How to Perform Intravesical Chemotherapy after Second TURBT for Non-Muscle-Invasive Bladder Cancer: A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Treatment Schedule
2.3. Pathology
2.4. Clinical and Pathological Characteristics
2.5. Statistical Analysis
3. Results
3.1. Clinical and Pathological Characteristics
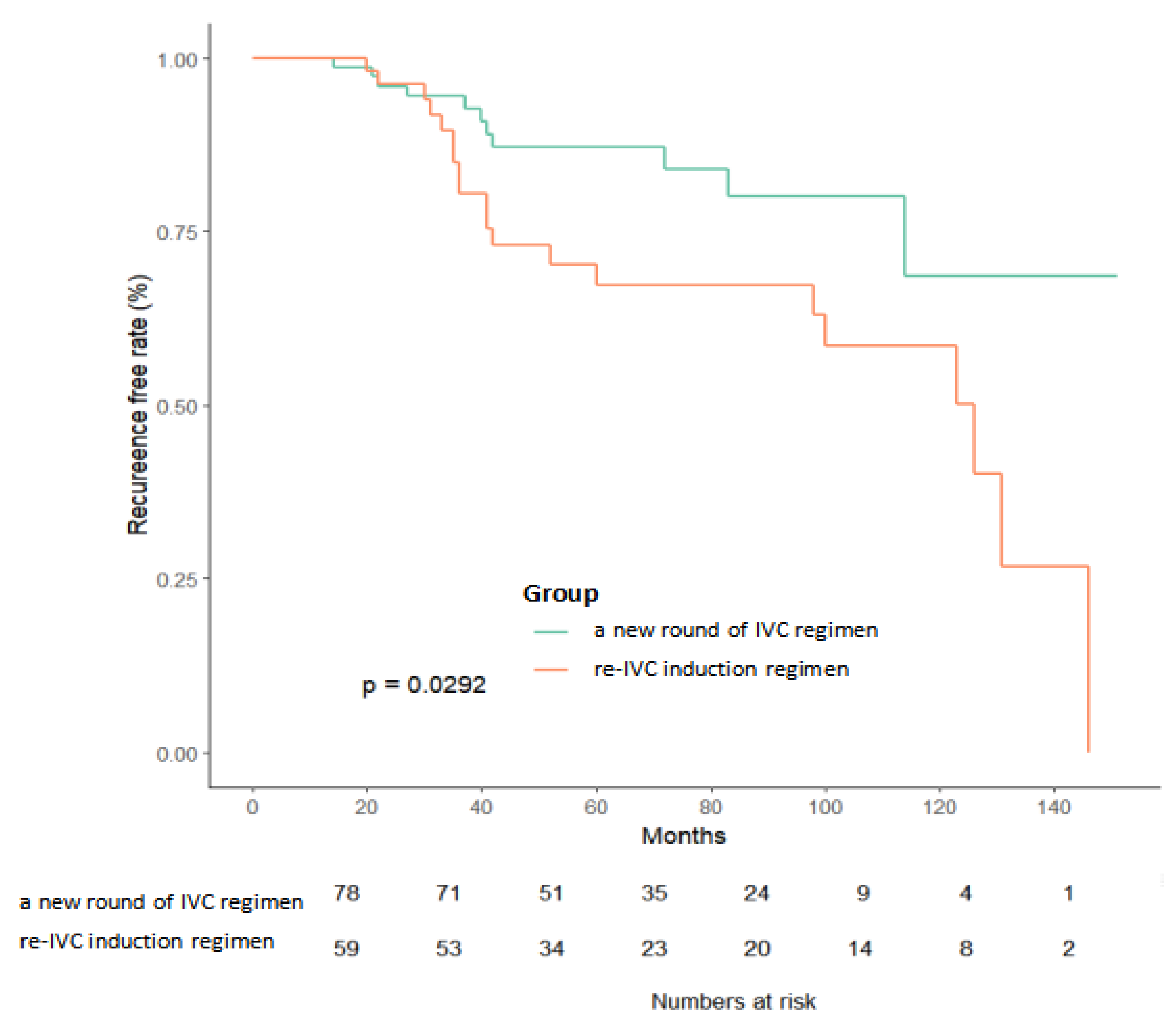
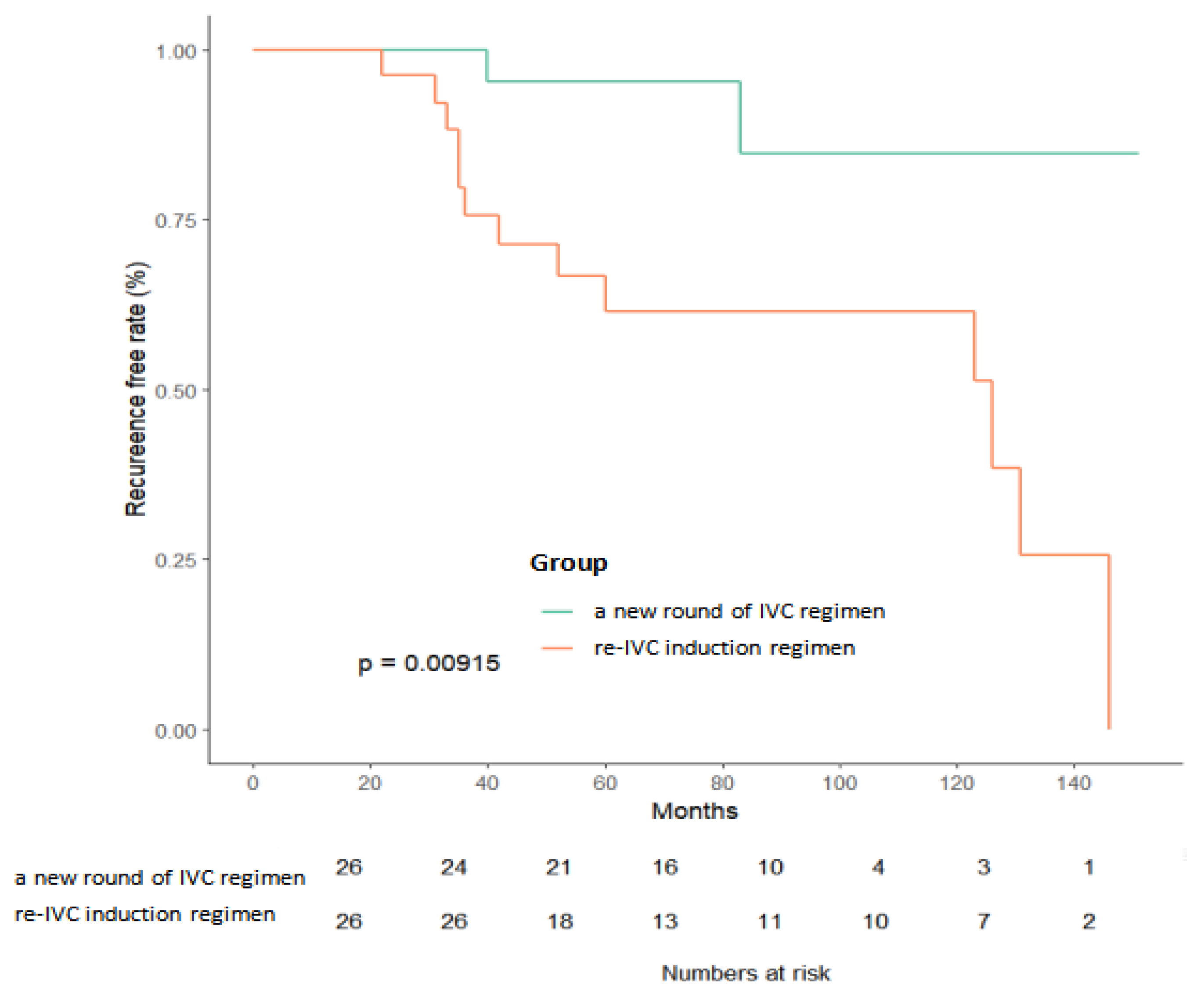
3.2. Kaplan–Meier Survival Analysis
3.3. Univariate and Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Compérat, E.; Larré, S.; Rouprêt, M.; Neuzillet, Y.; Pignot, G.; Quintens, H.; Houede, N.; Roy, C.; Durand, X.; Varinot, J.; et al. Clinicopathological characteristics of urothelial bladder cancer in patients less than 40 years old. Virchows Archiv. 2015, 466, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Isharwal, S.; Konety, B. Non-muscle invasive bladder cancer risk stratification. Indian J. Urol. 2015, 31, 289–296. [Google Scholar] [PubMed]
- Richterstetter, M.; Wullich, B.; Amann, K.; Haeberle, L.; Engehausen, D.G.; Goebell, P.J.; Krause, F.S. The value of extended transurethral resection of bladder tumour (TURBT) in the treatment of bladder cancer. BJU Int. 2012, 110, E76–E79. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Ibarrola, R.; Soria, F.; Abufaraj, M.; D’Andrea, D.; Preto, M.; Gust, K.M.; Briganti, A.; Shariat, S.; Gontero, P. Surgical checklist impact on recurrence-free survival of patients with non-muscle-invasive bladder cancer undergoing transurethral resection of bladder tumour. BJU Int. 2019, 123, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Badalament, R.A.; Farah, R.N. Treatment of superficial bladder cancer with intravesical chemotherapy. Semin Surg. Oncol. 1997, 13, 335–341. [Google Scholar] [CrossRef]
- Dïvrïk, R.T.; Yildirim; Zorlu, F.; Özen, H. The effect of repeat transurethral resection on recurrence and progression rates in patients with T1 tumors of the bladder who received intravesical mitomycin: A prospective, randomized clinical trial. J. Urol. 2006, 175, 1641–1644. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.-O.; Steinhoff, C.; Simon, X.; Spiegelhalder, P.; Ackermann, R.; Vögeli, T.A. Effect of routine repeat transurethral resection for superficial bladder cancer: A long-term observational study. J. Urol. 2003, 170, 433–437. [Google Scholar] [CrossRef]
- Cumberbatch, M.G.; Foerster, B.; Catto, J.W.; Kamat, A.M.; Kassouf, W.; Jubber, I.; Shariat, S.F.; Sylvester, R.J.; Gontero, P. Repeat Transurethral Resection in Non-muscle-invasive Bladder Cancer: A Systematic Review. Eur. Urol. 2018, 73, 925–933. [Google Scholar] [CrossRef]
