Marginal versus Standard Donors in Heart Transplantation: Proper Selection Means Heart Transplant Benefit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Outcomes and Definitions
- Status 1:
- a.
- mechanical circulatory support (MCS) due to acute haemodynamic deterioration; RVAD or biventricular assist device (BiVAD); LVAD with device-related complications; total artificial heart (TAH); intra-aortic balloon pump (IABP); extracorporeal membrane oxygenation (ECMO);
- b.
- mechanical ventilation.
- Status 2:
- a.
- uncomplicated LVAD; continuous inotrope infusion; patients with implantable cardioverter defibrillator (ICD) and malignant relapsing ventricular arrhythmias;
- b.
- outpatients, not included in the categories listed above.
- Status 3: temporarily inactive.
2.3. Cardiac Transplantation Protocol
2.4. Statistical Analysis
3. Results
3.1. MD Recipients
3.2. SD Recipients versus MDs
4. Comment
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation and Acronymous
| BEM | Endomyocardial biopsies |
| CAV | Coronary graft vasculopathy |
| COPD | Chronic obstructive pulmonary disease |
| CVVH | Continuous venovenous hemofiltration |
| ECMO | Extracorporeal membrane oxygenator |
| ESRD | End-stage renal disease |
| HTx | Heart transplantation |
| ICU | Intensive care unit |
| ISHLT | International Society for Heart and Lung Transplantation |
| K-M | Kaplan–Meier |
| LVAD | Left ventricle assist device |
| MDs | Marginal donors |
| OCS | Organ care support |
| PGF | Primary graft failure |
| SDs | Standard donors |
References
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. International Society for Heart Lung Transplantation (ISHLT) Infectious Diseases, Pediatric and Heart Failure and Transplantation Councils. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transplant. 2016, 35, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.trapianti.salute.gov.it/imgs/C_17_cntPubblicazioni_415_allegato.pdf (accessed on 21 August 2021).
- Carrozzini, M.; Bejko, J.; Guariento, A.; Rubino, M.; Bianco, R.; Tarzia, V. Minimally Invasive Implantation of Continuous Flow Left Ventricular Assist Devices: The Evolution of Surgical Techniques in a Single-Center Experience. Artif. Organs 2019, 43, E41–E52. [Google Scholar] [CrossRef] [PubMed]
- Apostolo, A.; Paolillo, S.; Contini, M.; Vignati, C.; Tarzia, V.; Campodonico, J.; Mapelli, M.; Massetti, M.; Bejko, J.; Righini, F.; et al. Comprehensive effects of left ventricular assist device speed changes on alveolar gas exchange, sleep ventilatory pattern, and exercise performance. J. Heart Lung Transplant. 2018, 37, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Bejko, J.; Toto, F.; Gregori, D.; Gerosa, G.; Bottio, T. Left ventricle assist devices and driveline’s infection incidence: A single-centre experience. J. Artif. Organs 2017, 21, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Bottio, T.; Bagozzi, L.; Fiocco, A.; Nadali, M.; Caraffa, R.; Bifulco, O. COVID-19 in Heart Transplant Recipients: A Multicenter Analysis of the Northern Italian Outbreak. JACC Heart Fail. 2021, 9, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Guihaire, J.; Noly, P.E.; Martin, A.; Rojo, M.; Aymami, M.; Ingels, A.; Lelong, B.; Chabanne, C.; Verhoye, J.-P.; Flécher, E. Impact of donor comorbidities on heart transplant outcomes in the modern era. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 898–904. [Google Scholar] [CrossRef] [Green Version]
- Galeone, A.; Lebreton, G.; Coutance, G.; Demondion, P.; Schmidt, M.; Amour, J.; Varnous, S.; Leprince, P. A single-center long-term experience with marginal donor utilization for heart transplantation. Clin. Transplant. 2020, 34, e14057. [Google Scholar] [CrossRef]
- Prieto, D.; Correia, P.; Baptista, M.; Antunes, M.J. Outcome after heart transplantation from older donor age: Expanding the donor pool. Eur. J. Cardio-Thoracic Surg. 2014, 47, 672–678. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.trapianti.salute.gov.it/imgs/C_17_cntPubblicazioni_394_allegato.pdf (accessed on 5 November 2020).
