Comparison of Biological Agent Monotherapy and Associations Including Disease-Modifying Antirheumatic Drugs for Rheumatoid Arthritis: Literature Review and Meta-Analysis of Randomized Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Trial Selection
2.3. Participants
2.4. Types of Interventions
2.5. Outcome Measures
2.6. Data Collection and Handling of Missing Data
2.7. Risk of Bias
2.8. Statistical Analysis
3. Results
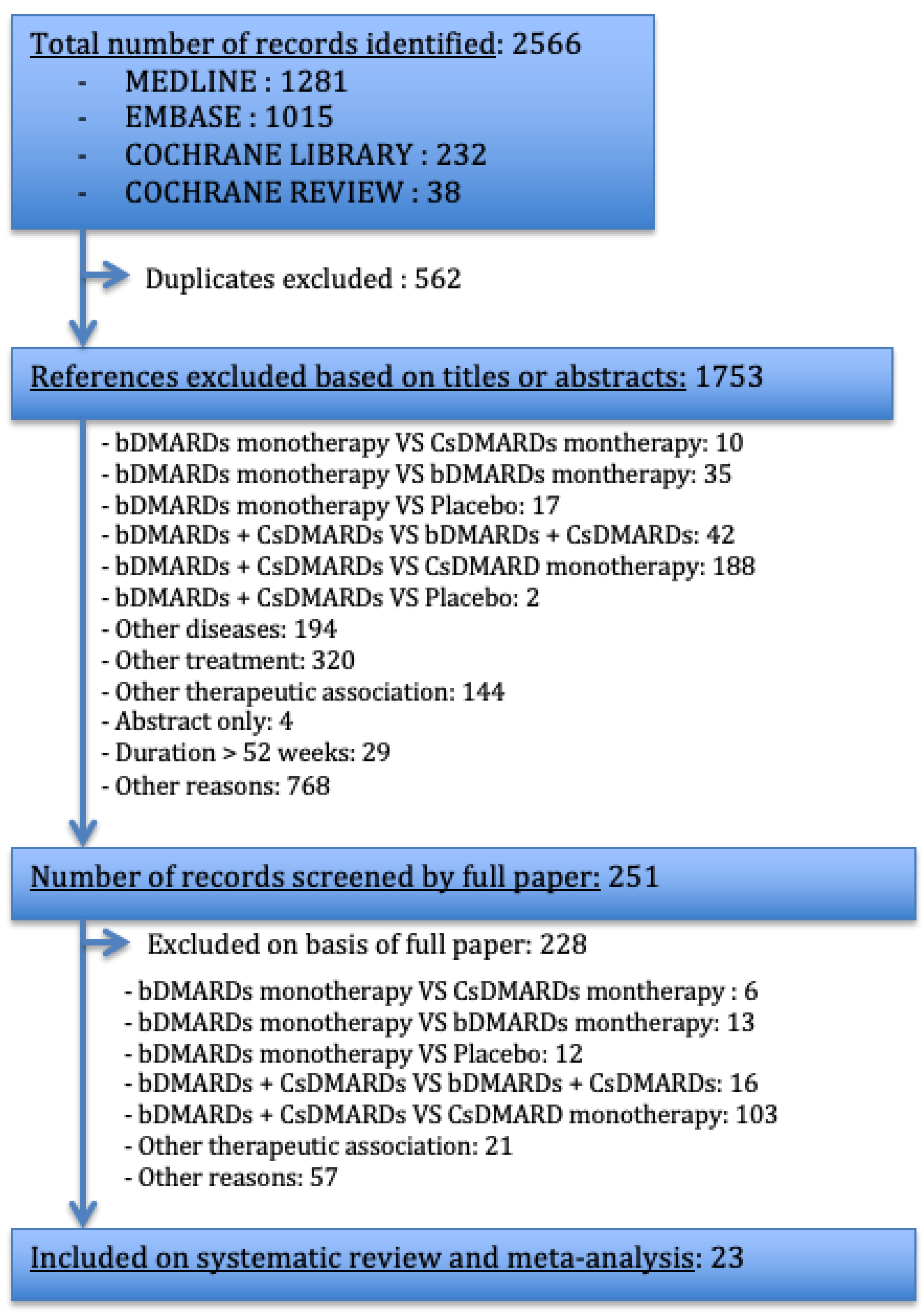
3.1. Study Selection Process
3.2. Study Characteristics
3.3. Principal Characteristics of the Patients
3.4. Primary Efficacy Endpoint: ACR 20 at 24 Weeks
3.5. Other Endpoints
3.5.1. ACR Reponses
3.5.2. Remission According to DAS 28 (Using ESR or CRP)
3.5.3. HAQ, CDAI and SDAI Scores
3.5.4. Subgroup Meta-Analysis
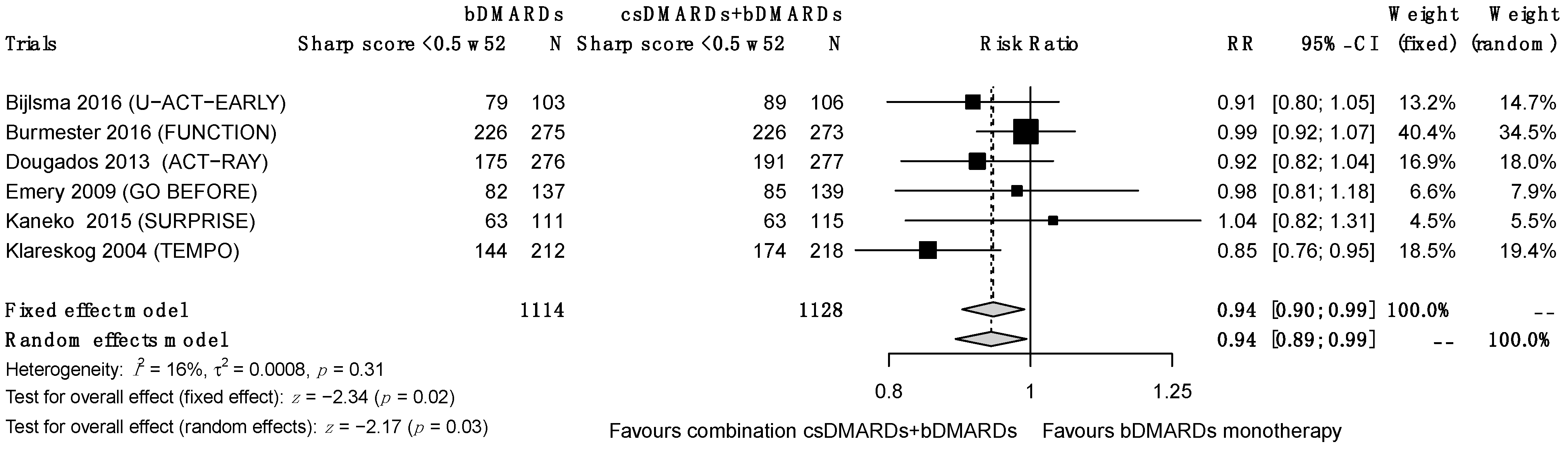
3.5.5. Structural Progression
3.5.6. Discontinuation Due to a Lack of Efficacy
3.5.7. Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gabriel, S.E.; Crowson, C.S.; O’Fallon, W.M. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis Rheum. 1999, 42, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Daien, C.; Hua, C.; Gaujoux-Viala, C.; Cantagrel, A.; Dubremetz, M.; Dougados, M.; Fautrel, B.; Mariette, X.; Nayral, N.; Richez, C.; et al. Update of the Recommendations of the French Society of Rheumatology for the 340 Management of Rheumatoid Arthritis. Jt. Bone Spine 2019, 86, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, W.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.; Bijlsma, J.; Burmester, G.; Chatzidionysiou, K.; Dougados, M.; Nam, J.; Ramiro, S.; Voshaar, M.; van Vollenhoven, R.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann. Rheum. Dis. 2017, 76, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.M.; Ashcroft, D.M.; Watson, K.D.; Lunt, M.; Symmons, D.P.; Hyrich, K.L. Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: Results from the British Society for Rheumatology Biologics Register. Ann. Rheum. Dis. 2011, 70, 583–589. [Google Scholar] [CrossRef]
- Listing, J.; Strangfeld, A.; Rau, R.; Kekow, J.; Gromnica-Ihle, E.; Klopsch, T.; Demary, W.; Burmester, G.R.; Zink, A. Clinical and functional remission: Even though biologics are superior to conventional DMARDs overall success rates remain low—Results from RABBIT, the German biologics register. Arthritis Res. Ther. 2006, 8, R66. [Google Scholar] [CrossRef]
- Mariette, X.; Gottenberg, J.-E.; Ravaud, P.; Combe, B. Registries in rheumatoid arthritis and autoimmune diseases: Data from the French registries. Rheumatol. Oxf. Engl. 2011, 50, 222–229. [Google Scholar] [CrossRef]
- Lee, S.J.; Chang, H.; Yazici, Y.; Greenberg, J.D.; Kremer, J.M.; Kavanaugh, A. Utilization trends of tumor necrosis factor inhibitors among patients with rheumatoid arthritis in a United States observational cohort study. J. Rheumatol. 2009, 36, 1611–1617. [Google Scholar] [CrossRef]
- Curtis, J.R.; Bykerk, V.P.; Aassi, M.; Schiff, M. Adherence and Persistence with Methotrexate in Rheumatoid Arthritis: A Systematic Review. J. Rheumatol. 2016, 43, 1997–2009. [Google Scholar] [CrossRef]
- Emery, P.; Sebba, A.; Huizinga, T.W.J. Biologic and oral disease-modifying antirheumatic drug monotherapy in rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 1897–1904. [Google Scholar] [CrossRef]
- Bliddal, H.; Eriksen, S.A.; Christensen, R.; Lorenzen, T.; Hansen, M.S.; Østergaard, M.; Dreyer, L.; Luta, G.; Vestergaard, P. Adherence to Methotrexate in Rheumatoid Arthritis: A Danish Nationwide Cohort Study. Arthritis 2015, 2015, 915142. [Google Scholar] [CrossRef]