- Eroglu, A.; Ekin, R.G.; Koc, G.; Divrik, R.T. The prognostic value of routine second transurethral resection in patients with newly diagnosed stage pT1 non-muscle-invasive bladder cancer: Results from randomized 10-year extension trial. Int. J. Clin Oncol. 2020, 25, 698–704. [Google Scholar] [CrossRef]
- Ayati, M.; Amini, E.; Damavand, R.S.; Nowroozi, M.R.; Soleimani, M.; Ranjbar, E.; Nowroozi, A. Second Transurethral Resection of Bladder Tumor: Is it Necessary in All T1 and/or High-Grade Tumors? Urol. J 2019, 16, 152–156. [Google Scholar]
- Chang, S.S.; Bochner, B.H.; Chou, R.; Dreicer, R.; Kamat, A.M.; Lerner, S.P.; Lotan, Y.; Meeks, J.J.; Michalski, J.M.; Morgan, T.M.; et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J. Urol. 2017, 198, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Roupret, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol 2019, 76, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Roupret, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol 2022, 81, 75–94. [Google Scholar] [CrossRef]
- Nomata, K.; Noguchi, M.; Kanetake, H.; Tsuda, N.; Hayashi, M.; Yamashita, S.; Sakuragi, T.; Kusaba, Y.; Shindo, K. Intravesical adjuvant chemotherapy for superficial transitional cell bladder carcinoma: Results of a randomized trial with epirubicin comparing short-term versus long-term maintenance treatment. Cancer Chemother. Pharmacol. 2002, 50, 266–270. [Google Scholar] [CrossRef]
- Ali-El-Dein, B.; Nabeeh, A.; El-Baz, M.; Shamaa, S.; Ashamallah, A. Single-dose versus multiple instillations of epirubicin as prophylaxis for recurrence after transurethral resection of pTa and pT1 transitional-cell bladder tumours: A prospective, randomized controlled study. Br. J. Urol. 1997, 79, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Schwaibold, H.; Pichlmeier, U.; Klingenberger, H.-J.; Huland, H. Long-Term follow-up of cytostatic intravesical instillation in patients with superficial bladder carcinoma. Is short-term, intensive instillation better than maintenance therapy? Eur. Urol. 1997, 31, 153–159. [Google Scholar] [CrossRef]
- Mant, J.; Doust, J.; Roalfe, A.; Barton, P.; Cowie, M.R.; Glasziou, P.; Mant, D.; McManus, R.J.; Holder, R.; Deeks, J.; et al. Systematic Review and Individual Patient Data Meta-analysis of Randomized Trials Comparing a Single Immediate Instillation of Chemotherapy After Transurethral Resection with Transurethral Resection Alone in Patients with Stage pTa-pT1 Urothelial Carcinoma of the Bladder: Which Patients Benefit from the Instillation? Eur. Urol 2016, 69, 231–244. [Google Scholar]
