A Meta-Analysis and Systematic Review of Normothermic and Hypothermic Machine Perfusion in Liver Transplantation
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Quality Assessment
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
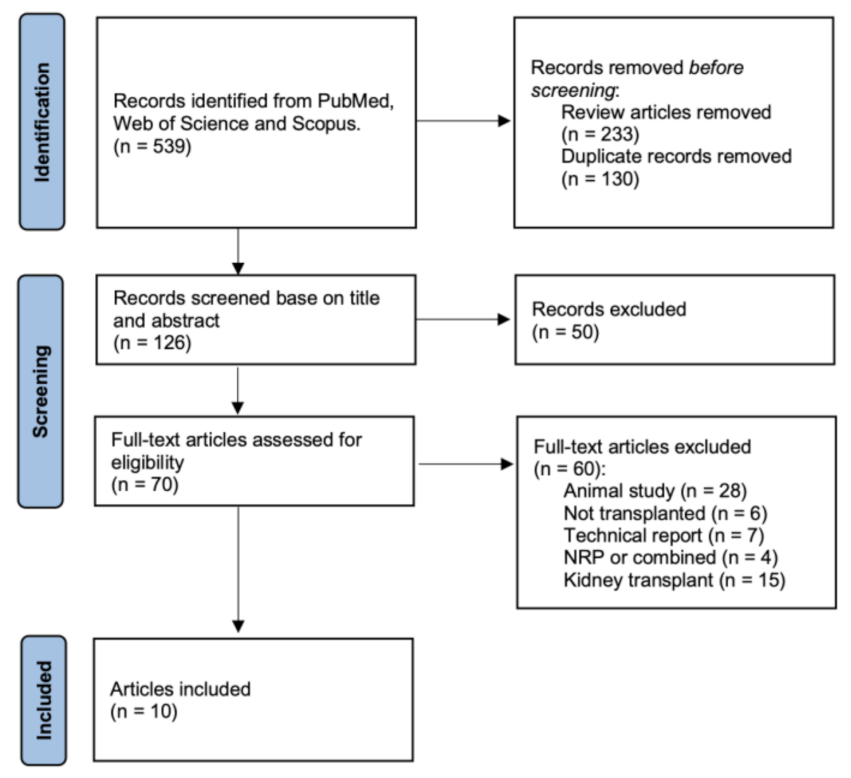
3.1. Literature Search Results
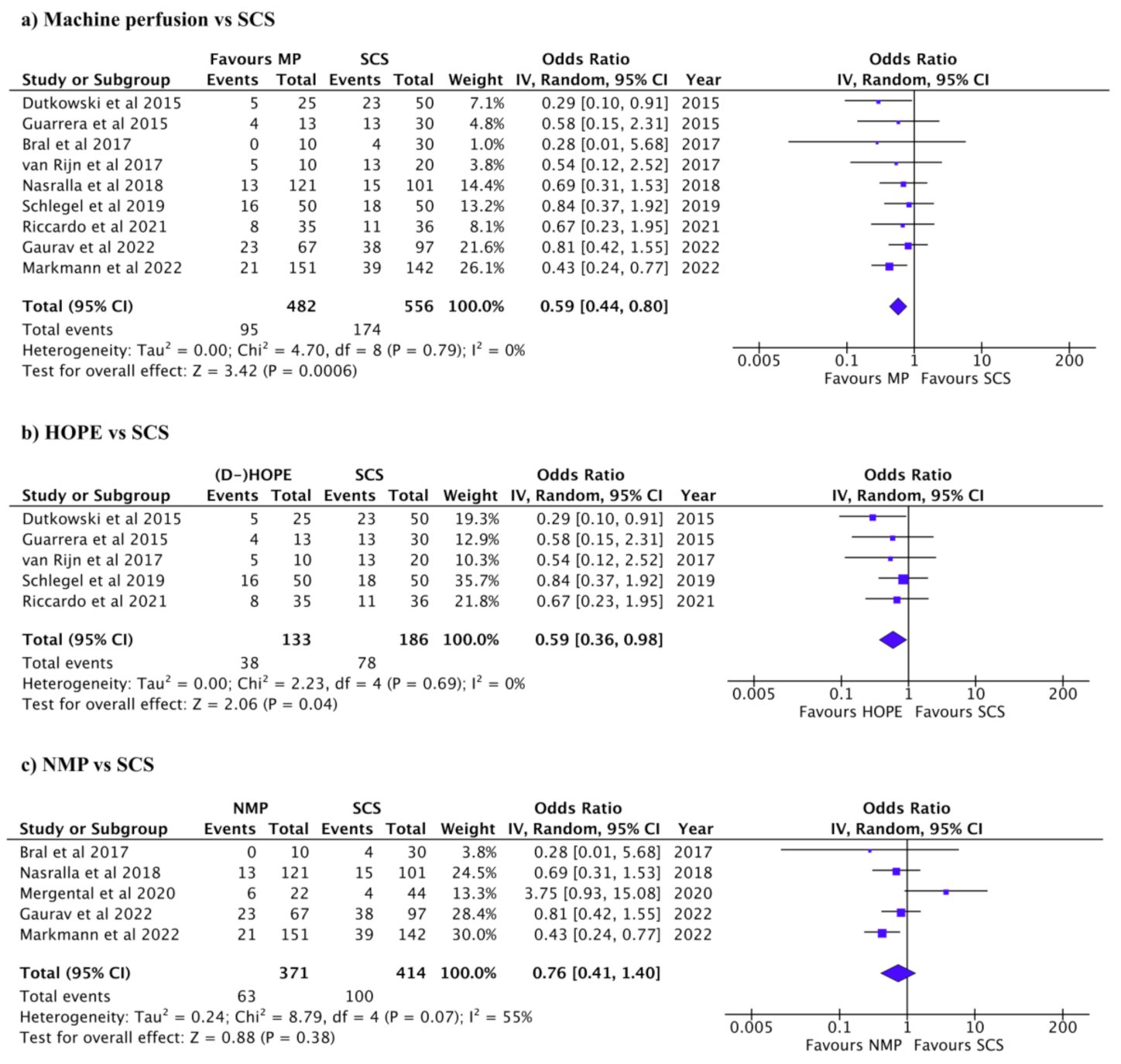
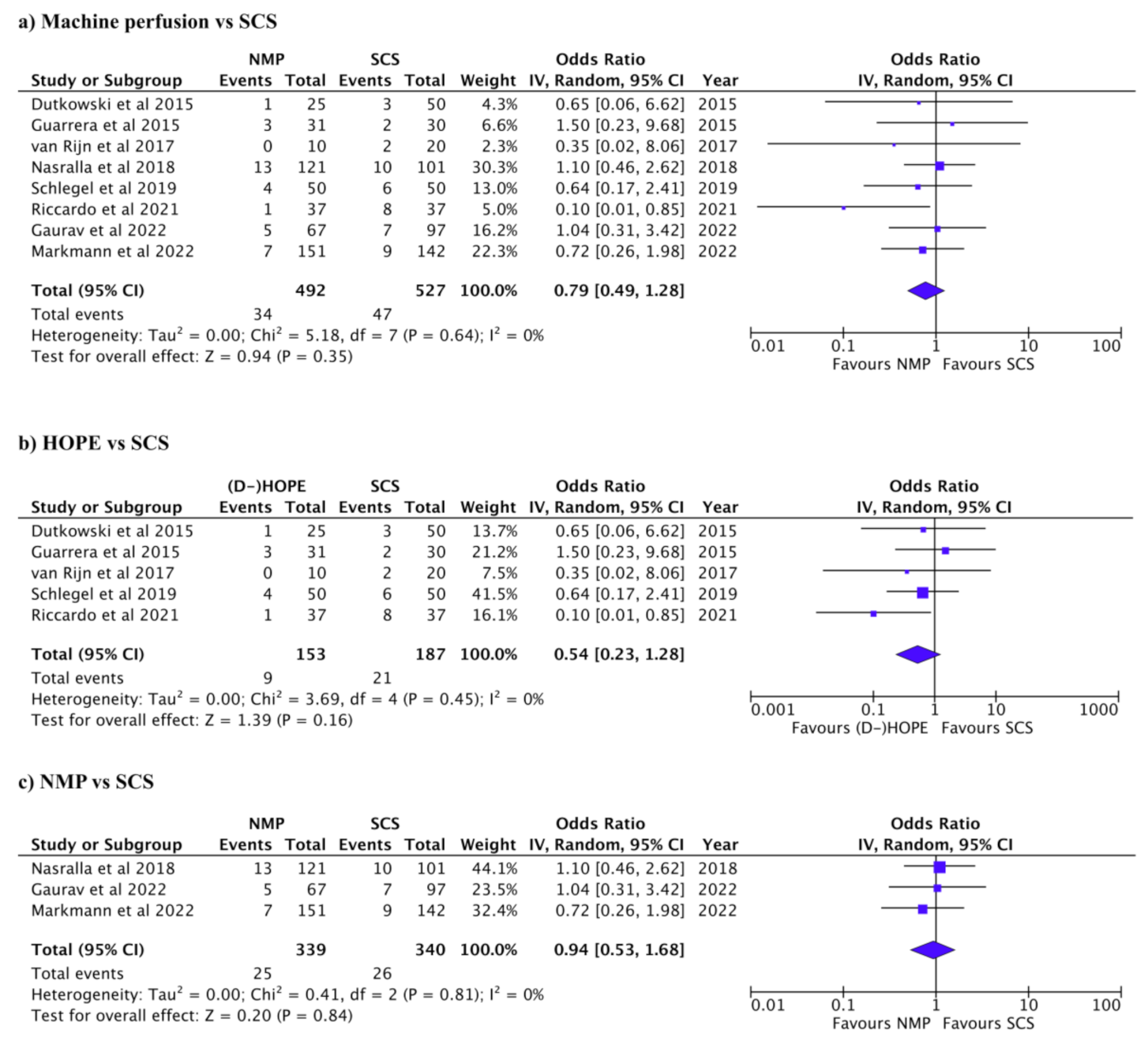
3.2. Complications after Liver Transplant
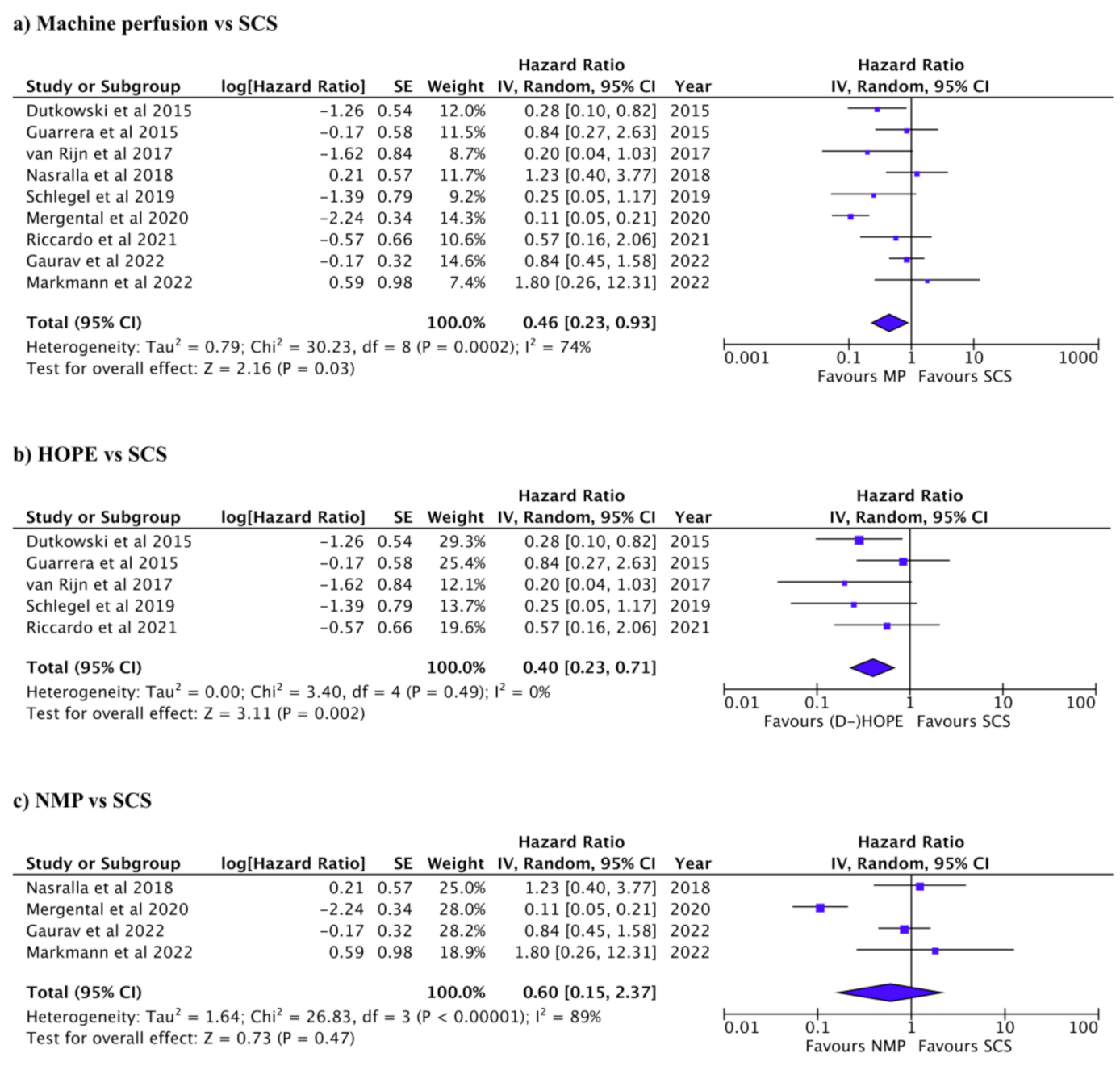
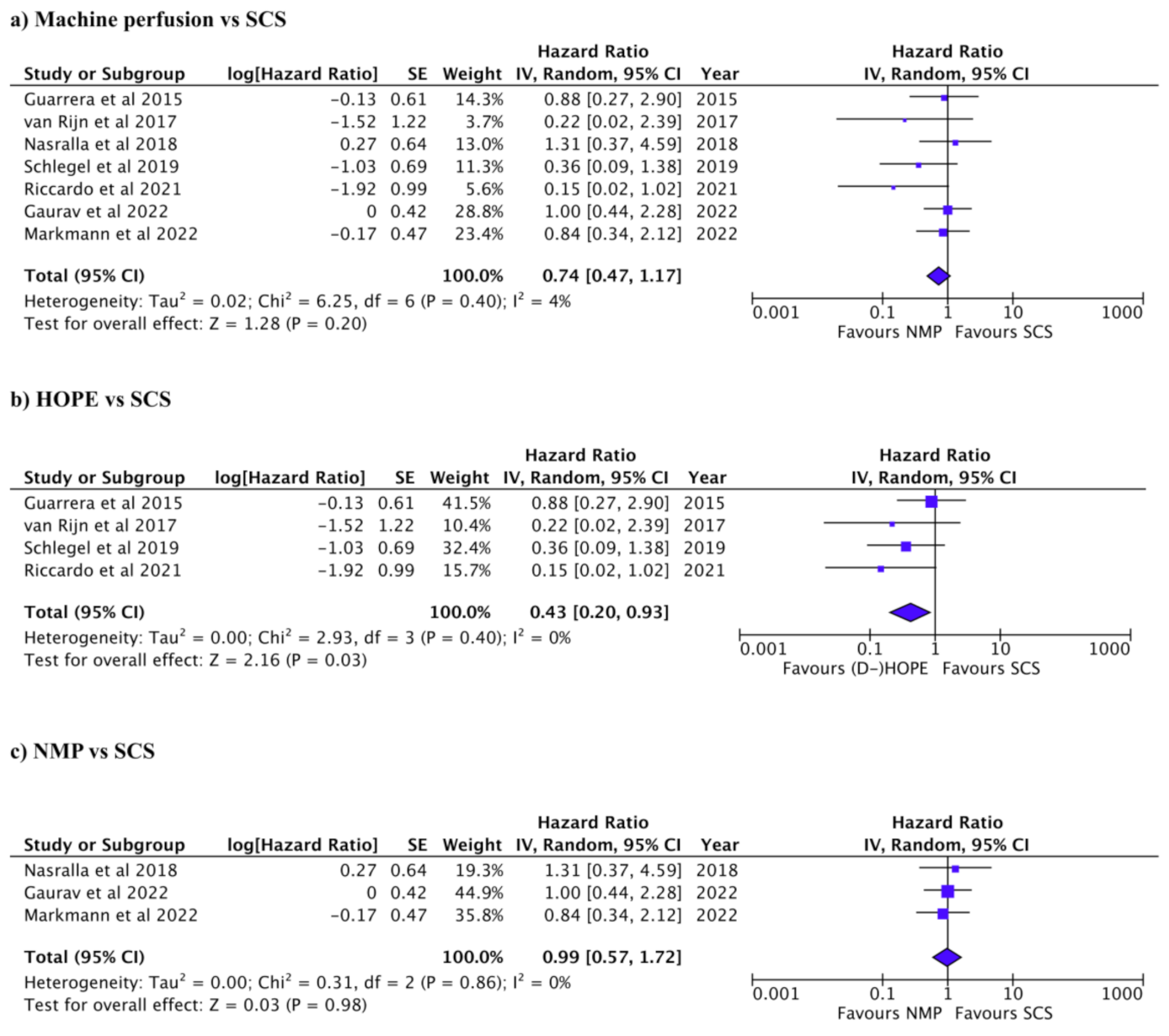
3.3. Graft and Patient Survival after Liver Transplant
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durand, F.; Renz, J.F.; Alkofer, B.; Burra, P.; Clavien, P.A.; Porte, R.J.; Freeman, R.B.; Belghiti, J. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transpl. 2008, 14, 1694–1707. [Google Scholar] [CrossRef]
- Morrissey, P.E.; Monaco, A.P. Donation after circulatory death: Current practices, ongoing challenges, and potential improvements. Transplantation 2014, 97, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Nemes, B.; Gámán, G.; Polak, W.G.; Gelley, F.; Hara, T.; Ono, S.; Baimakhanov, Z.; Piros, L.; Eguchi, S. Extended-criteria donors in liver transplantation Part II: Reviewing the impact of extended-criteria donors on the complications and outcomes of liver transplantation. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 841–859. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, C.J.; Charman, S.C.; Muiesan, P.; Powell, J.J.; Gimson1, A.E.; van der Meulen, J.H.P. Outcomes of transplantation of livers from donation after circulatory death donors in the UK: A cohort study. BMJ Open 2013, 3, e003287. [Google Scholar] [CrossRef] [PubMed]
- de Vries, Y.; von Meijenfeldt, F.A.; Porte, R.J. Post-transplant cholangiopathy: Classification, pathogenesis, and preventive strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1507–1515. [Google Scholar] [CrossRef]
