Elevated Circulating Lactate Levels and Widespread Expression of Its Cognate Receptor, Hydroxycarboxylic Acid Receptor 1 (HCAR1), in Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Samples and Lactate Analysis
2.2. Bioinformatic Analysis
2.3. Immunohistochemistry
2.4. Statistical Analysis
3. Results
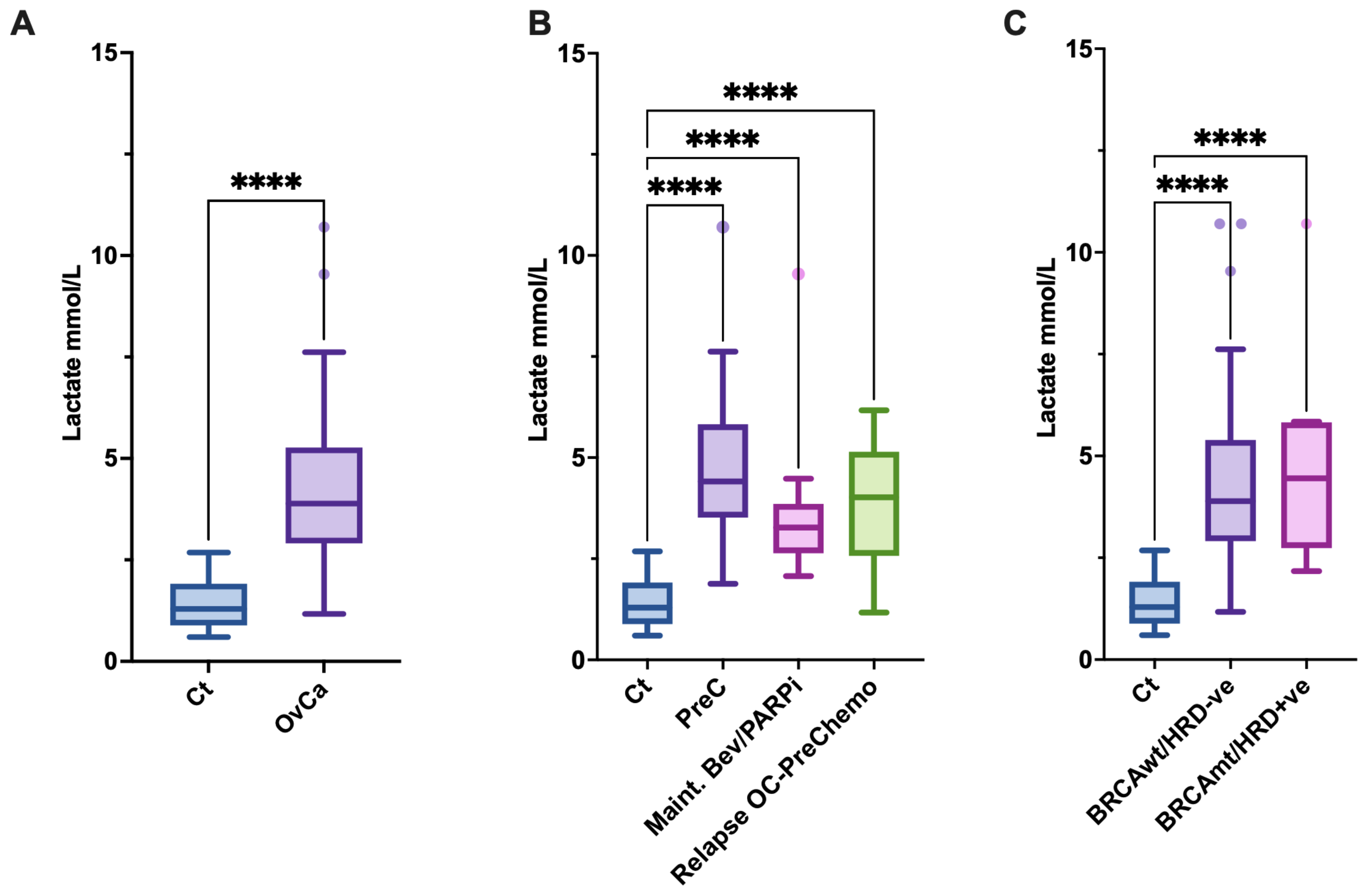
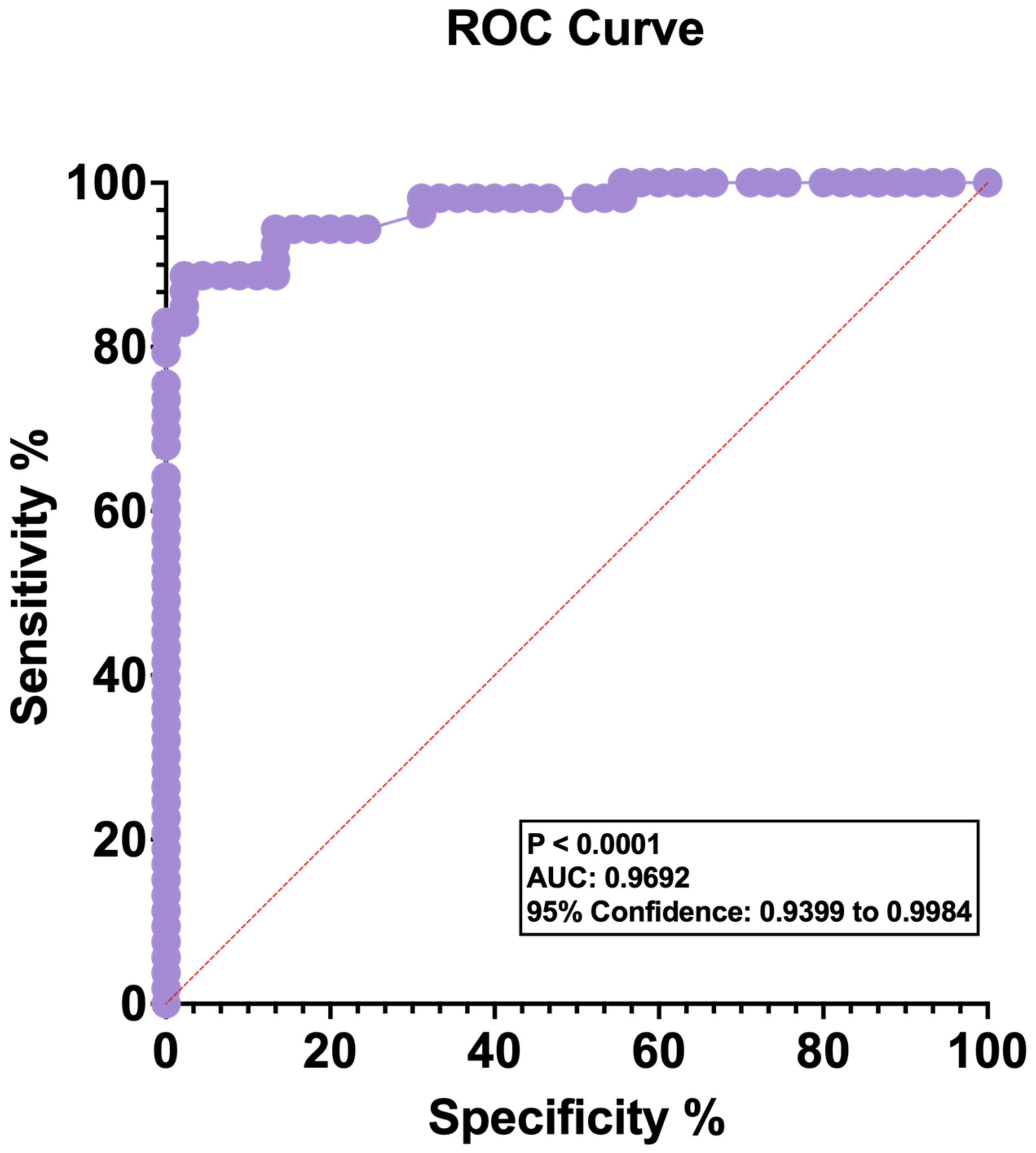
3.1. Blood Lactate Levels Are Elevated in Patients with OvCa
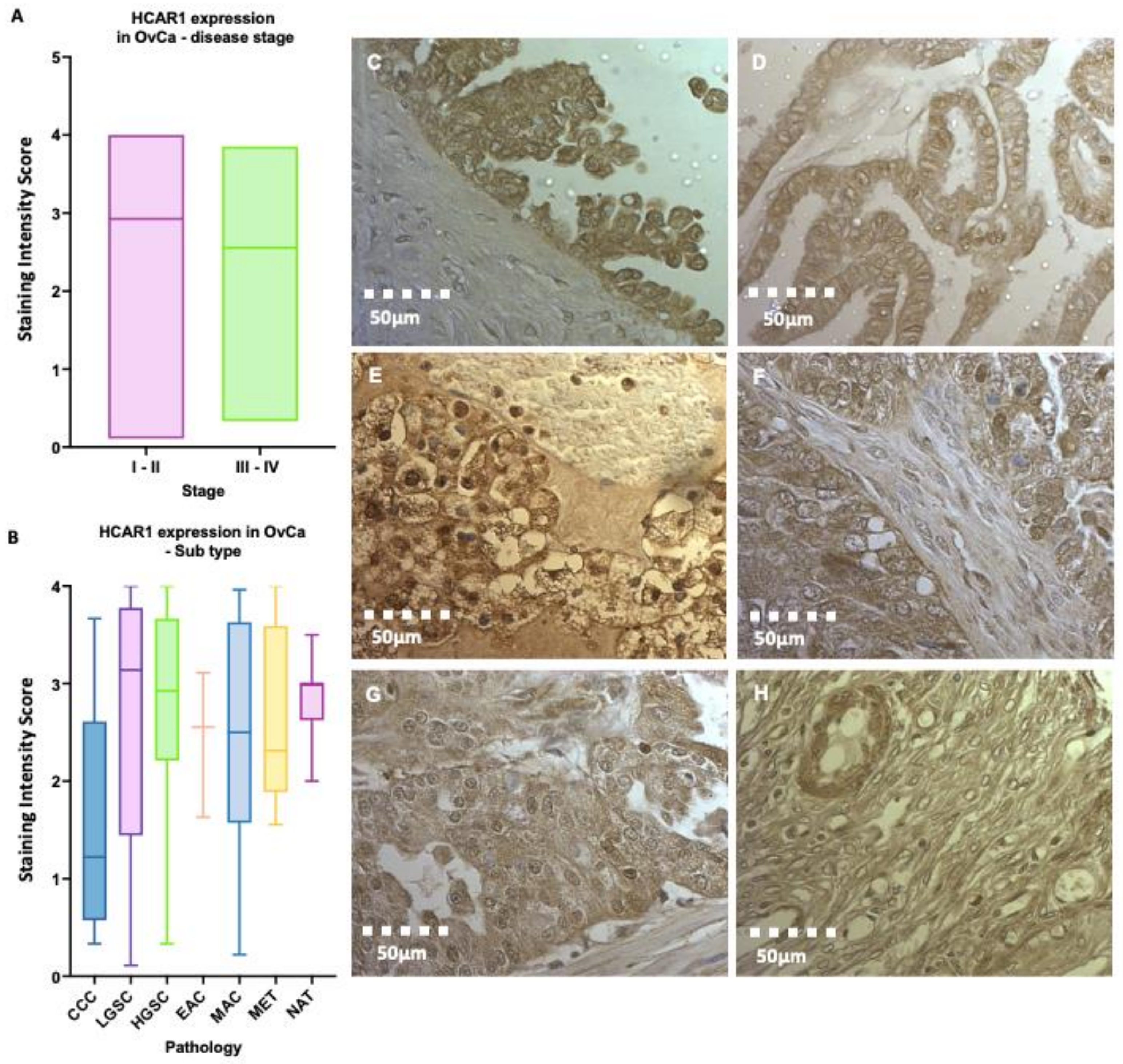
3.2. Elevated Gene Expression of the Lactate Receptor HCAR1 in OvCa