- Tarp, S.; Jørgensen, T.S.; Furst, D.E. Added value of combining methotrexate with a biological agent compared to biological monotherapy in rheumatoid arthritis patients: A systematic review and meta-analysis of randomised trials. Semin. Arthritis Rheum. 2019, 48, 958–966. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Drevon, D.; Fursa, S.R.; Malcolm, A.L. Intercoder Reliability and Validity of WebPlotDigitizer in Extracting Graphed Data. Behav. Modif. 2017, 41, 323–339. [Google Scholar] [CrossRef]
- Flower, A.; McKenna, J.W.; Upreti, G. Validity and Reliability of GraphClick and DataThief III for Data Extraction. Behav. Modif. 2016, 40, 396–413. [Google Scholar] [CrossRef]
- Rakap, S.; Rakap, S.; Evran, D.; Cig, O. Comparative evaluation of the reliability and validity of three data extraction programs: UnGraph, GraphClick, and DigitizeIt. Comput. Hum. Behav. 2016, 55, 159–166. [Google Scholar] [CrossRef]
- Shadish, W.R.; Brasil, I.C.C.; Illingworth, D.A.; White, K.D.; Galindo, R.; Nagler, E.D.; Rindskopf, D.M. Using UnGraph to extract data from image files: Verification of reliability and validity. Behav. Res. Methods 2009, 41, 177–183. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Fleiss, J. Review papers: The statistical basis of meta-analysis. Stat. Methods Med. Res. 1993, 2, 121–145. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Emery, P.; Burmester, G.R.; Bykerk, V.P.; Combe, B.G.; Furst, D.E.; Barré, E.; Karyekar, C.S.; Wong, D.A.; Huizinga, T.W. Evaluating drug-free remission with abatacept in early rheumatoid arthritis: Results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann. Rheum. Dis. 2015, 74, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, F.C.; Weisman, M.H.; Kavanaugh, A.F.; Cohen, S.B.; Pavelka, K.; van Vollenhoven, R.; Sharp, J.; Perez, J.L.; Spencer-Green, G.T. The PREMIER study: A multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006, 54, 26–37. [Google Scholar]
- Combe, B.; Codreanu, C.; Fiocco, U.; Gaubitz, M.; Geusens, P.P.; Kvien, T.K.; Pavelka, K.; Sambrook, P.N.; Smolen, J.S.; Wajdula, J.; et al. Etanercept and sulfasalazine, alone and combined, in patients with active rheumatoid arthritis despite receiving sulfasalazine: A double-blind comparison. Ann. Rheum. Dis. 2006, 68, 1146–1152. [Google Scholar] [CrossRef]
- Combe, B.; Codreanu, C.; Fiocco, U.; Gaubitz, M.; Geusens, P.P.; Kvien, T.K.; Pavelka, K.; Sambrook, P.N.; Smolen, J.S.; Khandker, R.; et al. Efficacy, safety and patient-reported outcomes of combination etanercept and sulfasalazine versus etanercept alone in patients with rheumatoid arthritis: A double-blind randomised 2-year study. Ann. Rheum. Dis. 2009, 68, 1146–1152. [Google Scholar] [CrossRef]
- Klareskog, L.; van der Heijde, D.; de Jager, J.P.; Gough, A.; Kalden, J.; Malaise, M.; Martín Mola, E.; Pavelka, K.; Sany, J.; Settas, L.; et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: Double-blind randomised controlled trial. Lancet 2004, 363, 675–681. [Google Scholar] [CrossRef]
- Emery, P.; Breedveld, F.C.; Hall, S.; Durez, P.; Chang, D.J.; Robertson, D.; Singh, A.; Pedersen, R.D.; Koenig, A.S.; Freundlich, B. Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): A randomised, double-blind, parallel treatment trial. Lancet 2008, 372, 375–382. [Google Scholar] [CrossRef]
