S.; Maj, M.; Maselko, J.; Phillips, M.R.; Rahman, A. No health without mental health. Lancet 2007, 370, 859–877. [Google Scholar] [CrossRef] [PubMed]
- Rummel-Kluge, C.; Komossa, K.; Schwarz, S.; Hunger, H.; Schmid, F.; Lobos, C.A.; Kissling, W.; Davis, J.M.; Leucht, S. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2010, 123, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Mond, J.; Morice, R.; Owen, C.; Korten, A. Use of antipsychotic medications in Australia between July 1995 and December 2001. Aust. N. Z. J. Psychiatry 2003, 37, 55–61. [Google Scholar] [CrossRef]
- Monshat, K.; Carty, B.; Olver, J.; Castle, D.; Bosanac, P. Trends in antipsychotic prescribing practices in an urban community mental health clinic. Australas. Psychiatry 2010, 18, 238–241. [Google Scholar] [CrossRef]
- Davis, W.A.; Gregg, E.W.; Davis, T.M.E. Temporal trends in cardiovascular complications in people with or without type 2 diabetes: The fremantle diabetes study. J. Clin. Endocrinol. Metab. 2020, 105, e2471–e2482. [Google Scholar] [CrossRef]
- Davis, T.M.; Zimmet, P.; Davis, W.A.; Bruce, D.G.; Fida, S.; Mackay, I.R. Autoantibodies to glutamic acid decarboxylase in diabetic patients from a multi-ethnic Australian community: The fremantle diabetes study. Diabet. Med. 2000, 17, 667–674. [Google Scholar] [CrossRef]
- Davis, T.M.E.; Bruce, D.G.; Davis, W.A. Cohort profile: The fremantle diabetes study. Int. J. Epidemiol. 2013, 42, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Holman, C.D.; Bass, A.J.; Rosman, D.L.; Smith, M.B.; Semmens, J.B.; Glasson, E.J.; Brook, E.L.; Trutwein, B.; Rouse, I.L.; Watson, C.R.; et al. A decade of data linkage in Western Australia: Strategic design, applications and benefits of the WA data linkage system. Aust. Health Rev. Publ. Aust. Hosp. Assoc. 2008, 32, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Ajani, U.A.; Croft, J.B.; Critchley, J.A.; Labarthe, D.R.; Kottke, T.E.; Giles, W.H.; Capewell, S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N. Engl. J. Med. 2007, 356, 2388–2398. [Google Scholar] [CrossRef]
- Gregg, E.W.; Li, Y.; Wang, J.; Burrows, N.R.; Ali, M.K.; Rolka, D.; Williams, D.E.; Geiss, L. Changes in diabetes-related complications in the United States, 1990–2010. N. Engl. J. Med. 2014, 370, 1514–1523. [Google Scholar] [CrossRef]
- Morton, J.I.; Lazzarini, P.A.; Shaw, J.E.; Magliano, D.J. Trends in the incidence of hospitalization for major diabetes-related complications in people with type 1 and type 2 diabetes in Australia, 2010–2019. Diabetes Care 2022, 45, 789–797. [Google Scholar] [CrossRef]
- Laursen, T.M. Causes of premature mortality in schizophrenia: A review of literature published in 2018. Curr. Opin. Psychiatry 2019, 32, 388–393. [Google Scholar] [CrossRef]
- Lee, E.E.; Liu, J.; Tu, X.; Palmer, B.W.; Eyler, L.T.; Jeste, D.V. A widening longevity gap between people with schizophrenia and general population: A literature review and call for action. Schizophr. Res. 2018, 196, 9–13. [Google Scholar] [CrossRef]
- Morgan, V.A.; Waterreus, A.; Jablensky, A.; Mackinnon, A.; McGrath, J.J.; Carr, V.; Bush, R.; Castle, D.; Cohen, M.; Harvey, C.; et al. People living with psychotic illness in 2010: The second Australian national survey of psychosis. Aust. N. Z. J. Psychiatry 2012, 46, 735–752. [Google Scholar] [CrossRef]
- World Health Organization. Shizophrenia. Available online: https://www.who.int/news-room/fact-sheets/detail/schizophrenia (accessed on 28 December 2022).