- den Dulk, A.C.; Sebib Korkmaz, K.; de Rooij, B.J.F.; Sutton, M.E.; Braat, A.E.; Inderson, A.; Dubbeld, J.; Verspaget, H.W.; Porte, R.J.; van Hoek, B. High peak alanine aminotransferase determines extra risk for nonanastomotic biliary strictures after liver transplantation with donation after circulatory death. Transpl. Int. 2015, 28, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Mergental, H.; Laing, R.W.; Kirkham, A.J.; Perera, M.T.P.R.; Boteon, Y.L.; Attard, J.; Barton, D.; Curbishley, S.; Wilkhu, M.; Neil, D.A.; et al. Transplantation of discarded livers following viability testing with normothermic machine perfusion. Nat. Commun. 2020, 11, 2939. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, O.B.; de Vries, Y.; Fujiyoshi, M.; Nijsten, M.W.; Ubbink, R.; Pelgrim, G.J.; Werner, M.J.; Reyntjens, K.M.; van den Berg, A.P.; de Boer, M.T.; et al. Transplantation of High-risk Donor Livers After Ex Situ Resuscitation and Assessment Using Combined Hypo- and Normothermic Machine Perfusion: A Prospective Clinical Trial. Ann. Surg. 2019, 270, 906–914. [Google Scholar] [CrossRef]
- Schlegel, A.; Kron, P.; Dutkowski, P. Hypothermic machine perfusion in liver transplantation. Curr. Opin. Organ Transplant. 2016, 21, 308–314. [Google Scholar] [CrossRef]
- Detelich, D.; Markmann, J.F. The dawn of liver perfusion machines. Curr. Opin. Organ Transplant. 2018, 23, 151–161. [Google Scholar] [CrossRef]
- Nasralla, D.; Coussios, C.C.; Mergental, H.; Akhtar, M.Z.; Butler, A.J.; Ceresa, C.D.; Chiocchia, V.; Dutton, S.J.; García-Valdecasas, J.C.; Heaton, N.; et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018, 557, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, B.G.; Yeh, H.; Özer, S.; Martins, P.N.; Farmer, A.; Wu, W.; Saeidi, N.; Op den Dries, S.; Berendsen, T.A.; Smith, R.N.; et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am. J. Transplant. 2014, 14, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, B.G.; Berendsen, T.A.; Izamis, M.L.; Yarmush, M.L.; Uygun, K. Determination and extension of the limits to static cold storage using subnormothermic machine perfusion. Int. J. Artif. Organs 2013, 36, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Gringeri, E.; Bonsignore, P.; Bassi, D.; D’Amico, F.E.; Mescoli, C.; Polacco, M.; Buggio, M.; Luisetto, R.; Boetto, R.; Noaro, G.; et al. Subnormothermic machine perfusion for non-heart-beating donor liver grafts preservation in a Swine model: A new strategy to increase the donor pool? Transplant. Proc. 2012, 44, 2026–2028. [Google Scholar] [CrossRef]
