Efficacy of AI-Assisted Personalized Microbiome Modulation by Diet in Functional Constipation: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
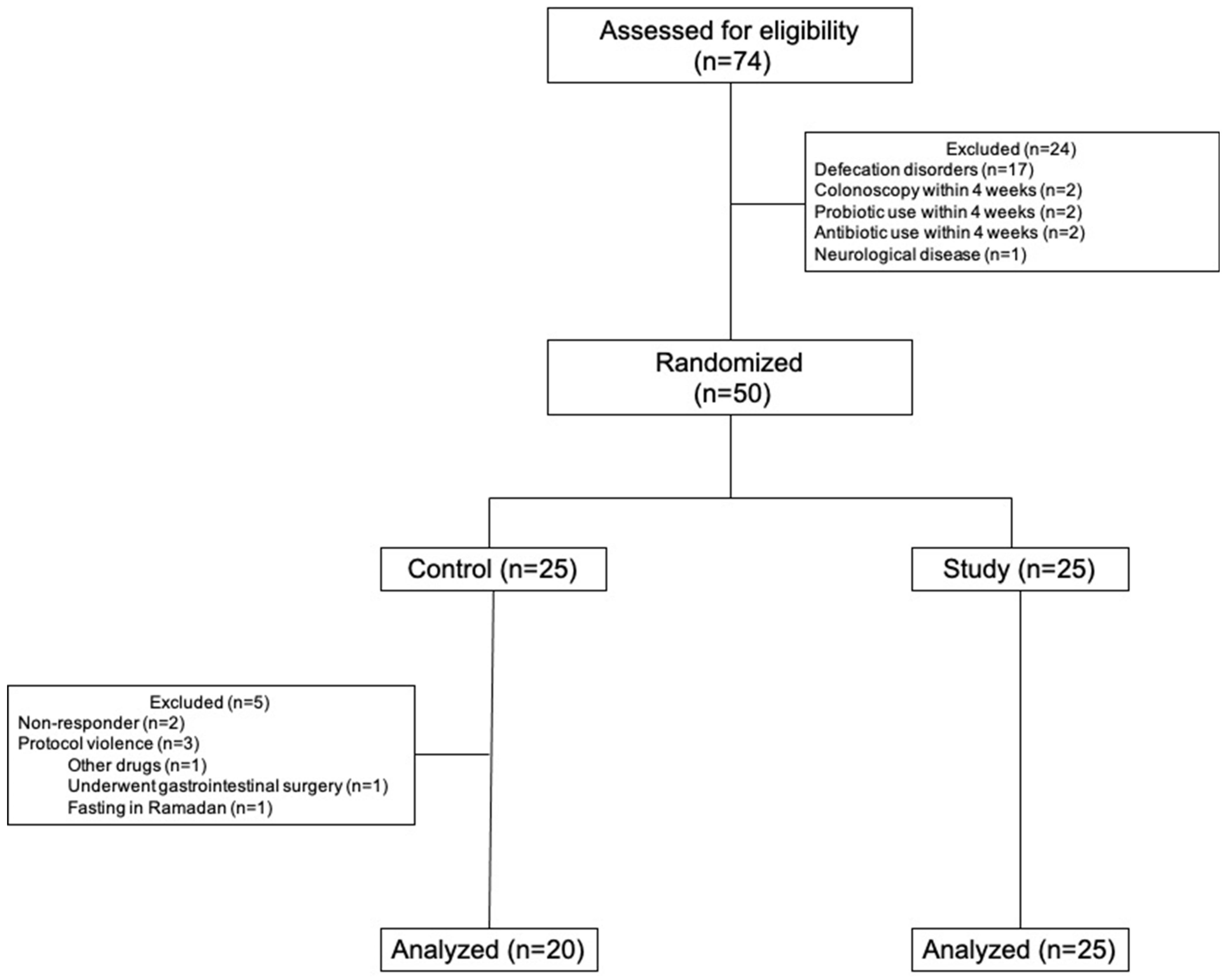
2.1. Study Design and Groups
2.2. Fecal Sampling and 16S Ribosomal RNA Gene Sequencing
2.3. The AI-Based Personalized Nutrition Model
2.4. Assessments and Follow-Up
2.5. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ford, A.C.; Suares, N.C. Effect of Laxatives and Pharmacological Therapies in Chronic Idiopathic Constipation: Systematic Review and Meta-Analysis. Gut 2011, 60, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Neri, L.; Basilisco, G.; Corazziari, E.; Stanghellini, V.; Bassotti, G.; Bellini, M.; Perelli, I.; Cuomo, R. Constipation Severity Is Associated with Productivity Losses and Healthcare Utilization in Patients with Chronic Constipation. United Eur. Gastroenterol. J. 2014, 2, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef] [PubMed]
- Luthra, P.; Camilleri, M.; Burr, N.E.; Quigley, E.M.M.; Black, C.J.; Ford, A.C. Efficacy of Drugs in Chronic Idiopathic Constipation: A Systematic Review and Network Meta-Analysis. Lancet Gastroenterol. Hepatol. 2019, 4, 831–844. [Google Scholar] [CrossRef]
- Aziz, I.; Whitehead, W.E.; Palsson, O.S.; Törnblom, H.; Simrén, M. An Approach to the Diagnosis and Management of Rome IV Functional Disorders of Chronic Constipation. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 39–46. [Google Scholar] [CrossRef]
- Belsey, J.; Greenfield, S.; Candy, D.; Geraint, M. Systematic Review: Impact of Constipation on Quality of Life in Adults and Children. Aliment. Pharmacol. Ther. 2010, 31, 938–949. [Google Scholar] [CrossRef]
- Johanson, J.F.; Kralstein, J. Chronic Constipation: A Survey of the Patient Perspective. Aliment. Pharmacol. Ther. 2007, 25, 599–608. [Google Scholar] [CrossRef]
- Sun, S.X.; Dibonaventura, M.; Purayidathil, F.W.; Wagner, J.S.; Dabbous, O.; Mody, R. Impact of Chronic Constipation on Health-Related Quality of Life, Work Productivity, and Healthcare Resource Use: An Analysis of the National Health and Wellness Survey. Dig. Dis. Sci. 2011, 56, 2688–2695. [Google Scholar] [CrossRef]