3.3. High HCAR1 Protein Expression across Epithelial Subtypes of OvCa
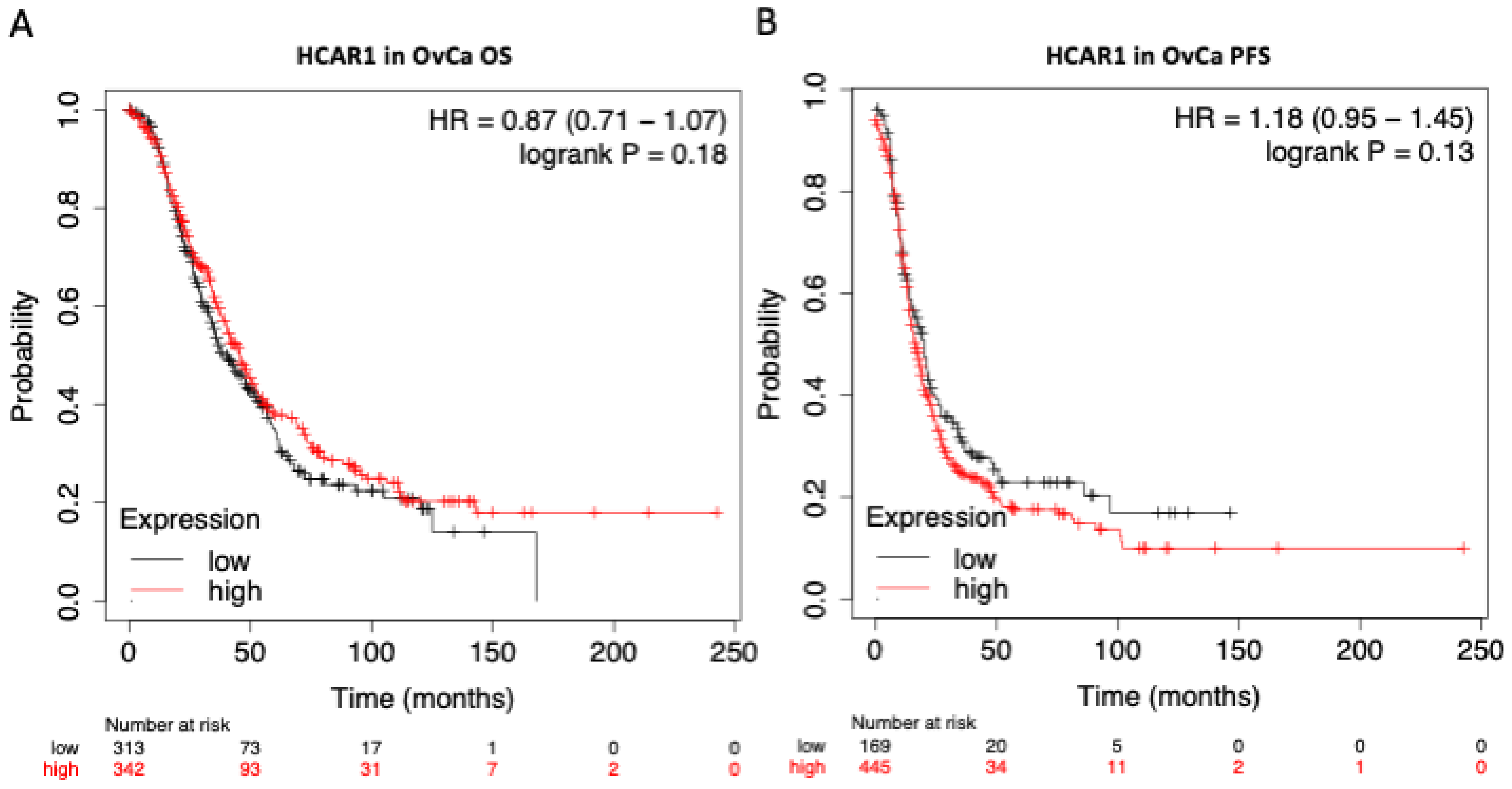
3.4. HCAR1 Expression Shows Little Influence on the Overall Survival (OS) or the Progression Free Survival (PFS) of Patients with OvCa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alberts, B. Molecular Biology of the Cell, 6th ed.; Garland Science, Taylor and Francis Group: Abingdon, UK, 2018; Available online: http://localhost/site/catalogue/7329 (accessed on 26 July 2020).