- Mergental, H.; Perera, M.T.P.R.; Laing, R.W.; Muiesan, P.; Isaac, J.R.; Smith, A.; Stephenson, B.T.F.; Cilliers, H.; Neil, D.A.H.; Hübscher, S.G.; et al. Transplantation of Declined Liver Allografts Following Normothermic Ex-Situ Evaluation. Am. J. Transplant. 2016, 16, 3235–3245. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; de Rougemont, O.; Graf, R.; Clavien, P.A.; Dutkowski, P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J. Hepatol. 2013, 58, 278–286. [Google Scholar] [CrossRef]
- Patrono, D.; Surra, A.; Catalano, G.; Rizza, G.; Berchialla, P.; Martini, S.; Tandoi, F.; Lupo, F.; Mirabella, S.; Stratta, C.; et al. Hypothermic Oxygenated Machine Perfusion of Liver Grafts from Brain-Dead Donors. Sci. Rep. 2019, 9, 9337. [Google Scholar] [CrossRef]
- Schlegel, A.; Muller, X.; Kalisvaart, M.; Muellhaupt, B.; Perera, M.T.P.; Isaac, J.R.; Clavien, P.A.; Muiesan, P.; Dutkowski, P. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J. Hepatol. 2019, 70, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Burlage, L.C.; Hessels, L.; van Rijn, R.; Matton, A.P.; Fujiyoshi, M.; van den Berg, A.P.; Reyntjens, K.M.; Meyer, P.; de Boer, M.T.; de Kleine, R.H.; et al. Opposite acute potassium and sodium shifts during transplantation of hypothermic machine perfused donor livers. Am. J. Transplant. 2019, 19, 1061–1071. [Google Scholar] [CrossRef]
- van Rijn, R.; Karimian, N.; Matton, A.P.; Burlage, L.C.; Westerkamp, A.C.; van den Berg, A.P.; de Kleine, R.H.; de Boer, M.T.; Lisman, T.; Porte, R.J. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br. J. Surg. 2017, 104, 907–917. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, R.; Schurink, I.J.; de Vries, Y.; van den Berg, A.P.; Cortes Cerisuelo, M.; Darwish Murad, S.; Erdmann, J.I.; Gilbo, N.; de Haas, R.J.; Heaton, N.; et al. Hypothermic Machine Perfusion in Liver Transplantation—A Randomized Trial. N. Engl. J. Med. 2021, 384, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, R.; van