- Dennison, C.; Prasad, M.; Lloyd, A.; Bhattacharyya, S.K.; Dhawan, R.; Coyne, K. The Health-Related Quality of Life and Economic Burden of Constipation. Pharmacoeconomics 2005, 23, 461–476. [Google Scholar] [CrossRef]
- Lindberg, G.; Hamid, S.S.; Malfertheiner, P.; Thomsen, O.O.; Fernandez, L.B.; Garisch, J.; Thomson, A.; Goh, K.-L.; Tandon, R.; Fedail, S.; et al. World Gastroenterology Organisation Global Guideline: Constipation—A Global Perspective. J. Clin. Gastroenterol. 2011, 45, 483–487. [Google Scholar] [CrossRef]
- Bassotti, G.; Usai-Satta, P.; Bellini, M. Linaclotide for the Treatment of Chronic Constipation. Expert Opin. Pharmacother. 2018, 19, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Vriesman, M.H.; Koppen, I.J.N.; Camilleri, M.; di Lorenzo, C.; Benninga, M.A. Management of Functional Constipation in Children and Adults. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 21–39. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pẽa, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; Desantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An Improved Greengenes Taxonomy with Explicit Ranks for Ecological and Evolutionary Analyses of Bacteria and Archaea. ISME J. 2011, 6, 610–618. [Google Scholar] [CrossRef]
- Karakan, T.; Gundogdu, A.; Alagözlü, H.; Ekmen, N.; Ozgul, S.; Tunali, V.; Hora, M.; Beyazgul, D.; Nalbantoglu, O.U. Artificial Intelligence-Based Personalized Diet: A Pilot Clinical Study for Irritable Bowel Syndrome. Gut Microbes 2022, 14, 2138672. [Google Scholar] [CrossRef]
- Bengi, G.; Yalçın, M.; Akpınar, H.; Keskinoğlu, P.; Ellidokuz, H. Validity and reliability of the patient assessment of constipation quality of life questionnaire for the Turkish population. Turk. J. Gastroenterol. 2015, 26, 309–314. [Google Scholar] [CrossRef]
- Forootan, M.; Bagheri, N.; Darvishi, M. Chronic Constipation: A Review of Literature. Medicine 2018, 97, e10631. [Google Scholar] [CrossRef]
- Sample Size Calculator by Raosoft, Inc. Available online: http://www.raosoft.com/samplesize.html (accessed on 5 October 2022).