- Klän, R.; Loy, V.; Huland, H. Residual tumor discovered in routine second transurethral resection in patients with stage T1 transitional cell carcinoma of the bladder. J. Urol. 1991, 146, 316–318. [Google Scholar] [CrossRef]
- Calò, B.; Falagario, U.; Sanguedolce, F.; Veccia, A.; Chirico, M.; Carvalho-Diaz, E.; Mota, P.; Lima, E.; Autorino, R.; Carrieri, G.; et al. Impact of time to second transurethral resection on oncological outcomes of patients with high-grade T1 bladder cancer treated with intravesical Bacillus Calmette-Guerin. World J. Urol 2020, 38, 3161–3167. [Google Scholar] [CrossRef]
- Chang, S.S. Re: Systematic Review and Individual Patient Data Meta-Analysis of Randomized Trials Comparing a Single Immediate Instillation of Chemotherapy after Transurethral Resection with Transurethral Resection Alone in Patients with Stage pTa-pT1 Urothelial Carcinoma of the Bladder: Which Patients Benefit from the Instillation? J. Urol. 2017, 197, 1219. [Google Scholar]
- Lu, J.; Xia, Q.; Lu, Y.; Liu, Z.; Zhou, P.; Hu, H.; Wang, S. Efficacy of intravesical therapies on the prevention of recurrence and progression of non-muscle-invasive bladder cancer: A systematic review and network meta-analysis. Cancer Med. 2020, 9, 7800–7809. [Google Scholar] [CrossRef] [PubMed]
- Tabayoyong, W.B.; Kamat, A.M.; O’Donnell, M.A.; McKiernan, J.M.; Ray-Zack, M.D.; Palou, J.; Brausi, M.; Black, P.C.; Williams, S.B. Systematic Review on the Utilization of Maintenance Intravesical Chemotherapy in the Management of Non-muscle-invasive Bladder Cancer. Eur. Urol. Focus 2018, 4, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Hermans, J.; Jokisch, F.; Volz, Y.; Eismann, L.; Pfitzinger, P.; Ebner, B.; Weinhold, P.; Schlenker, B.; Stief, C.G.; Tritschler, S.; et al. Impact of bacillus Calmette-Guerin intravesical therapy on the diagnostic efficacy of The Paris System for Reporting Urinary Cytology in patients with high-grade bladder cancer. Cancer Cytopathol. 2022, 130, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Zhu, D.; Barry, E.; Kovac, E.; Aboumohamed, A.; Agalliu, I.; Sankin, A. Intravesical Bacillus Calmette-Guerin Treatment Is Inversely Associated with the Risk of Developing Alzheimer Disease or Other Dementia Among Patients with Non-muscle-invasive Bladder Cancer. Clin. Genitourin. Cancer 2021, 19, e409–e416. [Google Scholar] [CrossRef] [PubMed]