Leeuwen, O.B.; Matton, A.P.; Burlage, L.C.; Wiersema-Buist, J.; van den Heuvel, M.C.; de Kleine, R.H.; de Boer, M.T.; Gouw, A.S.; Porte, R.J. Hypothermic oxygenated machine perfusion reduces bile duct reperfusion injury after transplantation of donation after circulatory death livers. Liver Transpl. 2018, 24, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P. Cochrane Handbook for Systematic Reviews of Interventions Version; John Wiley & Sons: Chichester, UK, 2022. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Bral, M.; Gala-Lopez, B.; Bigam, D.; Kneteman, N.; Malcolm, A.; Livingstone, S.; Andres, A.; Emamaullee, J.; Russell, L.; Coussios, C.; et al. Preliminary Single-Center Canadian Experience of Human Normothermic Ex Vivo Liver Perfusion: Results of a Clinical Trial. Am. J. Transplant. 2017, 17, 1071–1080. [Google Scholar] [CrossRef]
- De Carlis, R.; Schlegel, A.; Frassoni, S.; Olivieri, T.; Ravaioli, M.; Camagni, S.; Patrono, D.; Bassi, D.; Pagano, D.; Di Sandro, S.; et al. How to Preserve Liver Grafts from Circulatory Death With Long Warm Ischemia? A Retrospective Italian Cohort Study with Normothermic Regional Perfusion and Hypothermic Oxygenated Perfusion. Transplantation 2021, 105, 2385–2396. [Google Scholar] [CrossRef]
- Dutkowski, P.; Polak, W.G.; Muiesan, P.; Schlegel, A.; Verhoeven, C.J.; Scalera, I.; DeOliveira, M.L.; Kron, P.; Clavien, P.A. First Comparison of Hypothermic Oxygenated PErfusion Versus Static Cold Storage of Human Donation After Cardiac Death Liver Transplants: An International-matched Case Analysis. Ann. Surg. 2015, 262, 764–770. [Google Scholar] [CrossRef]
- Gaurav, R.; Butler, A.J.; Kosmoliaptsis, V.; Mumford, L.; Fear, C.; Swift, L.; Fedotovs, A.; Upponi, S.; Khwaja, S.; Richards, J.; et al. Liver Transplantation Outcomes from Controlled Circulatory Death Donors SCS vs in situ NRP vs ex situ NMP. Ann. Surg. 2022, 275, 1156–1164. [Google Scholar] [CrossRef]
- Guarrera, J.V.; Henry, S.D.; Samstein, B.; Reznik, E.; Musat, C.; Lukose, T.I.; Ratner, L.E.; Brown, R.S., Jr.; Kato, T.; Emond, J.C. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am. J. Transplant. 2015, 15, 161–169. [Google Scholar] [CrossRef]
