Patient Selection for Pemafibrate Therapy to Prevent Adverse Cardiovascular Events
Abstract
1. Background
2. Methods
2.1. Patient Selection
2.2. Study Protocol
2.3. Baseline Characteristics Data
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Impact of Pemafibrate on Lipid Parameters
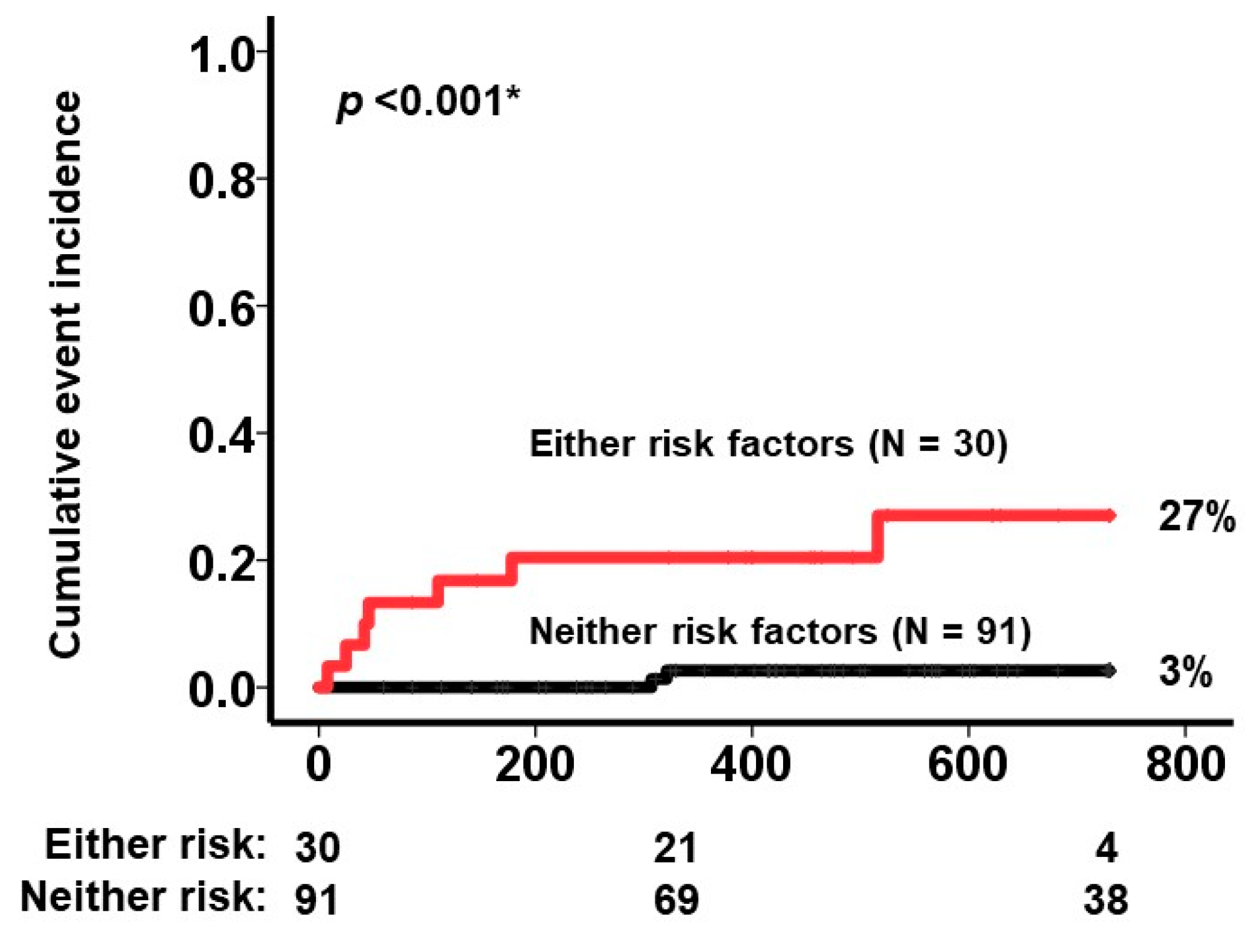
3.3. Baseline Characteristics That Were Associated with the Primary Endpoint
4. Discussion
4.1. Improvement in Lipid Parameters by Pemafibrate
4.2. Factors That Were Associated with Cardiovascular Events
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, F.C.; Huffman, M.; Ebrahim, S. Statin therapy for primary prevention of cardiovascular disease. JAMA 2013, 310, 2451–2452. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Varbo, A. Triglycerides and cardiovascular disease. Lancet 2014, 384, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Robillard, R.; Fontaine, C.; Chinetti, G.; Fruchart, J.C.; Staels, B. Fibrates. In Atherosclerosis: Diet and Drugs. Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 389–406. [Google Scholar]
- Pradhan, A.D.; Paynter, N.P.; Everett, B.M.; Glynn, R.J.; Amarenco, P.; Elam, M.; Ginsberg, H.; Hiatt, W.R.; Ishibashi, S.; Koenig, W.; et al. Rationale and design of the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) study. Am. Heart J. 2018, 206, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Masuda, D.; Matsuzawa, Y. Pemafibrate a New Selective PPARalpha Modulator: Drug Concept and Its Clinical Applications for Dyslipidemia and Metabolic Diseases. Curr. Atheroscler. Rep. 2020, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Glynn, R.J.; Fruchart, J.C.; MacFadyen, J.G.; Zaharris, E.S.; Everett, B.M.; Campbell, S.E.; Oshima, R.; Amarenco, P.; Blom, D.J.; et al. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N. Engl. J. Med. 2022, 387, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Araki, E.; Yamashita, S.; Arai, H.; Yokote, K.; Satoh, J.; Inoguchi, T.; Nakamura, J.; Maegawa, H.; Yoshioka, N.; Tanizawa, Y.; et al. Effects of Pemafibrate, a Novel Selective PPARalpha Modulator, on Lipid and Glucose Metabolism in Patients With Type 2 Diabetes and Hypertriglyceridemia: A Randomized, Double–Blind, Placebo-Controlled, Phase 3 Trial. Diabetes Care. 2018, 41, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Yamashita, S.; Yokote, K.; Araki, E.; Suganami, H.; Ishibashi, S.; Group, K.S. Efficacy and Safety of Pemafibrate Versus Fenofibrate in Patients with High Triglyceride and Low HDL Cholesterol Levels: A Multicenter, Placebo-Controlled, Double-Blind, Randomized Trial. J. Atheroscler. Thromb. 2018, 25, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Arai, H.; Yokote, K.; Araki, E.; Suganami, H.; Yamashita, S.; Group, K.S. Efficacy and safety of pemafibrate (K-877), a selective peroxisome proliferator-activated receptor alpha modulator, in patients with dyslipidemia: Results from a 24–week, randomized, double blind, active-controlled, phase 3 trial. J. Clin. Lipidol. 