- Damiano, R.; de Sio, M.; Quarto, G.; di Lorenzo, G.; Perdonà, S.; Palumbo, I.M.; Azzarito, G.; Giugliano, F.; Autorino, R. Short-term administration of prulifloxacin in patients with nonmuscle-invasive bladder cancer: An effective option for the prevention of bacillus Calmette-Guerin-induced toxicity? BJU Int. 2009, 104, 633–639. [Google Scholar] [CrossRef]
- Bohle, A.; Bock, P.R. Intravesical bacille Calmette-Guerin versus mitomycin C in superficial bladder cancer: Formal meta-analysis of comparative studies on tumor progression. Urology 2004, 63, 682–686, discussion 686-7. [Google Scholar] [CrossRef]
- Yates, D.R.; Rouprêt, M. Failure of bacille Calmette-Guerin in patients with high risk non-muscle-invasive bladder cancer unsuitable for radical cystectomy: An update of available treatment options. BJU Int. 2010, 106, 162–167. [Google Scholar] [CrossRef]
- Shore, N.D.; Boorjian, S.A.; Canter, D.J.; Ogan, K.; Karsh, L.I.; Downs, T.M.; Gomella, L.G.; Kamat, A.M.; Lotan, Y.; Svatek, R.S.; et al. Intravesical rAd-IFNalpha/Syn3 for Patients with High-Grade, Bacillus Calmette-Guerin-Refractory or Relapsed Non-Muscle-Invasive Bladder Cancer: A Phase II Randomized Study. J. Clin. Oncol. 2017, 35, 3410–3416. [Google Scholar] [CrossRef]
- Wright, K.M. FDA Approves Pembrolizumab for BCG-Unresponsive NMIBC. Oncology 2020, 34, 44. [Google Scholar]
- Chang, S.S. Re: Intravesical rAd-IFNalpha/Syn3 for Patients with High-Grade, bacillus Calmette-Guerin-Refractory or Relapsed Non-Muscle-Invasive Bladder Cancer: A Phase II Randomized Study. J. Urol. 2018, 199, 1110–1111. [Google Scholar]
- Larsen, E.S.; Nordholm, A.C.; Lillebaek, T.; Holden, I.K.; Johansen, I.S. The epidemiology of bacille Calmette-Guerin infections after bladder instillation from 2002 through 2017: A nationwide retrospective cohort study. BJU Int. 2019, 124, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Pyrgidis, N.; Lackner, J.; Schneidewind, L.; Sokolakis, I. Treatment of Ta and T1 intermediate or high risk bladder cancer with intravesical Bacillus Calmette-Guerin or mitomycin C. Urologe A 2021, 60, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Bandari, J.; Maganty, A.; MacLeod, L.C.; Davies, B.J. Manufacturing and the Market: Rationalizing the Shortage of Bacillus Calmette-Guerin. Eur. Urol. Focus 2018, 4, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Ourfali, S.; Ohannessian, R.; Fassi-Fehri, H.; Pages, A.; Badet, L.; Colombel, M. Recurrence Rate and Cost Consequence of the Shortage of Bacillus Calmette-Guerin Connaught Strain for Bladder Cancer Patients. Eur. Urol. Focus 2021, 7, 111–116. [Google Scholar] [CrossRef]