- Markmann, J.F.; Abouljoud, M.S.; Ghobrial, R.M.; Bhati, C.S.; Pelletier, S.J.; Lu, A.D.; Ottmann, S.; Klair, T.; Eymard, C.; Roll, G.R.; et al. Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant The OCS Liver PROTECT Randomized Clinical Trial. Jama Surg. 2022, 157, 189–198. [Google Scholar] [CrossRef]
- Vodkin, I.; Kuo, A. Extended Criteria Donors in Liver Transplantation. Clin. Liver Dis. 2017, 21, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Guorgui, J.; Ito, T.; Younan, S.; Agopian, V.G.; Dinorcia, J., III; Farmer, D.G.; Busuttil, R.W.; Kaldas, F.M. The Utility of Extended Criteria Donor Livers in High Acuity Liver Transplant Recipients. Am. Surg. 2021, 87, 1684–1689. [Google Scholar] [CrossRef]
- Seidita, A.; Longo, R.; Di Francesco, F.; Tropea, A.; Calamia, S.; Panarello, G.; Barbara, M.; Gruttadauria, S. The use of normothermic machine perfusion to rescue liver allografts from expanded criteria donors. Updates Surg. 2022, 74, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhong, Z.; Lin, D.; Liu, Z.; Zhang, Q.; Xia, H.; Peng, S.; Liu, A.; Lu, Z.; Wang, Y.; et al. Hypothermic oxygenated perfusion inhibits HECTD3-mediated TRAF3 polyubiquitination to alleviate DCD liver ischemia-reperfusion injury. Cell Death Dis. 2021, 12, 211. [Google Scholar] [CrossRef]
- Schlegel, A.; Graf, R.; Clavien, P.A.; Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) protects from biliary injury in a rodent model of DCD liver transplantation. J. Hepatol. 2013, 59, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.J.E.; Kosmoliaptsis, V.; Randle, L.V.; Gimson, A.E.; Brais, R.; Klinck, J.R.; Hamed, M.; Tsyben, A.; Butler, A.J. Normothermic Perfusion in the Assessment and Preservation of Declined Livers Before Transplantation: Hyperoxia and Vasoplegia-Important Lessons From the First 12 Cases. Transplantation 2017, 101, 1084–1098. [Google Scholar] [CrossRef]
- Czigany, Z.; Lurje, I.; Tolba, R.H.; Neumann, U.P.; Tacke, F.; Lurje, G. Machine perfusion for liver transplantation in the era of marginal organs-New kids on the block. Liver Int. 2019, 39, 228–249. [Google Scholar] [CrossRef]