- Kobashigawa, J.; Zuckermann, A.; Macdonald, P.; Leprince, P.; Esmailian, F.; Luu, M.; Mancini, D.; Patel, J.; Razi, R.; Reichenspurner, H.; et al. Consensus Conference participants. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J. Heart Lung Transplant. 2014, 33, 327–340. [Google Scholar] [CrossRef]
- Stewart, S.; Winters, G.L.; Fishbein, M.C.; Tazelaar, H.D.; Kobashigawa, J.; Abrams, J.; Andersen, C.B.; Angelini, A.; Berry, G.J.; Burke, M.M.; et al. Revision of the 1990 Working Formulation for the Standardization of Nomenclature in the Diagnosis of Heart Rejection. J. Heart Lung Transplant. 2005, 24, 1710–1720. [Google Scholar] [CrossRef]
- Mehra, M.R.; Crespo-Leiro, M.G.; Dipchand, A.; Ensminger, S.M.; Hiemann, N.E.; Kobashigawa, J.A. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac graft vasculopathy-2010. J. Heart Lung Transplant. 2010, 29, 717–727. [Google Scholar] [CrossRef]
- Listijono, D.R.; Watson, A.; Pye, R.; Keogh, A.M.; Kotlyar, E.; Spratt, P. Usefulness of extracorporeal membrane oxygenation for early cardiac graft dysfunction. J. Heart Lung Transplant. 2011, 30, 783–789. [Google Scholar] [CrossRef]
- Mastroianni, C.; Nenna, A.; Lebreton, G.; D’Alessandro, C.; Greco, S.M.; Lusini, M.; Leprince, P.; Chello, M. Extracorporeal membrane oxygenation as treatment of graft failure after heart transplantation. Ann. Cardiothorac. Surg. 2019, 8, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Pozzi, M.; Bottin, C.; Armoiry, X.; Sebbag, L.; Boissonnat, P.; Hugon-Vallet, E.; Koffel, C.; Flamens, C.; Paulus, S.; Fellahi, J.L.; et al. Extracorporeal life support for primary graft dysfunction after heart transplantation. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 778–784. [Google Scholar] [CrossRef]
- Wittwer, T.; Wahlers, T. Marginal donor grafts in heart transplantation: Lessons learned from 25 years of experience. Transplant. Int. 2007, 21, 113–125. [Google Scholar] [CrossRef]
- Piperata, A.; Caraffa, R.; Bifulco, O.; Avesani, M.; Apostolo, A.; Gerosa, G. Marginal donors and organ shortness: Concomitant surgical procedures during heart transplantation: A literature review. J. Cardiovasc. Med. 2021, 23, 167–175. [Google Scholar] [CrossRef]
- Lietz, K.; John, R.; Mancini, D.M.; Edwards, N.M. Outcomes in cardiac transplant recipients using grafts from older donors versus mortality on the transplant waiting list; Implications for donor selection criteria. J. Am. Coll. Cardiol. 2004, 43, 1553–1561. [Google Scholar] [CrossRef] [Green Version]
- Goda, A.; Williams, P.; Mancini, D.; Lund, L.H. Selecting patients for heart transplantation: Comparison of the Heart Failure Survival Score (HFSS) and the Seattle Heart Failure Model (SHFM). J. Heart Lung Transplant. 2011, 30, 1236–1243. [Google Scholar] [CrossRef]