- Pope, J.E.; Haraoui, B.; Thorne, J.C.; Vieira, A.; Poulin-Costello, M.; Keystone, E.C. The Canadian Methotrexate and Etanercept Outcome Study: A randomised trial of discontinuing versus continuing methotrexate after 6 months of etanercept and methotrexate therapy in rheumatoid arthritis. Ann. Rheum. Dis. 2014, 73, 2144–2151. [Google Scholar] [CrossRef]
- Kameda, H.; Kanbe, K.; Sato, E.; Ueki, Y.; Saito, K.; Nagaoka, S.; Hidaka, T.; Atsumi, T.; Tsukano, M.; Kasama, T.; et al. Continuation of Methotrexate Resulted in Better Clinical and Radiographic Outcomes Than Discontinuation upon Starting Etanercept in Patients with Rheumatoid Arthritis: 52-week Results from the JESMR Study. J. Rheumatol. 2011, 38, 1585–1592. [Google Scholar] [CrossRef]
- Kameda, H.; Ueki, Y.; Saito, K.; Nagaoka, S.; Hidaka, T.; Atsumi, T.; Tsukano, M.; Kasama, T.; Shiozawa, S.; Tanaka, Y.; et al. Etanercept (ETN) with methotrexate (MTX) is better than ETN monotherapy in patients with active rheumatoid arthritis despite MTX therapy: A randomized trial. Mod. Rheumatol. 2010, 20, 531–538. [Google Scholar] [CrossRef]
- van Riel, P.L.; Taggart, A.J.; Sany, J.; Gaubitz, M.; Nab, H.W.; Pedersen, R.; Freundlich, B.; MacPeek, D. Efficacy and safety of combination etanercept and methotrexate versus etanercept alone in patients with rheumatoid arthritis with an inadequate response to methotrexate: The ADORE study. Ann. Rheum. Dis. 2006, 65, 1478–1483. [Google Scholar] [CrossRef]
- Chen, X.X.; Dai, Q.; Huang, A.B.; Wu, H.X.; Zhao, D.B.; Li, X.F.; Hu, S.X.; Yang, N.P.; Tao, Y.; Xu, J.H.; et al. A multicenter, randomized, double-blind clinical trial of combination therapy with Anbainuo, a novel recombinant human TNFRII:Fc fusion protein, plus methotrexate versus methotrexate alone or Anbainuo alone in Chinese patients with moderate to severe rheumatoid arthritis. Clin. Rheumatol. 2013, 32, 99–108. [Google Scholar]
- Emery, P.; Fleischmann, R.; Van der Heijde, D.; Keystone, E.C.; Genovese, M.C.; Conaghan, P.G.; Hisa, E.C.; Xu, W.; Baratelle, A.; Beutler, A.; et al. The Effects of Golimumab on Radiographic Progression in Rheumatoid Arthritis. Arthritis Rheum. 2011, 63, 1200–1210. [Google Scholar] [CrossRef]
- Emery, P.; Fleischmann, R.M.; Moreland, L.W.; Hsia, E.C.; Strusberg, I.; Durez, P.; Nash, P.; Amante, E.J.; Churchill, M.; Park, W.; et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: Twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009, 60, 2272–2283. [Google Scholar]
- Emery, P.; Fleischmann, R.M.; Doyle, M.K. Golimumab, a Human Anti–Tumor Necrosis Factor Monoclonal Antibody, Injected Subcutaneously Every 4 Weeks in Patients with Active Rheumatoid Arthritis Who Had Never Taken Methotrexate: 1-Year and 2-Year Clinical, Radiologic, and Physical Function Findings of a Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study. Arthritis Care Res. 2013, 65, 1732–1742. [Google Scholar]
- Kremer, J.; Ritchlin, C.; Mendelsohn, A.; Baker, D.; Kim, L.; Xu, Z.; Han, J.; Taylor, P. Golimumab, a new human anti-tumor necrosis factor alpha antibody, administered intravenously in patients with active rheumatoid arthritis: Forty-eight-week efficacy and safety results of a phase III randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2010, 62, 917–928. [Google Scholar] [CrossRef]
- Keystone, E. Golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: 52-week results of the GO-FORWARD study. Ann. Rheum. Dis. 2010, 69, 1129–1135. [Google Scholar] [CrossRef]
- Keystone, E.C.; Genovese, M.C.; Klareskog, L.; Hsia, E.C.; Hall, S.T.; Miranda, P.C.; Pazdur, J.; Bae, S.C.; Palmer, W.; Zrubek, J.; et al. Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: The GO-FORWARD Study. Ann. Rheum. Dis. 2009, 68, 789–796. [Google Scholar] [CrossRef]