- Boteon, Y.L.; Boteon, Y.L.; Laing, R.W.; Schlegel, A.; Wallace, L.; Smith, A.; Attard, J.; Bhogal, R.H.; Neil, D.A.; Hübscher, S.; et al. Combined Hypothermic and Normothermic Machine Perfusion Improves Functional Recovery of Extended Criteria Donor Livers. Liver Transpl. 2018, 24, 1699–1715. [Google Scholar] [CrossRef] [PubMed]
- Matinlauri, I.H.; Nurminen, M.M.; Hockerstedt, K.A.; Isoniemi, H.M. Risk factors predicting survival of liver transplantation. Transplant. Proc. 2005, 37, 1155–1160. [Google Scholar] [CrossRef]
- Moore, D.E.; Feurer, I.D.; Speroff, T.; Gorden, D.L.; Wright, J.K.; Chari, R.S.; Pinson, C.W. Impact of donor, technical, and recipient risk factors on survival and quality of life after liver transplantation. Arch. Surg. 2005, 140, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Bastos-Neves, D.; Salvalaggio, P.R.O.; Almeida, M.D. Risk factors, surgical complications and graft survival in liver transplant recipients with early allograft dysfunction. Hepatobiliary Pancreat. Dis. Int. 2019, 18, 423–429. [Google Scholar] [CrossRef] [PubMed]

| References | Reporting | External Validity | Internal Validity (Risk of Bias) | Internal Validity (Confounding) | Power | Total Points |
|---|---|---|---|---|---|---|
| Dutkowski et al., 2015 | 10 | 3 | 5 | 3 | 1 | 22 |
| Guarrera et al., 2015 | 9 | 2 | 5 | 2 | 1 | 19 |
| Bral et al., 2017 | 8 | 3 | 6 | 2 | 1 | 20 |
| Van Rijn et al., 2017 | 8 | 3 | 6 | 4 | 1 | 22 |
| Nasralla et al., 2018 | 9 | 3 | 6 | 4 | 1 | 23 |
| Schlegel et al., 2019 | 8 | 3 | 5 | 4 | 1 | 21 |
| Mergental et al., 2020 | 8 | 3 | 5 | 2 | 1 | 19 |
| Riccardo et al., 2021 | 7 | 3 | 5 | 1 | 1 | 17 |
| Gaurav et al., 2022 | 8 | 3 | 5 | 1 | 1 | 18 |
| Markmann et al., 2022 | 8 | 3 | 5 | 3 | 1 | 20 |
| Maximum score | 11 | 3 | 7 | 6 | 5 | 32 |
| References | n | Age | MELD | CIT | Perfusion Time | ||||
|---|---|---|---|---|---|---|---|---|---|
| HOPE | SCS | HOPE | SCS | HOPE | SCS | HOPE | SCS | ||
| Dutkowski et al., 2015 | 25 | 50 | 60 (57–64) | 56 (49–59) | 13 (9–15) | 16 (10–21) | 188 (141–264) | 395 (349–447) | 317 (280–391) |