- AbouEzzeddine, O.F.; Redfield, M.M. Who Has Advanced Heart Failure? Definition and Epidemiology. Congest. Heart Fail. 2011, 17, 160–168. [Google Scholar] [CrossRef]
- Pouteil-Noble, C.; Hemdawy, A.; Villar, E.; Boissonnat, P.; Sebbag, L. Chronic Renal Failure and End-Stage Renal Disease Are Associated With a High Rate of Mortality After Heart Transplantation. Transplant. Proc. 2005, 37, 1352–1354. [Google Scholar] [CrossRef]
- Shahin, J.; Devarennes, B.; Tse, C.W.; Amarica, D.-A.; Dial, S. The relationship between inotrope exposure, six-hour postoperative physiological variables, hospital mortality and renal dysfunction in patients undergoing cardiac surgery. Crit. Care 2011, 15, R162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, J.R.; Cheng, A.; Ising, M.; Lenneman, A.; Birks, E.; Slaughter, M.S. Heart Transplant Survival Based on Recipient and Donor Risk Scoring: A UNOS Database Analysis. ASAIO J. 2016, 62, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Amarelli, C.; De Santo, L.S.; Marra, C.; Maiello, C.; Bancone, C.; Della Corte, A.; Nappi, G.; Romano, G. Early graft failure after heart transplant: Risk factors and implications for improved donor-recipient matching. Interact. Cardiovasc. Thorac. Surg. 2012, 15, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaac, D. Primary cardiac graft failure-defining, predicting, preventing. J. Heart Lung Transplant. 2013, 32, 1168–1169. [Google Scholar] [CrossRef]
- Collins, M.J.; Moainie, S.L.; Griffith, B.P.; Poston, R.S. Preserving and evaluating hearts with ex vivo machine perfusion: An avenue to improve early graft performance and expand the donor pool. Eur. J. Cardio-Thorac. Surg. 2008, 34, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Schroder, J.; D’Alessandro, D.; Esmailian, F.; Boeve, T.; Tang, P.; Liao, K.; Wang, I.; Anyanwu, A.; Shah, A.; Mudy, K.; et al. Successful Utilization of Extended Criteria Donor (ECD) Hearts for Transplantation-Results of the OCS™ Heart EXPAND Trial to Evaluate the Effectiveness and Safety of the OCS Heart System to Preserve and Assess ECD Hearts for Transplantation. J. Heart Lung Transplant. 2019, 38, S42. [Google Scholar] [CrossRef]
- Piperata, A.; Caraffa, R.; Bifulco, O.; Avesani, M.; Gerosa, G.; Bottio, T. Heart transplantation in the new era of extended donor criteria. J. Card. Surg. 2021, 36, 4828–4829. [Google Scholar] [CrossRef]
- Sugimura, Y.; Immohr, M.B.; Aubin, H.; Mehdiani, A.; Rellecke, P.; Tudorache, I.; Lichtenberg, A.; Boeken, U.; Akhyari, P. Impact of Reported Donor Ejection Fraction on Outcome after Heart Transplantation. Thorac. Cardiovasc. Surg. 2021, 69, 490–496. [Google Scholar] [CrossRef]