- Edwards, J.C.; Szczepanski, L.; Szechinski, J.; Filipowicz-Sosnowska, A.; Emery, P.; Close, D.R.; Stevens, R.M.; Shaw, T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 2004, 350, 2572–2581. [Google Scholar] [CrossRef] [PubMed]
- Strand, V.; Balbir-Gurman, A.; Pavelka, K.; Emery, P.; Li, N.; Yin, M.; Lehane, P.B.; Agarwal, S. Sustained benefit in rheumatoid arthritis following one course of rituximab: Improvements in physical function over 2 years. Rheumatology 2006, 45, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Dougados, M.; Kissel, K.; Sheeran, T.; Tak, P.P.; Conaghan, P.G.; Mola, E.M.; Schett, G.; Amital, H.; Navarro-Sarabia, F.; Hou, A.; et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann. Rheum. Dis. 2013, 72, 43–50. [Google Scholar] [CrossRef]
- Dougados, M.; Kissel, K.; Conaghan, P.G.; Mola, E.M.; Schett, G.; Gerli, R.; Hansen, M.S.; Amital, H.; Xavier, R.M.; Troum, O.; et al. Clinical, radiographic and immunogenic effects after 1 year of tocilizumab-based treatment strategies in rheumatoid arthritis: The ACT-RAY study. Ann. Rheum. Dis. 2014, 73, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Maini, R.N.; Taylor, P.C.; Szechinski, J.; Pavelka, K.; Bröll, J.; Balint, G.; Emery, P.; Raemen, F.; Petersen, J.; Smolen, J.; et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006, 54, 2817–2829. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Rigby, W.F.; Vollenhoven, R.F.; Kay, J.; Rubbert-Roth, A.; Kelman, A.; Dimonaco, S.; Mitchell, N. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann. Rheum. Dis. 2016, 5, 1081–1091. [Google Scholar] [CrossRef]
- Bijlsma, J.W.J.; Welsing, P.M.J.; Woodworth, T.G.; Middelink, L.M.; Pethö-Schramm, A.; Bernasconi, C.; Borm, M.E.A.; Wortel, C.H.; Ter Borg, E.J.; Jahangier, Z.N.; et al. Early rheumatoid arthritis treated with tocilizumab, methotrexate, or their combination (U-Act-Early): A multicentre, randomised, double-blind, double-dummy, strategy trial. Lancet 2016, 388, 343–355. [Google Scholar] [CrossRef]
- Teitsma, X.M.; Jacobs, J.W.G.; Welsing, P.M.J.; Pethö-Schramm, A.; Borm, M.E.A.; van Laar, J.M.; Lafeber, F.P.J.G.; Bijlsma, J.W.J. Radiographic joint damage in early rheumatoid arthritis patients: Comparing tocilizumab- and methotrexate-based treat-to-target strategies. Rheumatol. Oxf. Engl. 2018, 57, 309–317. [Google Scholar] [CrossRef]
- Pablos, J.L.; Navarro, F.; Blanco, J.F. Efficacy of tocilizumab monotherapy after response to combined tocilizumab and methotrexate in patients with rheumatoid arthritis: The randomised JUST-ACT study. Clin. Exp. Rheumatol. 2019, 37, 437–444. [Google Scholar]
- Kremer, J.M.; Rigby, W.; Singer, N.G. Sustained Response Following Discontinuation of Methotrexate in Patients with Rheumatoid Arthritis Treated with Subcutaneous Tocilizumab Results from a Randomized, Controlled Trial. Arthritis Rheumatol. 2018, 70, 1200–1208. [Google Scholar] [CrossRef]
- Edwards, C.J.; Ostor, A.J.K.; Naisbett-Groet, B.; Kiely, P. Tapering versus steady-state methotrexate in combination with tocilizumab for rheumatoid arthritis: A randomized, double-blind trial. Rheumatology 2018, 57, 84–91. [Google Scholar] [CrossRef]
- Kaneko, Y.; Atsumi, T.; Tanaka, Y.; Inoo, M.; Kobayashi-Haraoka, H.; Amano, K.; Miyata, M.; Murakawa, Y.; Yasuoka, H.; Hirata, S.; et al. Comparison of adding tocilizumab to methotrexate with switching to tocilizumab in patients with rheumatoid arthritis with inadequate response to methotrexate: 52-week results from a prospective, randomised, controlled study (SURPRISE study). Ann. Rheum. Dis. 2016, 75, 1917–1923. [Google Scholar] [CrossRef]