2018, 12, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Vaz, A.J.; Fayyad, R.; Boekholdt, S.M.; Hovingh, G.K.; Kastelein, J.J.; Melamed, S.; Barter, P.; Waters, D.D.; Ray, K.K. Triglyceride-Rich Lipoprotein Cholesterol and Risk of Cardiovascular Events Among Patients Receiving Statin Therapy in the TNT Trial. Circulation 2018, 138, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, C.; Imamura, K.; Teramoto, T. Assessment of LDL particle size by triglyceride/HDL-cholesterol ratio in non-diabetic, healthy subjects without prominent hyperlipidemia. J. Atheroscler. Thromb. 2003, 10, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Sekimoto, T.; Koba, S.; Mori, H.; Matsukawa, N.; Sakai, R.; Yokota, Y.; Sato, S.; Tanaka, H.; Masaki, R.; et al. Impact of small dense low-density lipoprotein cholesterol and triglyceride-rich lipoproteins on plaque rupture with ST-segment elevation myocardial infarction. J. Clin. Lipidol. 2022, 16, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Kokubo, Y.; Watanabe, M.; Sawamura, T.; Ito, Y.; Minagawa, A.; Okamura, T.; Miyamato, Y. Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: The Suita study. J. Atheroscler. Thromb. 2013, 20, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Keech, A.; Simes, R.J.; Barter, P.; Best, J.; Scott, R.; Taskinen, M.R.; Forder, P.; Pillai, A.; Davis, T.; Glasziou, P.; et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005, 366, 1849–1861. [Google Scholar] [PubMed]
- ACCORD Study Group; Ginsberg, H.N.; Elam, M.B.; Lovato, L.C.; Crouse, J.R., 3rd; Leiter, L.A.; Linz, P.; Friedewald, W.T.; Buse, J.B.; Gerstein, H.C.; et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 2010, 362, 1563–1574. [Google Scholar]
| Total (N = 121) | Events (N = 9) | No Events (N = 112) | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 62 (51, 71) | 59 (51, 71) | 62 (51, 69) | 0.74 |
| Men | 88 (73%) | 8 (89%) | 80 (71%) | 0.26 |
| Body mass index | 21.2 (20.1, 22.0) | 21.1 (20.1, 21.9) | 21.3 (20.2, 22.1) | 0.33 |
| Comorbidity | ||||
| Heart failure | 10 (8%) | 4 (44%) | 6 (5%) | <0.001 * |
| Atrial fibrillation | 13 (11%) | 1 (11%) | 12 (11%) | 0.97 |
| Diabetes mellitus | 70 (58%) | 3 (33%) | 67 (60%) | 0.12 |
| Coronary disease | 21 (17%) | 4 (44%) | 17 (15%) | 0.026 * |
| History of stroke | 14 (12%) | 0 (0%) | 14 (13%) | 0.26 |
| Dose of pemafibrate | <0.001 * | |||
| 0.1 mg/day | 4 (3%) | 3 (33%) | 1 (1%) | - |
| 0.2 mg/day | 114 (94%) | 6 (67%) | 108 (96%) | - |
| 0.4 mg/day | 3 (3%) | 0 (0%) | 3 (3%) | - |
| Concomitant medication | ||||
| Statin | 40 (33%) | 4 (44%) | 36 (32%) | 0.45 |
| Ezetimib | 12 (10%) | 1 (11%) | 11 (10%) | 0.90 |
| Anti-platelet | 29 (24%) | 3 (33%) | 26 (23%) | 0.49 |
| Renin-angiotensin-aldosterone inhibitor | 72 (60%) | 5 (56%) | 67 (60%) | 0.80 |
| Laboratory data | ||||
| Hemoglobin, g/dL | 14.3 (12.3, 15.7) | 13.3 (11.8, 15.5) | 14.5 (12.7, 15.9) | 0.52 |