- Perlis, N.; Zlotta, A.R.; Beyene, J.; Finelli, A.; Fleshner, N.E.; Kulkarni, G.S. Immediate post-transurethral resection of bladder tumor intravesical chemotherapy prevents non-muscle-invasive bladder cancer recurrences: An updated meta-analysis on 2548 patients and quality-of-evidence review. Eur. Urol. 2013, 64, 421–430. [Google Scholar] [CrossRef]
- Akand, M.; Muilwijk, T.; Raskin, Y.; De Vrieze, M.; Joniau, S.; Van Der Aa, F. Quality Control Indicators for Transurethral Resection of Non-Muscle-Invasive Bladder Cancer. Clin. Genitourin. Cancer 2019, 17, e784–e792. [Google Scholar] [CrossRef]
- Mahran, A.; Bukavina, L.; Mishra, K.; Buzzy, C.; Fish, M.L.; Bobrow, A.; Ponsky, L. Bladder irrigation after transurethral resection of superficial bladder cancer: A systematic review of the literature. Can. J. Urol. 2018, 25, 9579–9584. [Google Scholar]

| Variable, n (%) | Level | Total (n = 137) | Patient with no Residual Tumor (n = 85) | Patient with Residual Tumor (n = 52) | p |
|---|---|---|---|---|---|
| IVC regimen after 2nd TURBT | New round of IVC | 78(57) | 52(61) | 26(50.000) | 0.2 |
| Re-IVC induction regimen | 59(43) | 33(39) | 26(50.000) | ||
| BMI | <25 | 74(54) | 46(54) | 28(54) | 0.975 |
| ≥25 | 63(46) | 39(46) | 24(46) | ||
| Age | ≤70 | 89(65) | 54(64) | 35(67) | 0.653 |
| >70 | 48(35) | 31(36) | 17(33) | ||
| Gender | Female | 32(23) | 19(22) | 13(25.000) | 0.722 |
| Male | 105(77) | 66(78) | 39(75.000) | ||
| Smoking history | No | 24(18) | 18(21) | 6(12) | 0.150 |
| Yes | 113(82) | 67(79) | 46(88) | ||
| Risk group | Intermediate-risk | 99(72) | 61(72) | 38(73) | 0.569 |
| High-risk | 38(28) | 24(28) | 14(27) | ||
| Gross hematuria | No | 20(15) | 12(14) | 8(15) | 0.839 |
| Yes | 117(85) | 73(86) | 44(85) | ||
| 1st T stage/Pathology grade | Ta/LG | 21(15) | 13(15) | 8(15) | 0.998 |
| Ta/HG | 36(26) | 23(27) | 13(25) | ||
| T1/LG | 7(5) | 4(4) | 3(6) | ||
| T1/HG | 73(53) | 45(53) | 28(54) | ||
| 1st Tumor number | Single | 83(61) | 56(66) | 27(52) | 0.105 |
| Multiple | 54(39) | 29(34) | 25(48) | ||
| 1st Maximum tumor diameter | <3cm | 80(58) | 54(64) | 26(50) | 0.119 |
| ≥3cm | 57(42) | 31(36) | 26(50) | ||
| 2nd T stage/Pathology grade | Ta/LG | 20(15) | 13(15) | 7(13) | 0.827 |
| Ta/HG | 37(27) | 23(27) | 14(27) | ||
| T1/LG | 5(4) | 4(4) | 1(2) | ||
| T1/HG | 75(55) | 45(53) | 30(58) |