- Larsen, T. Fluorometric determination of d-lactate in biological fluids. Anal. Biochem. 2017, 539, 152–157. [Google Scholar] [CrossRef]
- Wacharasint, P.; Nakada, T.A.; Boyd, J.H.; Russell, J.A.; Walley, K.R. Normal-range blood lactate concentration in septic shock is prognostic and predictive. Shock 2012, 38, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, M.L.; Harris, J.E.; Hernández, A.; Gladden, L.B. Blood Lactate Measurements and Analysis during Exercise: A Guide for Clinicians. J. Diabetes Sci. Technol. 2007, 1, 558. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Nijsten, M.W.N.; Jansen, T.C. Clinical use of lactate monitoring in critically ill patients. Ann. Intensive Care 2013, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- San-Millán, I.; Brooks, G.A. Reexamining cancer metabolism: Lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 2017, 38, 119–133. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? In Trends in Biochemical Sciences; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; Volume 41, pp. 211–218. [Google Scholar] [CrossRef]
- Tanner, L.B.; Goglia, A.; Wei, M.; Sehgal, T.; Parsons, L.R.; Park, J.; White, E.; Toettcher, J.E.; Rabinowitz, J.D. Four key steps control glycolytic flux in mammalian cells. Cell Syst. 2018, 7, 49. [Google Scholar] [CrossRef]
- Pizzuti, L.; Sergi, D.; Mandoj, C.; Antoniani, B.; Sperati, F.; Chirico, A.; Di Lauro, L.; Valle, M.; Garofalo, A.; Vizza, E.; et al. GLUT 1 receptor expression and circulating levels of fasting glucose in high grade serous ovarian cancer. J. Cell. Physiol. 2018, 233, 1396–1401. [Google Scholar] [CrossRef]
- Rattigan, Y.I.; Patel, B.B.; Ackerstaff, E.; Sukenick, G.; Koutcher, J.A.; Glod, J.W.; Banerjee, D. Lactate is a mediator of metabolic cooperation between stromal carcinoma associated fibroblasts and glycolytic tumor cells in the tumor microenvironment. Exp. Cell Res. 2012, 318, 326–335. [Google Scholar] [CrossRef]
- Dhup, S.; Dadhich, R.K.; Porporato, P.E.; Sonveaux, P. Multiple biological activities of lactic acid in cancer: Influences on tumor growth, angiogenesis and metastasis. Curr. Pharm. Des. 2012, 18, 1319–1330. [Google Scholar] [CrossRef]
- Miao, P.; Sheng, S.; Sun, X.; Liu, J.; Huang, G. Lactate dehydrogenase A in cancer: A promising target for diagnosis and therapy. IUBMB Life 2013, 65, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Di Cello, A.; Borelli, M.; Marra, M.L.; Franzon, M.; D’Alessandro, P.; Di Carlo, C.; Venturella, R.; Zullo, F. A more accurate method to interpret lactate dehydrogenase (LDH) isoenzymes’ results in patients with uterine masses. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 236, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, Y.; Quan, Q.; Meng, Y.; Mu, X. Diagnostic Value of Preoperative CA125, LDH and HE4 for Leiomyosarcoma of the Female Reproductive System. Cancer Manag. Res. 2021, 13, 4657–4664. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Escuredo, J.; Dadhich, R.K.; Dhup, S.; Cacace, A.; Van Hée, V.F.; De Saedeleer, C.J.; Sboarina, M.; Rodriguez, F.; Fontenille, M.-J.; Brisson, L.; et al. Lactate promotes glutamine uptake and metabolism in oxidative cancer cells. Cell Cycle 2016, 15, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Cao, J.; Gao, X.; Zhang, J.; Wang, L.; Wang, B.; Guo, L.; Hu, X.; Wang, Z. Overall survival of cancer patients with serum lactate dehydrogenase greater than 1000 IU/L. Tumor Biol. 2016, 37, 14083–14088. [Google Scholar] [CrossRef]
- Mollo, A.; Raffone, A.; Travaglino, A.; Di Cello, A.; Saccone, G.; Zullo, F.; De Placido, G. Increased LDH5/LDH1 ratio in preoperative diagnosis of uterine sarcoma with inconclusive MRI and LDH total activity but suggestive CT scan: A case report. BMC Womens Health 2018, 18. [Google Scholar] [CrossRef]
- Chen, L.; Huang, L.; Gu, Y.; Cang, W.; Sun, P.; Xiang, Y. Lactate-Lactylation Hands between Metabolic Reprogramming and Immunosuppression. Int. J. Mol. Sci. 2022, 23, 11943. [Google Scholar] [CrossRef]
- Pardella, E.; Ippolito, L.; Giannoni, E.; Chiarugi, P. Nutritional and metabolic signalling through GPCRs. FEBS Lett. 2022, 596, 2364–2381. [Google Scholar] [CrossRef]
- Hirschhaeuser, F.; Sattler, U.G.A.; Mueller-Klieser, W. Lactate: A metabolic key player in cancer. Cancer Res. 2011, 71, 6921–6925. [Google Scholar] [CrossRef]
- Brizel, D.M.; Schroeder, T.; Scher, R.L.; Walenta, S.; Clough, R.W.; Dewhirst, M.W.; Mueller-Klieser, W. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 349–353. [Google Scholar] [CrossRef]
- Gu, J.; Zhou, J.; Chen, Q.; Xu, X.; Gao, J.; Li, X.; Shao, Q.; Zhou, B.; Zhou, H.; Wei, S.; et al. Tumor metabolite lactate promotes tumorigenesis by modulating MOESIN lactylation and enhancing TGF-β signaling in regulatory T cells. Cell Rep. 2022, 39, 110986. [Google Scholar] [CrossRef]
- Roland, C.L.; Arumugam, T.; Deng, D.; Liu, S.H.; Philip, B.; Gomez, S.; Burns, W.R.; Ramachandran, V.; Wang, H.; Cruz-Monserrate, Z.; et al. Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res. 2014, 74, 5301–5310. [Google Scholar] [CrossRef]
- Wellbourne-wood, J.; Briquet, M.; Alessandri, M.; Binda, F.; Touya, M.; Chatton, J.Y. Evaluation of Hydroxycarboxylic Acid Receptor 1 (HCAR1) as a Building Block for Genetically Encoded Extracellular Lactate Biosensors. Biosensors 2022, 12, 143. [Google Scholar] [CrossRef]
- Jin, L.; Guo, Y.; Chen, J.; Wen, Z.; Jiang, Y.; Qian, J. Lactate receptor HCAR1 regulates cell growth, metastasis and maintenance of cancer-specific energy metabolism in breast cancer cells. Mol. Med. Rep. 2022, 26, 268. [Google Scholar] [CrossRef]
- Wagner, W.; Kania, K.D.; Blauz, A.; Ciszewski, W.M. The lactate receptor (HCAR1/GPR81) contributes to doxorubicin chemoresistance via ABCB1 transporter up-regulation in human cervical cancer HeLa cells. J. Physiol. Pharmacol. 2017, 68, 555–564. Available online: https://pubmed.ncbi.nlm.nih.gov/29151072/ (accessed on 18 September 2022).