- Marietta, E.; Horwath, I.; Taneja, V. Microbiome, Immunomodulation, and the Neuronal System. Neurotherapeutics 2018, 15, 23–30. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, G.; Cao, H.; Yu, D.; Fang, X.; de Vos, W.M.; Wu, H. Gut Dysbacteriosis and Intestinal Disease: Mechanism and Treatment. J. Appl. Microbiol. 2020, 129, 787–805. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Choi, S.C.; Park, K.S.; Park, M.I.; Shin, J.E.; Lee, T.H.; Jung, K.W.; Koo, H.S.; Myung, S.J. Change of Fecal Flora and Effectiveness of the Short-Term VSL#3 Probiotic Treatment in Patients with Functional Constipation. J. Neurogastroenterol. Motil. 2015, 21, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, W.; Alkhouri, R.; Baker, R.D.; Bard, J.E.; Quigley, E.M.; Baker, S.S. Structural Changes in the Gut Microbiome of Constipated Patients. Physiol. Genom. 2014, 46, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.T.A.; Milke-García, M.P.; Argüello-Arévalo, G.A.; de la Barca, A.M.C.; Carmona-Sánchez, R.I.; Consuelo-Sánchez, A.; Coss-Adame, E.; García-Cedillo, M.F.; Hernández-Rosiles, V.; Icaza-Chávez, M.E.; et al. Dietary Fiber and the Microbiota: A Narrative Review by a Group of Experts from the Asociación Mexicana de Gastroenterología. Rev. Gastroenterol. Mex. 2021, 86, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Mearin, F.; Ciriza, C.; Mínguez, M.; Rey, E.; Mascort, J.J.; Peña, E.; Cañones, P.; Júdez, J. Clinical Practice Guideline: Irritable Bowel Syndrome with Constipation and Functional Constipation in the Adult. Rev. Esp. Enferm. Dig. 2016, 108, 332–363. [Google Scholar] [CrossRef]
| Variables | Total (n = 45) | Control Group (n = 20) | Study Group (n = 25) | p |
|---|---|---|---|---|
| Age (years, mean ± SD) | 31.5 ± 10.2 | 34.5 ± 11.4 | 29.1 ± 8.6 | 0.76 * |
| Gender | 0.608 ** | |||
| Male | 5 (11.1) | 2 (10) | 3 (12) | |
| Female | 40 (88.9) | 18 (90) | 22 (88) | |
| BMI (kg/m2 mean ± SD) | 26.3 ± 5.1 | 26.1 ± 5 | 26.5 ± 5.3 | 0.786 * |
| Constipation duration (months, mean ± SD) | 88.8 ± 66.9 | 91.9 ± 75.9 | 86.2 ± 60.2 | 0.778 * |
| CBMpW (n, mean ± SD) | 1.9 ± 1.92 | 2.1 ± 2.2 | 1.7 ± 1.6 | 0.374 *** |
| CBMpW ≥ 3 (n, %) | 6 (13.3) | 4 (20) | 2 (8) | 0.383 * |
| PAC-QoL subscales (points, mean ± SD) | ||||
| Physical discomfort | 10.33 ± 2.5 | 10 ± 2.4 | 10.5 ± 2.5 | 0.494 * |
| Psychosocial discomfort | 17.33 ± 5.3 | 20.3 ± 4 | 15 ± 5.1 | 0.001 * |
| Worries and discomfort | 30.8 ± 9.4 | 32.3 ± 5.8 | 29.6 ± 11.4 | 0.314 *** |
| Satisfaction | 3.2 ± 2.1 | 3.3 ± 2.2 | 3.1 ± 2.1 | 0.736 * |
| Total PAC-QoL score (points, mean ± SD) | 55.3 ± 14.6 | 59.3 ± 10.4 | 52.1 ± 16.9 | 0.101 * |
| Control Group | Study Group | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | After 10 Weeks | T | p * | Baseline | After 10 Weeks | T | p * | |