| Variables | Univariate HR | 95%CI | p | Multivariate HR | 95% CI | p |
|---|---|---|---|---|---|---|
| IVC regimen after 2nd TURBT | ||||||
| New round of IVC | 1 | 1 | ||||
| Re-IVC induction regimen | 5.834 | (1.303, 26.123) | 0.021 | 7.687 | (1.555, 37.994) | 0.012 |
| BMI | ||||||
| <25 | 1 | |||||
| ≥25 | 2.341 | (0.829, 6.610) | 0.108 | |||
| Age | ||||||
| ≤70 | 1 | |||||
| >70 | 0.568 | (0.203, 1.591) | 0.282 | |||
| Gender | ||||||
| Female | 1 | |||||
| Male | 1.464 | (0.407, 5.273) | 0.560 | |||
| Smoking history | ||||||
| No | 1 | |||||
| Yes | 0.472 | (0.101, 2.205) | 0.340 | |||
| Gross hematuria | ||||||
| No | 1 | |||||
| Yes | 6.958 | (0.903, 53.627) | 0.063 | |||
| 1st T stage | ||||||
| Ta | 1 | |||||
| T1 | 0.727 | (0.248, 2.128) | 0.561 | |||
| 1st Pathology Grade | ||||||
| Low | 1 | |||||
| High | 1.122 | (0.312, 4.035) | 0.860 | |||
| 1st Tumor number | ||||||
| Single | 1 | |||||
| Multiple | 3.101 | (0.924, 10.411) | 0.067 | |||
| 1st Maximum tumor diameter | ||||||
| <3 cm | 1 | |||||
| ≥3 cm | 1.407 | (0.479, 4.134) | 0.535 | |||
| 2nd T stage | ||||||
| Ta | 1 | 1 | ||||
| T1 | 7.281 | (1.631, 32.496) | 0.009 | 9.218 | (1.928–44.085) | 0.005 |
| 2nd Pathology Grade | ||||||
| Low | 1 | |||||
| High | 0.863 | (0.192, 3.883) | 0.848 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Qi, N.; Gao, Z.; Ding, L.; Zhu, J.; Guo, Q.; Wang, J.; Wen, R.; Li, H. How to Perform Intravesical Chemotherapy after Second TURBT for Non-Muscle-Invasive Bladder Cancer: A Single-Center Experience. J. Clin. Med. 2023, 12, 169. https://doi.org/10.3390/jcm12010169
Li Z, Qi N, Gao Z, Ding L, Zhu J, Guo Q, Wang J, Wen R, Li H. How to Perform Intravesical Chemotherapy after Second TURBT for Non-Muscle-Invasive Bladder Cancer: A Single-Center Experience. Journal of Clinical Medicine. 2023; 12(1):169. https://doi.org/10.3390/jcm12010169
Chicago/Turabian StyleLi, Zhen, Nienie Qi, Zhimin Gao, Li Ding, Jiawei Zhu, Qingxiang Guo, Junqi Wang, Rumin Wen, and Hailong Li. 2023. "How to Perform Intravesical Chemotherapy after Second TURBT for Non-Muscle-Invasive Bladder Cancer: A Single-Center Experience" Journal of Clinical Medicine 12, no. 1: 169. https://doi.org/10.3390/jcm12010169
APA StyleLi, Z., Qi, N., Gao, Z., Ding, L., Zhu, J., Guo, Q., Wang, J., Wen, R., & Li, H. (2023). How to Perform Intravesical Chemotherapy after Second TURBT for Non-Muscle-Invasive Bladder Cancer: A Single-Center Experience. Journal of Clinical Medicine, 12(1), 169. https://doi.org/10.3390/jcm12010169