- Kameda, H.; Wada, K.; Takahashi, Y.; Hagino, O.; van Hoogstraten, H.; Graham, N.; Tanaka, Y. Sarilumab monotherapy or in combination with non-methotrexate disease-modifying antirheumatic drugs in active rheumatoid arthritis: A Japan phase 3 trial (HARUKA). Mod Rheumatol. 2020, 30, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Weinblatt, M.E.; Mease, P.; Mysler, E.; Takeuchi, T.; Drescher, E.; Berman, A.; Xing, J.; Zilberstein, M.; Banerjee, S.; Emery, P. The efficacy and safety of subcutaneous clazakizumab in patients with moderate-to-severe rheumatoid arthritis and an inadequate response to methotrexate: Results from a multinational, phase IIb, randomized, double-blind, placebo/active-controlled, dose-ranging study. Arthritis Rheumatol. 2015, 67, 2591–2600. [Google Scholar]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Singh, J.A.; Christensen, R.; Wells, G.A.; Suarez-Almazor, M.E.; Buchbinder, R.; Lopez-Olivo, M.A.; Ghogomu, E.T.; Tugwell, P. A network meta-analysis of randomized controlled trials of biologics for rheumatoid arthritis: A Cochrane overview. Can. Med. Assoc. J. 2009, 181, 787–796. [Google Scholar] [CrossRef]
- Alfonso-Cristancho, R.; Armstrong, N.; Arjunji, R.; Riemsma, R.; Worthy, G.; Ganguly, R.; Kleijnen, J. Comparative effectiveness of biologics for the management of rheumatoid arthritis: Systematic review and network meta-analysis. Clin. Rheumatol. 2017, 36, 25–34. [Google Scholar] [CrossRef]
- Buckley, F.; Finckh, A.; Huizinga, T.W.J.; Dejonckheere, F.; Jansen, J.P. Comparative Efficacy of Novel DMARDs as Monotherapy and in Combination with Methotrexate in Rheumatoid Arthritis Patients with Inadequate Response to Conventional DMARDs: A Network Meta-Analysis. J. Manag. Care Spec. Pharm. 2015, 21, 409–423. [Google Scholar] [CrossRef]
- Donahue, K.E.; Schulman, E.R.; Gartlehner, G.; Jonas, B.L.; Coker-Schwimmer, E.; Patel, S.V.; Weber, R.P.; Bann, C.M.; Viswanathan, M. Comparative Effectiveness of Combining MTX with Biologic Drug Therapy Versus Either MTX or Biologics Alone for Early Rheumatoid Arthritis in Adults: A Systematic Review and Network Meta-analysis. J. Gen. Intern. Med. 2019, 34, 2232–2245. [Google Scholar] [CrossRef]
- Migliore, A.; Bizzi, E.; Egan, C.G.; Bernardi, M.; Petrella, L. Efficacy of biological agents administered as monotherapy in rheumatoid arthritis: A Bayesian mixed-treatment comparison analysis. Ther. Clin. Risk Manag. 2015, 11, 1325–1335. [Google Scholar]
- Tarp, S.; Furst, D.E.; Dossing, A.; Østergaard, M.; Lorenzen, T.; Hansen, M.S.; Singh, J.A.; Choy, E.H.; Boers, M.; Suarez-Almazor, M.E.; et al. Defining the optimal biological monotherapy in rheumatoid arthritis: A systematic review and meta-analysis of randomised trials. Semin. Arthritis Rheum. 2017, 46, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Orme, M.E.; Macgilchrist, K.S.; Mitchell, S.; Spurden, D.; Bird, A. Systematic review and network meta-analysis of combination and monotherapy treatments in disease-modifying antirheumatic drug-experienced patients with rheumatoid arthritis: Analysis of American College of Rheumatology criteria scores 20, 50, and 70. Biologics 2012, 6, 429–464. [Google Scholar] [PubMed]
- Gabay, C.; Riek, M.; Hetland, M.L.; Hauge, E.M.; Pavelka, K.; Tomšič, M.; Canhao, H.; Chatzidionysiou, K.; Lukina, G.; Nordström, D.C.; et al. Effectiveness of tocilizumab with and without synthetic disease-modifying antirheumatic drugs in rheumatoid arthritis: Results from a European collaborative study. Ann. Rheum. Dis. 2016, 75, 1336–1342. [Google Scholar] [CrossRef]