| CBMpW (n, mean ± SD) | 2.1 ± 2.2 | 2.8 ± 2 | −3.462 | 0.003 | 1.7 ± 1.6 | 4.3 ± 1.8 | −10.718 | <0.001 |
| PAC-QoL (points, mean ± SD) | ||||||||
| Physical discomfort | 10.1 ± 2.4 | 9.8 ± 2.3 | 0.677 | 0.506 | 10.6 ± 2.5 | 5 ± 3.9 | 6.551 | <0.001 |
| Psychosocial discomfort | 20.3 ± 4 | 19.4 ± 3.5 | 1.294 | 0.211 | 15 ± 5.1 | 6.5 ± 5.3 | 6.987 | <0.001 |
| Worries and discomfort | 32.3 ± 5.9 | 29.8 ± 5.7 | 2.708 | 0.014 | 29.6 ± 11.5 | 15.2 ± 8.1 | 6.982 | <0.001 |
| Satisfaction | 3.3 ± 2.2 | 3.9 ± 2.3 | −1.332 | 0.199 | 3.1 ± 2.1 | 10.7 ± 3.5 | −9.553 | <0.001 |
| Total PAC-QoL score (points, mean ± SD) | 59.3 ± 10.4 | 55 ± 8.5 | 3.155 | 0.005 | 52.1 ± 16.9 | 15.9 ± 16 | 9.317 | <0.001 |
| Variables | Total (n = 45) | Control Group (n = 20) | Study Group (n = 25) | p |
|---|---|---|---|---|
| CBMpW (n, mean ± SD) | 3.6 ± 2 | 2.8 ± 2 | 4.3 ± 1.8 | 0.013 * |
| PAC-QoL (points, mean ± SD) | ||||
| Physical discomfort | 7.1 ± 4.1 | 9.8 ± 2.3 | 5 ± 3.9 | <0.001 * |
| Psychosocial discomfort | 12.2 ± 7.9 | 19.3 ± 3.5 | 6.5 ± 5.4 | <0.001 ** |
| Worries and discomfort | 21.7 ± 10.2 | 29.9 ± 5.6 | 15.2 ± 8.1 | <0.001 * |
| Satisfaction | 7.7 ± 4.5 | 3.9 ± 2.3 | 10.7 ± 3.5 | <0.001 ** |
| Total PAC-QoL score (points, mean ± SD) | 33.3 ± 23.6 | 55.1 ± 8.5 | 15.9 ± 16 | <0.001 ** |
| 50% improvement in total score (n, %) | 30 (66.7) | 8 (40) | 22 (88) | 0.001 *** |
| CBMpW ≥ 3 (n, %) | 29 (64.4) | 8 (40) | 21 (84) | 0.003 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arslan, N.Ç.; Gündoğdu, A.; Tunali, V.; Topgül, O.H.; Beyazgül, D.; Nalbantoğlu, Ö.U. Efficacy of AI-Assisted Personalized Microbiome Modulation by Diet in Functional Constipation: A Randomized Controlled Trial. J. Clin. Med. 2022, 11, 6612. https://doi.org/10.3390/jcm11226612
Arslan NÇ, Gündoğdu A, Tunali V, Topgül OH, Beyazgül D, Nalbantoğlu ÖU. Efficacy of AI-Assisted Personalized Microbiome Modulation by Diet in Functional Constipation: A Randomized Controlled Trial. Journal of Clinical Medicine. 2022; 11(22):6612. https://doi.org/10.3390/jcm11226612
Chicago/Turabian StyleArslan, Naciye Çiğdem, Aycan Gündoğdu, Varol Tunali, Oğuzhan Hakan Topgül, Damla Beyazgül, and Özkan Ufuk Nalbantoğlu. 2022. "Efficacy of AI-Assisted Personalized Microbiome Modulation by Diet in Functional Constipation: A Randomized Controlled Trial" Journal of Clinical Medicine 11, no. 22: 6612. https://doi.org/10.3390/jcm11226612
APA StyleArslan, N. Ç., Gündoğdu, A., Tunali, V., Topgül, O. H., Beyazgül, D., & Nalbantoğlu, Ö. U. (2022). Efficacy of AI-Assisted Personalized Microbiome Modulation by Diet in Functional Constipation: A Randomized Controlled Trial. Journal of Clinical Medicine, 11(22), 6612. https://doi.org/10.3390/jcm11226612