| Estimated GFR, mL/min/1.73m2 | 63.4 (39.3, 75.3) | 62.5 (56.4, 67.1) | 63.4 (38.9, 77.0) | 0.084 |
| Total cholesterol, mg/dL | 201 (165, 227) | 182 (137, 206) | 204 (167, 228) | 0.21 |
| LDL-cholesterol, mg/dL | 109 (66, 124) | 71 (53, 124) | 111 (74, 126) | 0.13 |
| HDL-cholesterol, mg/dL | 44 (35, 46) | 38 (36, 41) | 44 (34, 47) | 0.084 |
| Triglyceride, mg/dL | 302 (205, 581) | 428 (201, 651) | 302 (207, 466) | 0.62 |
| Triglyceride/HDL-cholesterol ratio | 7.0 (4.6, 16.1) | 11.4 (4.7, 17.0) | 7.0 (4.5, 14.5) | 0.37 |
| Triglyceride-rich lipoprotein, mg/dL | 49 (33, 69) | 53 (33, 70) | 49 (33, 68) | 0.63 |
| Baseline | 3 Months Follow-Up | p Value | |
|---|---|---|---|
| Total cholesterol, mg/dL | 201 (165, 227) | 182 (158, 212) | <0.001 * |
| LDL-cholesterol, mg/dL | 109 (66, 124) | 104 (88, 136) | 0.18 |
| HDL-cholesterol, mg/dL | 44 (35, 46) | 49 (37, 52) | <0.001 * |
| Triglyceride, mg/dL | 302 (205, 581) | 178 (117, 253) | <0.001 * |
| Triglyceride/HDL-cholesterol ratio | 7.0 (4.6, 16.1) | 4.3 (2.5, 5.7) | <0.001 * |
| Triglyceride-rich lipoprotein, mg/dL | 49 (33, 69) | 27 (22, 45) | <0.001 * |
| Variables | Hazard Ratio (95% Confidence Interval) | p Value |
|---|---|---|
| Heart failure | 7.38 (1.72–35.3) | 0.012 * |
| Coronary disease | 7.76 (1.69–34.3) | 0.013 * |
| Dose of pemafibrate as 0.1 mg/day decrease | 17.6 (3.13–94.4) | 0.001 * |
| Age, years | 1.02 (0.96–1.07) | 0.58 |
| Male sex | 3.21 (0.40–25.6) | 0.27 |
| Anti-platelet | 0.96 (0.94–10.5) | 0.44 |
| Renin-angiotensin-aldosterone inhibitor | 0.97 (0.95–13.1) | 0.27 |
| Events (N = 9) | No Events (N = 112) | p Value | |
|---|---|---|---|
| Total cholesterol, mg/dL | 171 (151, 201) | 188 (162, 218) | 0.37 |
| LDL-cholesterol, mg/dL | 98 (79, 116) | 106 (83, 134) | 0.51 |
| HDL-cholesterol, mg/dL | 42 (37, 49) | 49 (40, 55) | 0.34 |
| Triglyceride, mg/dL | 196 (116, 295) | 167 (120, 252) | 0.60 |
| Triglyceride/HDL-cholesterol ratio | 5.6 (3.1, 6.0) | 3.4 (2.4, 5.6) | 0.36 |
| Triglyceride-rich lipoprotein, mg/dL | 36 (27, 42) | 30 (24, 46) | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izumida, T.; Imamura, T.; Narang, N.; Kinugawa, K. Patient Selection for Pemafibrate Therapy to Prevent Adverse Cardiovascular Events. J. Clin. Med. 2023, 12, 21. https://doi.org/10.3390/jcm12010021
Izumida T, Imamura T, Narang N, Kinugawa K. Patient Selection for Pemafibrate Therapy to Prevent Adverse Cardiovascular Events. Journal of Clinical Medicine. 2023; 12(1):21. https://doi.org/10.3390/jcm12010021
Chicago/Turabian StyleIzumida, Toshihide, Teruhiko Imamura, Nikhil Narang, and Koichiro Kinugawa. 2023. "Patient Selection for Pemafibrate Therapy to Prevent Adverse Cardiovascular Events" Journal of Clinical Medicine 12, no. 1: 21. https://doi.org/10.3390/jcm12010021
APA StyleIzumida, T., Imamura, T., Narang, N., & Kinugawa, K. (2023). Patient Selection for Pemafibrate Therapy to Prevent Adverse Cardiovascular Events. Journal of Clinical Medicine, 12(1), 21. https://doi.org/10.3390/jcm12010021