- Pavanello, S.; Campisi, M.; Fabozzo, A.; Cibin, G.; Tarzia, V.; Toscano, G.; Gerosa, G. The biological age of the heart is consistently younger than chronological age. Sci. Rep. 2020, 10, 10752. [Google Scholar] [CrossRef]

| SDs (n = 174) | MDs (n = 64) | p | |
|---|---|---|---|
| n (%) or Median (IQR) | n (%) or Median (IQR) | ||
| (a) | |||
| Gender (Female) | 39 (22.4%) | 17 (26.6%) | 0.5 |
| Age (years) | 56.4 (46.8–63.3) | 63.6 (55.3–66.8) | <0.001 |
| Cardiac diagnosis | 0.5 | ||
| Dilatative | 67 (38.5%) | 18 (28.1%) | |
| Ischemic | 71 (40.8%) | 33 (51.6%) | |
| Congenital | 12 (6.9%) | 3 (4.7%) | |
| Valvular | 3 (1.7%) | 2 (3.1%) | |
| Hypertrophic | 10 (5.7%) | 2 (3.1%) | |
| Other | 11 (6.3%) | 6 (9.4%) | |
| BSA (m2) | 1.7 (1.7–2.0) | 1.8 (1.7–1.9) | 0.6 |
| Dyslipidemia | 63 (36.2%) | 25 (39.1%) | 0.8 |
| Hypertension | 62 (35.6%) | 33 (51.6%) | 0.04 |
| Cancer | 9 (5.2%) | 2 (3.1%) | 0.7 |
| Diabetes | 31 (17.8%) | 16 (25.0%) | 0.3 |
| PVD | 12 (6.9%) | 7 (10.9%) | 0.2 |
| COPD | 10 (5.7%) | 13 (20.3%) | 0.002 |
| ICD | 129 (74.1%) | 54 (84.4%) | 0.1 |
| Cerebral event | 28 (16.1%) | 16 (25.0%) | 0.1 |
| Smoker | 63 (36.2%) | 23 (35.9%) | 0.6 |
| Genetic syndrome | 11 (6.3%) | 3 (4.7%) | 0.8 |
| Previous cardiac surgery | 67 (38.5%) | 32 (50.0%) | 0.1 |
| Bilirubin (µmol/L) | 14.6 (9.3–22.6) | 15.0 (7.2–22.7) | 0.3 |
| GFR (mL/min/mq) | 71.0 (49.0–89.0) | 51.0 (44.0–68.0) | 0.03 |
| (b) | |||
| Intracorporeal LVAD | 49 (28.2%) | 19 (29.7%) | |
| Length of LVAD support (months) | 13.1 (4.8–31.6) | 19.0 (13.7–28.0) | 0.4 |
| List status | 0.02 | ||
| 2B | 74 (42.5%) | 28 (43.8%) | |
| 2A | 51 (29.3%) | 21 (32.8%) | |
| 1 | 5 (2.9%) | 7 (10.9%) | |
| HU | 44 (25.3%) | 8 (12.5%) | |
| Status 1 + HU | 49 (28.2%) | 15 (23.4%) | 0.5 |
| Waiting list time (months) | 4.8 (1.1–18.1) | 6.5 (2.2–21.6) | 0.5 |
| ICU-stay | 62 (35.6%) | 15 (23.4%) | 0.2 |
| Inotropic support | 47 (27.0%) | 15 (23.4%) | 0.6 |
| Mechanical ventilation | 7 (4.0%) | 4 (6.3%) | 0.5 |
| CVVH | 8 (4.6%) | 3 (4.7%) | 0.9 |
| Temporary-MCS | 42 (24.14%) | 8 (12.5%) | 0.2 |
| IABP | 1 (0.6%) | 0 (0.0%) | 0.9 |
| ECMO | 18 (10.3%) | 3 (4.7%) | 0.2 |
| Paracorporeal-LVAD | 12 (6.9%) | 3 (4.7%) | 0.8 |
| Paracorporeal-RVAD | 3 (1.7%) | 1 (1.6%) | 0.8 |
| Paracorporeal-BiVAD | 8 (4.6%) | 1 (1.6%) | 0.5 |
| Standard (n = 174) | Marginal (n = 64) | p | |
|---|---|---|---|
| n (%) or Median (IQR) | n (%) or Median (IQR) | ||
| Age (years) | 45.0 (29.5–53.0) | 64.0 (62.0–66.0) | <0.001 |
| Match age | −10.0 (−23.3–1.2) | 2.8 (−1.2–10.1) | <0.001 |
| Gender (female) | 71 (40.8%) | 33 (51.6%) | 0.2 |