- Jablensky, A.V.; Morgan, V.; Zubrick, S.R.; Bower, C.; Yellachich, L.A. Pregnancy, delivery, and neonatal complications in a population cohort of women with schizophrenia and major affective disorders. Am. J. Psychiatry 2005, 162, 79–91. [Google Scholar] [CrossRef]
- Holt, R.I.; Mitchell, A.J. Diabetes mellitus and severe mental illness: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2015, 11, 79–89. [Google Scholar] [CrossRef] [PubMed]
| FDS1 | FDS2 | |||||||
|---|---|---|---|---|---|---|---|---|
| No Diabetes, No Schizophrenia | T2D, No Schizophrenia | Schizophrenia, No Diabetes | T2D, Schizophrenia | No Diabetes, No Schizophrenia | T2D, No Schizophrenia | Schizophrenia, No Diabetes | T2D, Schizophrenia | |
| Number (% by Phase) | 5138 (79.7) | 1286 (19.9) | 21 (0.3) | 5 (0.1) | 6007 (79.6) | 1497 (19.8) | 29 (0.4) | 12 (0.2) |
| Age at FDS entry (years) | 64.0±11.2 | 64.0±11.2 | 56.4±11.6 | 61.7±8.0 | 65.4±11.7 | 65.6±11.6 | 62.4±12.3 | 47.8±10.9 |
| Sex (% male) | 48.8 | 48.7 | 38.1 | 60.0 | 51.8 | 51.8 | 48.3 | 58.3 |
| CCI (%): | ||||||||
| 0 | 85.5 | 71.8 | 90.5 | 40.0 | 86.5 | 75.0 | 89.7 | 91.7 |
| 1 or 2 | 11.2 | 21.9 | 9.5 | 60.0 | 9.8 | 17.0 | 10.3 | 0 |
| ≥3 | 3.3 | 6.4 | 0 | 0 | 3.7 | 8.0 | 0 | 8.3 |
| Follow-up (person-years) | 75,253 | 16,672 | 287 | 51 | 39,807 | 9,995 | 164 | 75 |
| Deaths (n (%)) | 2151 (41.9) | 729 (56.7) | 8 (38.1) | 5 (100) | 725 (12.1) | 265 (17.7) | 5 (17.2) | 4 (33.3) |
| Mortality rate (/1000 person years) | 28.6 (27.4, 29.8) | 43.7 (40.6, 47.0) | 27.9 (12.0, 54.9) | 98.0 (31.8, 228.8) | 18.2 (16.9, 19.6) | 26.5 (23.4, 29.9) | 30.5 (9.9, 71.2) | 53.3 (14.5, 136.6) |
| Mortality rate difference (/1000 person years) | - | 15.1 (11.8, 18.5) | −0.71 (−20.1, 18.6) | 69.5 (−16.5, 155) | - | 8.30 (4.84, 11.8) | 12.3 (−14.5, 39.0) | 35.1 (−17.2, 87.4) |
| Mortality rate ratio (95% CI) (reference no diabetes, no schizophrenia) | 1.00 | 1.53 (1.40, 1.66) | 0.98 (0.42, 1.92) | 3.43 (1.11, 8.02) | 1.00 | 1.46 (1.26, 1.68) | 1.67 (0.54, 3.92) | 2.93 (0.80, 7.53) |
| HR (95% CI) unadjusted | 1.00 | 1.56 (1.44, 1.70) | 0.97 (0.48, 1.94) | 3.71 (1.54, 8.93) | 1.00 | 1.45 (1.26, 1.67) | 1.74 (0.72, 4.18) | 2.96 (1.11, 7.91) |
| HR (95% CI) adjusted for age | 1.00 | 1.71 (1.57, 1.86) | 2.27 (1.13, 4.54) | 5.61 (2.33, 13.5) | 1.00 | 1.46 (1.27, 1.69) | 2.30 (0.95, 5.54) | 26.9 (9.94, 72.6) |
| HR (95% CI) adjusted for age, sex and CCI | 1.00 | 1.55 (1.42, 1.69) | 2.87 (1.43, 5.75) | 4.77 (1.98, 11.5) | 1.00 | 1.23 (1.07, 1.42) | 2.85 (1.18, 6.89) | 25.6 (9.46, 69.3) |
| HR (95% CI) adjusted for age, sex, CCI and their time-varying interactions with ln(time) | 1.00 | 1.54 (1.41, 1.68) | 2.82 (1.40, 5.65) | 4.52 (1.88, 10.9) | 1.00 | 1.24 (1.07, 1.43) | 2.90 (1.20, 6.99) | 24.7 (9.09, 67.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, W.A.; Bruce, D.G.; Starkstein, S.E.; Davis, T.M.E. Temporal Trends in Mortality Associated with Comorbid Type 2 Diabetes and Schizophrenia: The Fremantle Diabetes Study. J. Clin. Med. 2023, 12, 300. https://doi.org/10.3390/jcm12010300
Davis WA, Bruce DG, Starkstein SE, Davis TME. Temporal Trends in Mortality Associated with Comorbid Type 2 Diabetes and Schizophrenia: The Fremantle Diabetes Study. Journal of Clinical Medicine. 2023; 12(1):300. https://doi.org/10.3390/jcm12010300
Chicago/Turabian StyleDavis, Wendy A., David G. Bruce, Sergio E. Starkstein, and Timothy M. E. Davis. 2023. "Temporal Trends in Mortality Associated with Comorbid Type 2 Diabetes and Schizophrenia: The Fremantle Diabetes Study" Journal of Clinical Medicine 12, no. 1: 300. https://doi.org/10.3390/jcm12010300
APA StyleDavis, W. A., Bruce, D. G., Starkstein, S. E., & Davis, T. M. E. (2023). Temporal Trends in Mortality Associated with Comorbid Type 2 Diabetes and Schizophrenia: The Fremantle Diabetes Study. Journal of Clinical Medicine, 12(1), 300. https://doi.org/10.3390/jcm12010300