| Guarrera et al., 2015 * | 31 | 30 | 57.5 ± 8 | 58.4 ± 9.6 | 19.5 ± 5.9 | 21.4 ± 6.3 | 553 ± 96 | 516 ± 114 | 228 ± 54 |
| Van Rijn et al., 2017 | 10 | 20 | 57 (54–62) | 52 (42–60) | 16 (15–22) | 22 (17–27) | - | 503 (476–526) | 126 (123–135) |
| Schlegel et al., 2019 | 50 | 50 | 58 (56–62) | 57 (51–61) | 11 (8–14) | 11.8(8.5–15.8) | 264 (210–312) | 282 (258–318) | 120 (96–144) |
| Riccardo et al., 2021 | 37 | 37 | 58 (37–70) | 56 (38–66) | 9 (6–25) | 13 (6–19) | 411 (330–660) | 390 (240–583) | 120 (42–380) |
| References | n | Age | MELD | CIT | Perfusion Time | ||||
| NMP | SCS | NMP | SCS | NMP | SCS | NMP | SCS | ||
| Bral et al., 2017 | 10 | 30 | 53(28–67) | 59(43–69) | 13 (9–32) | 19 (7–34) | 167 (95–293) | 233 (64–890) | 690 (198–1350) |
| Nasralla et al., 2018 | 121 | 101 | 55(48–62) | 55(48–62) | 13 (10–18) | 14 (9–18) | 126 (106.5–143) | 465 (375–575) | 547.5(372.5–710.5) |
| Mergental et al., 2020 | 22 | 44 | 56(46–65) | - | 12 (9–16) | - | 452 (389–600) | - | 587 (450–705) |
| Gaurav et al., 2022 | 67 | 97 | 59(51–63) | 56(50–62) | 14 (10–18) | 16 (13–20) | 396 (346–441) | 430 (397–474) | 460 (330–569) |
| Markmann et al., 2022 * | 151 | 142 | 57 ± 10.3 | 58.4 ± 10.1 | 28.4 ± 6.9 | 28 ± 5.7 | 175. 4 ± 43.5 | 338.8 ± 91.5 | 276.6 ± 117.4 |
| References | Biliary Complications | Vascular Complications | PNF | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | MP | SCS | Total | MP | SCS | Total | MP | SCS | |
| Dutkowski et al., 2015 | 28 | 5 | 23 | 4 | 1 | 3 | 10 | 7 | 3 |
| Guarrera et al., 2015 | 17 | 4 | 13 | 5 | 3 | 2 | 3 | 1 | 2 |
| Bral et al., 2017 | 4 | 0 | 4 | - | - | - | - | - | - |
| Van Rijn et al., 2017 | 18 | 5 | 13 | 2 | 0 | 2 | - | - | - |
| Nasralla et al., 2018 | 28 | 13 | 15 | 23 | 13 | 10 | 2 | 2 | 0 |
| Schlegel et al., 2019 | 34 | 16 | 18 | 10 | 4 | 6 | 1 | 1 | 0 |
| Mergental et al., 2020 | - | - | - | - | - | - | 1 | 0 | 1 |
| Riccardo et al., 2021 | 19 | 8 | 11 | 9 | 1 | 8 | - | - | - |
| Gaurav et al., 2022 | 61 | 23 | 38 | 12 | 5 | 7 | 6 | 1 | 5 |
| Markmann et al., 2022 | 60 | 21 | 39 | 16 | 7 | 9 | - | - | - |
| References | Proportion (%) Graft Survival | ||||