- Lauper, K.; Nordström, D.C.; Pavelka, K.; Hernández, M.V.; Kvien, T.K.; Kristianslund, E.K.; Santos, M.J.; Rotar, Ž.; Iannone, F.; Codreanu, C.; et al. Comparative effectiveness of tocilizumab versus TNF inhibitors as monotherapy or in combination with conventional synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis after the use of at least one biologic disease-modifying antirheumatic drug: Analyses from the pan-European TOCERRA register collaboration. Ann. Rheum. Dis. 2018, 77, 1276–1282. [Google Scholar]
- Gabay, C.; Emery, P.; van Vollenhoven, R.; Dikranian, A.; Alten, R.; Pavelka, K.; Klearman, M.; Musselman, D.; Agarwal, S.; Green, J.; et al. ADACTA Study Investigators. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): A randomised, double-blind, controlled phase 4 trial. Lancet. 2013, 381, 1541–1550. [Google Scholar] [CrossRef]
- Burmester, G.R.; Lin, Y.; Patel, R.; van Adelsberg, J.; Mangan, E.K.; Graham, N.M.; van Hoogstraten, H.; Bauer, D.; Ignacio Vargas, J.; Lee, E.B. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): A randomised, double-blind, parallel-group phase III trial. Ann. Rheum. Dis. 2017, 76, 840–847. [Google Scholar] [CrossRef]
- Ramiro, S.; Sepriano, A.; Chatzidionysiou, K.; Nam, J.L.; Smolen, J.S.; van der Heijde, D.; Dougados, M.; van Vollenhoven, R.; Bijlsma, J.W.; Burmester, G.; et al. Safety of synthetic and biological DMARDs: A systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann. Rheum. Dis. 2017, 76, 1101–1136. [Google Scholar] [CrossRef]
- Raaschou, P.; Simard, J.F.; Holmqvist, M.; Askling, J. ARTIS Study Group. Rheumatoid arthritis, anti-tumour necrosis factor therapy, and risk of malignant melanoma: Nationwide population based prospective cohort study from Sweden. BMJ 2013, 346, f1939. [Google Scholar] [CrossRef]



| Studies | Follow-Up | CsDMARD History | WO Period | bDMARD History | WO Period | RA Duration | Treatment | Doses (mg) | Dose Adjustment Defined a Priori | N |
|---|---|---|---|---|---|---|---|---|---|---|
| Anbainuo, Chen, 2013 [33] | 24 weeks | Naive | / | Naive | / | ND | Abainuo + MTX Abainuo | Abainuo: 25 mg SC 2x/Week MTX: 10–15 mg/Week PO | No | N = 132 N = 132 |
| Abatacept (AVERT), Emery, 2015 [23] | 48 weeks | Naive: MTX-naive or received MTX (≤10 mg/week) for ≤4 weeks | MTX 1 month | Naive | / | <2 years | ABA + MTX ABA | ABA: 125 mg SC/Week MTX: 7.5–20 mg/Week PO | No | N = 119 N = 116 |
| Adalimumab (PREMIER), Breedveld, 2006 [24] | 48/104 weeks | Naive or not: MTX, cyclophosphamid, CYC, AZA, or 2 other CsDMARDs were excluded | 4 weeks | Naive | / | <3 years | ADA + MTX ADA | ADA: 40 mg SC/2 weeks MTX: 7.5–20 mg/Week PO | Increased dosing with ADA/placebo to weekly if ACR 20 not achieved in 2 consecutive visits after week 16. | N = 268 N = 274 |
| Clazakizumab, Weinblatt, 2015 [53] | 24 weeks | Non naive: MTX failure (>3 months treatment) | / | Naive | / | >16 weeks | CLZ + MTX CLZ | CLZ: 100 mg SC/4 wks MTX: 10–22 mg/Week PO | If <20% reduction SJC/TJC: receive open-label CLZ 200 mg SC/4 wk + MTX | N = 60 N = 60 |
| Etanercept, (ADORE), van Riel, 2006 [32] | 16 weeks | Non naive: MTX >12.5 mg/week for >3 months | 12 weeks | Naive | / | ND | ETN + MTX ETN | ETN: 25 mg SC 2x/Week MTX: >12.5 mg/week PO or SC | No | N = 155 N = 159 |