- Paul, A.; Paul, S. The breast cancer susceptibility genes (BRCA) in breast and ovarian cancers. Front. Biosci. 2014, 19, 605–618. [Google Scholar] [CrossRef]
- Cheung, S.M.; Husain, E.; Masannat, Y.; Miller, I.D.; Wahle, K.; Heys, S.D.; He, J. Lactate concentration in breast cancer using advanced magnetic resonance spectroscopy. Br. J. Cancer 2020, 123, 261–267. [Google Scholar] [CrossRef]
- Luker, G.D. Imaging Hyperpolarized Lactate in Prostate Cancer. Radiol. Imaging Cancer 2019, 1, e194007. [Google Scholar] [CrossRef]
- Graboń, W.; Otto-Ślusarczyk, D.; Chrzanowska, A.; Mielczarek-Puta, M.; Joniec-Maciejak, I.; Słabik, K.; Barańczyk-Kuźma, A. Lactate Formation in Primary and Metastatic Colon Cancer Cells at Hypoxia and Normoxia. Cell Biochem. Funct. 2016, 34, 483–490. [Google Scholar] [CrossRef]
- Kellenberger, L.D.; Bruin, J.E.; Greenaway, J.; Campbell, N.E.; Moorehead, R.A.; Holloway, A.C.; Petrik, J. The Role of Dysregulated Glucose Metabolism in Epithelial Ovarian Cancer. J. Oncol. 2010, 2010, 514310. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Hall, M.; Cruickshank, D.; Gabra, H.; Ganesan, R.; Hughes, C.; Kehoe, S.; Ledermann, J.; Morrison, J.; Naik, R.; et al. British Gynaecological Cancer Society (BGCS) epithelial ovarian/fallopian tube/primary peritoneal cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 213, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Vanderstichele, A.; Busschaert, P.; Olbrecht, S.; Lambrechts, D.; Vergote, I. Genomic signatures as predictive biomarkers of homologous recombination deficiency in ovarian cancer. Eur. J. Cancer 2017, 86, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Kerslake, R.; Hall, M.; Vagnarelli, P.; Jeyaneethi, J.; Randeva, H.S.; Pados, G.; Kyrou, I.; Karteris, E. A pancancer overview of FBN1, asprosin and its cognate receptor OR4M1 with detailed expression profiling in ovarian cancer. Oncol. Lett. 2021, 22, 650. [Google Scholar] [CrossRef] [PubMed]
- Okorie, O.N.; Dellinger, P. Lactate: Biomarker and potential therapeutic target. Crit. Care Clin. 2011, 27, 299–326. [Google Scholar] [CrossRef]
- Pérez-Tomás, R.; Pérez-Guillén, I. Lactate in the Tumor Microenvironment: An Essential Molecule in Cancer Progression and Treatment. Cancers 2020, 12, 3244. [Google Scholar] [CrossRef]
- Heneberg, P. Lactic Acidosis in Patients with Solid Cancer. Antioxid. Redox Signal. 2022, 37, 1130–1152. [Google Scholar] [CrossRef]
- Maher, S.A.; Temkit, M.; Buras, M.; McLemore, R.Y.; Butler, R.K.; Chowdhury, Y.; Lipinski, C.A.; Traub, S.J. Serum Lactate and Mortality in Emergency Department Patients with Cancer. West. J. Emerg. Med. 2018, 19, 827–833. [Google Scholar] [CrossRef]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Weinberger, J.; Klompas, M.; Rhee, C. What Is the Utility of Measuring Lactate Levels in Patients with Sepsis and Septic Shock? Semin. Respir. Crit. Care Med. 2021, 42, 650–661. [Google Scholar] [CrossRef]