|---|---|---|---|---|---|
| 6 months | 1 Year | ||||
| MP | SCS | MP | SCS | ||
| Dutkowski et al., 2015 | - | - | 90 | 69 | |
| Guarrera et al., 2015 | - | - | 81 | 80 | |
| Bral et al., 2017 | 80 | 100 | - | - | |
| Van Rijn et al., 2017 | 100 | 80 | 100 | 67 | |
| Nasralla et al., 2018 | - | - | 95 | 96 | |
| Schlegel et al., 2019 | - | - | 90 | 82 | |
| Mergental et al., 2020 | - | - | 86.4 | 86.4 | |
| Riccardo et al., 2021 | - | - | 91.8 | 83.8 | |
| Gaurav et al., 2022 | 90 | 87 | 75 | 83 | |
| Markmann et al., 2022 | 99 | 99 | 98 | 99 | |
| References | Proportion (%) Patient Survival | ||
|---|---|---|---|
| 1 Year | |||
| MP | SCS | ||
| Dutkowski et al., 2015 | - | - | |
| Guarrera et al., 2015 | 84 | 80 | |
| Bral et al., 2017 | 100 | 85 | |
| Van Rijn et al., 2017 | 100 | 67 | |
| Nasralla et al., 2018 | 95 | 97 | |
| Schlegel et al., 2019 | 98 | 86 | |
| Mergental et al., 2020 | 100 | 95.5 | |
| Riccardo et al., 2021 | 100 | 91.8 | |
| Gaurav et al., 2022 | 80 | 94 | |
| Markmann et al., 2022 | 94 | 93.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mugaanyi, J.; Dai, L.; Lu, C.; Mao, S.; Huang, J.; Lu, C. A Meta-Analysis and Systematic Review of Normothermic and Hypothermic Machine Perfusion in Liver Transplantation. J. Clin. Med. 2023, 12, 235. https://doi.org/10.3390/jcm12010235
Mugaanyi J, Dai L, Lu C, Mao S, Huang J, Lu C. A Meta-Analysis and Systematic Review of Normothermic and Hypothermic Machine Perfusion in Liver Transplantation. Journal of Clinical Medicine. 2023; 12(1):235. https://doi.org/10.3390/jcm12010235
Chicago/Turabian StyleMugaanyi, Joseph, Lei Dai, Changjiang Lu, Shuqi Mao, Jing Huang, and Caide Lu. 2023. "A Meta-Analysis and Systematic Review of Normothermic and Hypothermic Machine Perfusion in Liver Transplantation" Journal of Clinical Medicine 12, no. 1: 235. https://doi.org/10.3390/jcm12010235
APA StyleMugaanyi, J., Dai, L., Lu, C., Mao, S., Huang, J., & Lu, C. (2023). A Meta-Analysis and Systematic Review of Normothermic and Hypothermic Machine Perfusion in Liver Transplantation. Journal of Clinical Medicine, 12(1), 235. https://doi.org/10.3390/jcm12010235