| Etanercept, (CAMEO), Pope, 2013 [29] | 24/104 weeks | Non naive: MTX therapy for >12 weeks | / | Non naive: ETN + MTX for 6 months | / | > 6 months | ETN + MTX ETN | ETN: 50 mg SC/Week MTX: ≥15 mg/week | No | N = 107 N = 98 |
| Etanercept, Combe, 2006 [25] | 24/48/104 weeks | Non naive: SSZ for >4 months | Other than SSZ: 3 month | Naive or not: ineligible if they had received ETN or other TNF antagonists | bDMARDs or CTX: 6 months | <20 years | ETN + SSZ ETN | ETN: 25 mg SC 2x/Week SSZ: 2–2.5–3 g/day PO | No | N = 101 N = 103 |
| Etanercept (COMET), Emery, 2010 [28] | 52 weeks | Non naive: ETN + MTX for 52 weeks before new randomization. | No | Non naive: ETN + MTX during 52 weeks before new randomization. | No | 4 months until 2 years | ETN + MTX ETN | ETN: 25 mg SC 2x/week MTX 7.5–20 mg/Week PO | No | N = 111 N = 111 |
| Etanercept, (JESMR), Kameda, 2010 and 2011 [30,31] | 24/52 weeks | Non naive: MTX 6 mg/week for >3 months | / | Naive | / | ND | ETN + MTX ETN | ETN: 25 mg SC 2x/Week MTX: 6–8 mg/week | No | N = 76 N = 71 |
| Etanercept (TEMPO), Klareskog, 2004 [27] | 24/52/ 104 weeks | CsDMARD non naive, but MTX naive or not | MTX 6 month | Naive or not: Ineligible if previously received ETN or other TNF antagonists. | ISD: 6 months bDMARD: 3 months | 6 months until 20 years. | ETN + MTX ETN | ETN: 25 mg SC 2x/week MTX: 7.5–20 mg/Week PO | No | N = 231 N = 223 |
| Golimumab (GO BEFORE), Emery, 2009 [35] | 24/52/104 weeks | Naive or not: had not received more than 3 weekly doses of oral MTX | / | Naive or not: IFX, ETN, ADA, RTX, NTZ, or cytotoxic agents, and alkylating agents, were excluded | ANK: 4 weeks alefacept/ efalizumab: 3 months, other: 5 half-lives | 3 months until 3 years | GOL + MTX GOL | GOL: 100 mg SC/4 weeks MTX: 10–20 mg/Week PO | If <20% improvement from baseline SJC/TJC entered early escape any time after week 24. | N = 159 N = 159 |
| Golimumab (GO FORWARD), Keystone, 2009 [38] | 24/52/104 weeks | Non naive: had been receiving a stable dose of MTX 15–25 mg/week for at least 4 weeks | Other than MTX 4 weeks | Naive or not: excluded if used anti- TNF agent, RTX, NTZ or cytotoxic agents | ANK: 4 weeks alefacept efalizumab: 3 months | NR | GOL + MTX GOL | GOL: 100 mg SC/4 weeks MTX: 15–20 mg/week PO | If <20% improvement from baseline TJC/SJC escape any time after week 24. | N = 89 N = 133 |
| Golimumab (GO LIVE), Kremer, 2010 [37] | 24/48 weeks | Non naive: MTX for >3 months | / | Naive or not: limited to 20% of the study population. (Excluded if RTX, ABA, or NTZ). | IFX, alefa- Cept/efalizumab: 3 months, ETN/ADA 2 monthsANK/ABA/NTZ. 4 weeks | <8 years | GOL 2 mg/kg + MTX GOL 4 mg/kg + MTX GOL 2 mg/kg GOL 4 mg/kg | GOL: 2 mg/kg OR 4 mg/kg IV/12 weeks MTX: 15 mg/Week PO | At weeks 16 and 24, patients with <20% improvement from baseline in both the SJC and TJC entered early escape and dose regimen | N = 128 N = 129 N = 129 N = 128 |
| Rituximab, Edwards, 2004, Strand, 2006 [40,41] | 24/48/104 weeks | Non naive: had failed 1–5 CsDMARDs and MTX with treatment for >16 weeks | / | ND | / | ND | RTX + MTX RTX | RTX: 1000 mg IV on days 1 and 15 all 6 months MTX: 12.5–15 mg/Week PO | No | N = 40 N = 40 |
| Sarilumab, (HARUKA)Kameda, 2019 [52] | 24/52 weeks | Naive or not: -monotherapy: CsDMARDs naive -combination: CsDMARDs non naive | / | Naive or not | CYC, MFMAZA, CTX, bDMARD: 4–12 weeks | ND | SLM 150 mg + non-MTX CsDMARDs SLM 200 mg + non-MTX CsDMARDs SLM 150 mg SLM 200 mg | SLM 150 or 200 mg/2 Weeks SC | No | N = 15 N = 15 N = 30 N = 31 |