- White, N.J.; Looareesuwan, S.; Phillips, R.E.; Warrell, D.A.; Chanthavanich, P.; Pongpaew, P. Pathophysiological and prognostic significance of cerebrospinal-fluid lactate in cerebral malaria. Lancet 1985, 1, 776–778. [Google Scholar] [CrossRef]
- Aran, D.; Camarda, R.; Odegaard, J.; Paik, H.; Oskotsky, B.; Krings, G.; Goga, A.; Sirota, M.; Butte, A.J. Comprehensive analysis of normal adjacent to tumor transcriptomes. Nat. Commun. 2017, 8, 1077. [Google Scholar] [CrossRef]
- Lundø, K.; Trauelsen, M.; Pedersen, S.F.; Schwartz, T.W. Why Warburg Works: Lactate Controls Immune Evasion through GPR81. Cell Metab. 2020, 31, 666–668. [Google Scholar] [CrossRef]
- Yang, K.; Xu, J.; Fan, M.; Tu, F.; Wang, X.; Ha, T.; Williams, D.L.; Li, C. Lactate Suppresses Macrophage Pro-Inflammatory Response to LPS Stimulation by Inhibition of YAP and NF-κB Activation via GPR81-Mediated Signaling. Front. Immunol. 2020, 11, 587913. [Google Scholar] [CrossRef]
- TBrown, P.; Ganapathy, V. Lactate/GPR81 signaling and proton motive force in cancer: Role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol. Ther. 2020, 206, 107451. [Google Scholar] [CrossRef]
- Brown, T.P.; Bhattacharjee, P.; Ramachandran, S.; Sivaprakasam, S.; Ristic, B.; Sikder, M.O.F.; Ganapathy, V. The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene 2020, 39, 3292–3304. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerslake, R.; Panfilov, S.; Mustafa, N.; Hall, M.; Kyrou, I.; Randeva, H.S.; Karteris, E.; Godfrey, R. Elevated Circulating Lactate Levels and Widespread Expression of Its Cognate Receptor, Hydroxycarboxylic Acid Receptor 1 (HCAR1), in Ovarian Cancer. J. Clin. Med. 2023, 12, 217. https://doi.org/10.3390/jcm12010217
Kerslake R, Panfilov S, Mustafa N, Hall M, Kyrou I, Randeva HS, Karteris E, Godfrey R. Elevated Circulating Lactate Levels and Widespread Expression of Its Cognate Receptor, Hydroxycarboxylic Acid Receptor 1 (HCAR1), in Ovarian Cancer. Journal of Clinical Medicine. 2023; 12(1):217. https://doi.org/10.3390/jcm12010217
Chicago/Turabian StyleKerslake, Rachel, Suzana Panfilov, Nashrah Mustafa, Marcia Hall, Ioannis Kyrou, Harpal S. Randeva, Emmanouil Karteris, and Richard Godfrey. 2023. "Elevated Circulating Lactate Levels and Widespread Expression of Its Cognate Receptor, Hydroxycarboxylic Acid Receptor 1 (HCAR1), in Ovarian Cancer" Journal of Clinical Medicine 12, no. 1: 217. https://doi.org/10.3390/jcm12010217
APA StyleKerslake, R., Panfilov, S., Mustafa, N., Hall, M., Kyrou, I., Randeva, H. S., Karteris, E., & Godfrey, R. (2023). Elevated Circulating Lactate Levels and Widespread Expression of Its Cognate Receptor, Hydroxycarboxylic Acid Receptor 1 (HCAR1), in Ovarian Cancer. Journal of Clinical Medicine, 12(1), 217. https://doi.org/10.3390/jcm12010217