| Tocilizumab (ACT RAY), Dougados, 2013 [42] | 24/52/104 weeks | Non naive: MTX for at least 12 weeks | LEF: 3 moth Other 1 month | Naive or not | bDMARD 1 month | ND | TCZ + MTX TCZ | TCZ: 8 mg/kg IV/4 weeks MTX: 15–20 mg/Week PO | At week 24, if DAS28 > 3.2; an open-label CsDMARD was added. At week 36, if DAS28 > 3.2, an additional CsDMARD added. | N = 277 N = 276 |
| Tocilizumab (ACT-TAPER), Edwards, 2017 [50] | 24 weeks | Non naive: had inadequately responded to 2 CsDMARDs, including MTX. | / | Non naive | Had have TCZ 8 mg/kg/4 weeks for 24 weeks | ND | TCZ + MTX stable dose TCZ + MTX Tapering dose | TCZ: 8 mg/kg IV/4 weeks MTX stable dose: 10–15 mg/Week MTX tapering dose S24 to S40: 5 mg /week; S40 to S48: 0 mg. | No | N = 136 N = 136 |
| Tocilizumab (CHARISMA), Maini, 2006 [54] | 16/20 weeks | Non naive: MTX failure >6 months of treatment | LEF: 6 months Other 4 weeks | Naive or not | anti-TNF agents: 12 weeks | ND | TCZ + MTX TCZ | TCZ: 8 mg/kg IV/4 weeks MTX: 10–25 mg/Week PO | No | N = 50 N = 52 |
| Tocilizumab (COMP-ACT), Kremer, 2018 [49] | 24 weeks | Non naive: TCZ + MTX during 24 weeks before new randomization. | / | Non naive: TCZ + MTX for 24 weeks before new randomization. | / | ND | TCZ + MTX TCZ | TCZ: 162 mg/week (≥100 kg) or /2 weeks (<100 kg) MTX: >15 mg/week PO | No | N = 147 N = 147 |
| Tocilizumab (FUNCTION), Burmester, 2016 [45] | 24/52/104 weeks | CsDMARD-naive or not but MTX-naive | / | Naive | / | <2 years | TCZ + MTX TCZ | TCZ:8 mg/kg IV/4 wks MTX: 7.5–20 mg/Week PO | No | N = 291 N = 292 |
| Tocilizumab (JUST ACT), Pablos, 2019 [48] | 12 weeks | Non naive: TCZ + MTX 16 weeks before randomization. | / | Non naive: TCZ + MTX 16 weeks before randomization. | / | NR | TCZ + MTX TCZ | TCZ: 8 mg/kg IV/4 wks MTX: >15 mg/Wek PO | No | N = 83 N = 82 |
| Tocilizumab, (SURPRISE), Kaneko, 2015 [51] | 24/52/104 weeks | Non naive: MTX ≥6 mg/week for at least 8 weeks | LEF: 12 weeks, other: 8 weeks | Naive | Tacrolimus: 4 weeks | <10 years | TCZ + MTX TCZ | TCZ: 8 mg/ kg IV/4 wks MTX: >6 mg/Week PO | No | N = 118 N = 115 |
| Tocilizumab (U-ACT-EARLY), Bijlsma, 2016 [46] | 24/52/104 weeks | Naive | / | Naive | / | <1 year | TCZ + MTX TCZ | TCZ:8 mg/ kg IV/4 wks MTX: 10–30 mg/Week PO | No | N = 106 N = 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delpech, C.; Laborne, F.-X.; Hilliquin, P. Comparison of Biological Agent Monotherapy and Associations Including Disease-Modifying Antirheumatic Drugs for Rheumatoid Arthritis: Literature Review and Meta-Analysis of Randomized Trials. J. Clin. Med. 2023, 12, 286. https://doi.org/10.3390/jcm12010286
Delpech C, Laborne F-X, Hilliquin P. Comparison of Biological Agent Monotherapy and Associations Including Disease-Modifying Antirheumatic Drugs for Rheumatoid Arthritis: Literature Review and Meta-Analysis of Randomized Trials. Journal of Clinical Medicine. 2023; 12(1):286. https://doi.org/10.3390/jcm12010286
Chicago/Turabian StyleDelpech, Célia, François-Xavier Laborne, and Pascal Hilliquin. 2023. "Comparison of Biological Agent Monotherapy and Associations Including Disease-Modifying Antirheumatic Drugs for Rheumatoid Arthritis: Literature Review and Meta-Analysis of Randomized Trials" Journal of Clinical Medicine 12, no. 1: 286. https://doi.org/10.3390/jcm12010286
APA StyleDelpech, C., Laborne, F.-X., & Hilliquin, P. (2023). Comparison of Biological Agent Monotherapy and Associations Including Disease-Modifying Antirheumatic Drugs for Rheumatoid Arthritis: Literature Review and Meta-Analysis of Randomized Trials. Journal of Clinical Medicine, 12(1), 286. https://doi.org/10.3390/jcm12010286