| Mismatch gender | 55 (31.6%) | 20 (31.3%) | 0.9 |
| Inotropic support | 130 (74.7%) | 46 (71.9%) | 0.6 |
| Cardiac arrest | 33 (19.0%) | 9 (14.1%) | 0.4 |
| Cath abnormalities | 1 (0.6%) | 4 (6.3%) | 0.08 |
| Hypertension | 17 (9.8%) | 12 (18.8%) | 0.08 |
| Smoker | 51 (29.3%) | 18 (28.1%) | 0.9 |
| Dyslipidemia | 3 (1.7%) | 4 (6.3%) | 0.1 |
| Diabetes | 1 (0.6%) | 4 (6.3%) | 0.02 |
| Cold ischemic time (minutes) | 220.0 (160.0–250.0) | 200.0 (156.3–234.3) | 0.2 |
| SDs (n = 174) | MDs (n = 64) | p | |
|---|---|---|---|
| n (%) or Median (IQR) | n (%) or Median (IQR) | ||
| Severe PGF | 36 (20.7%) | 18 (28.1%) | 0.2 |
| Postoperative ECMO support (days) | 5.0 (3.0–6.5) | 3.5 (2.0–6.8) | 0.2 |
| CVVH | 54 (31.0%) | 32 (50.0%) | 0.01 |
| Intrahospital infection | 61 (35.1%) | 30 (46.9%) | 0.1 |
| Clinical cellular rejection | 62 (35.6%) | 24 (37.5%) | 0.9 |
| CAV | 28 (16.1%) | 14 (21.9%) | 0.6 |
| Cerebral event | 17 (9.8%) | 12 (18.8%) | 0.07 |
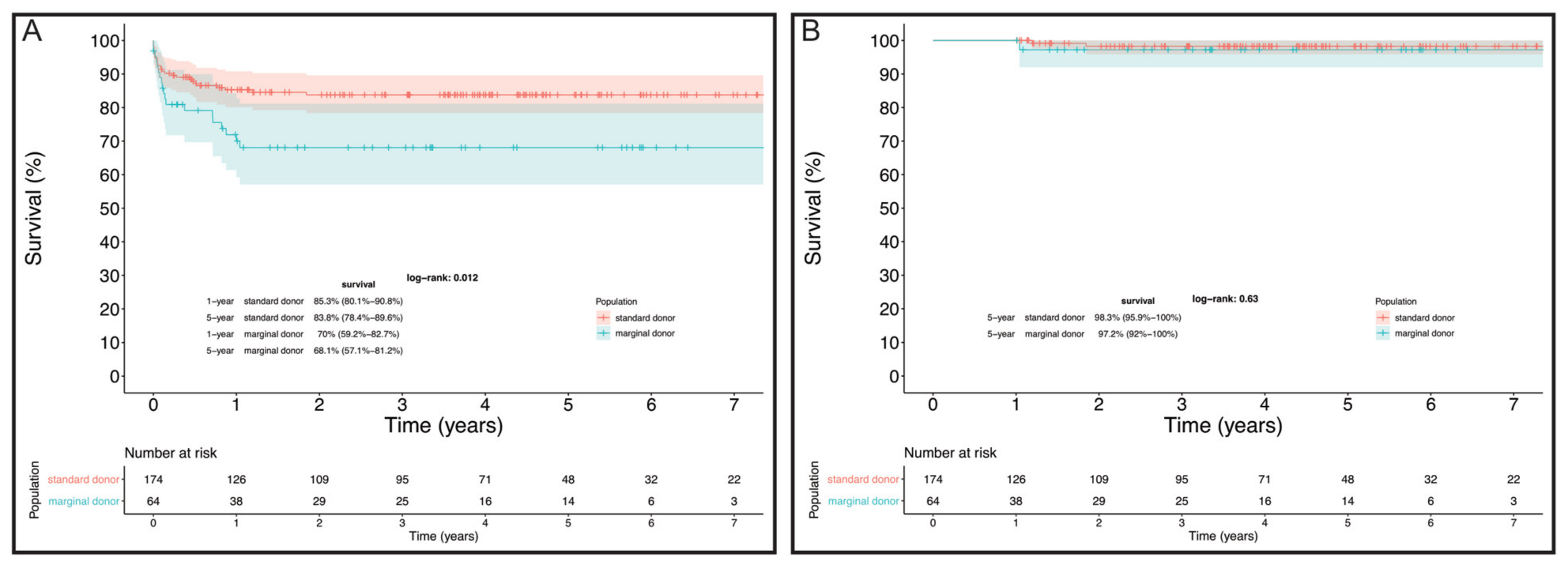
| 30-day mortality | 13 (7.5%) | 9 (14.1%) | 0.1 |
| Hospital mortality | 21 (12.1%) | 15 (23.4%) | 0.04 |
| Cause of hospital mortality | |||
| MOF | 10 (47.6%) | 11 (73.3%) | |
| Neurologic | 2 (9.5%) | 1 (6.7%) | |
| Infection | 4 (19.1%) | 3 (20.0%) | |
| Other | 5 (23.8%) | 0 (0.0%) |
| Recipient Characteristics | MDs Alive (n = 44) | MDs Mortality (n = 19) | p | Multivariate Analysis |
|---|---|---|---|---|
| n (%) or Median (IQR) | n (%) or Median (IQR) | |||
| Listing status | 0.12 | |||
| 2B | 24 (55%) | 3 (16%) | ||
| 2A | 12 (27%) | 9 (47%) | ||
| 1 | 3 (7%) | 4 (21%) | ||
| HU | 5 (11%) | 3 (16%) | ||
| Peripheral vascular disease | 2 (5%) | 4 (21%) | 0.01 | |
| Platelets count (103/mm3) | 187 (167–249) | 189 (128–255) | 0.13 | |
| Bilirubin (µmol/L) | 13 (7–21) | 20 (10–44) | 0.08 | |
| C-reactive Protein (mg/L) | 3.8 (2.9–15.1) | 26 (4–99) | 0.12 | |
| eGFR (mL/min/m2) | 60 (49–84) | 47 (42–53) | 0.02 | HR 0.98 (0.95–0.99; p = 0.04) |
| Inotropic support | 5 (11%) | 10 (53%) | <0.01 | HR 3.96 (1.17–13.44; p = 0.03) |
| CVVH | 0 (0%) | 3 (16%) | <0.01 | |
| Mechanical ventilation | 1 (2%) | 3 (16%) | 0.08 | |
| ICU stay | 8 (18%) | 7 (37%) | 0.13 | |
| Paracorporeal-LVAD | 1 (2%) | 2 (11%) | 0.17 | |
| Donors Characteristics | MDs Alive (n = 44) | MDs Mortality (n = 19) | p | Multivariate Analysis |
| n (%) or Median (IQR) | n (%) or Median (IQR) | |||
| Cold ischemic time (min) | 200 (149–234) | 216 (169–240) | 0.12 | |
| Hypertension | 6 (14%) | 6 (32%) | 0.13 | |
| Donor/Recipient BSA | 0.0 (−0.2–0.1) | 0.1 (0.0–0.2) | 0.12 |
| Recipients’ Characteristics | Marginal Donors w/o PGF (n = 46) | Marginal Donors with PGF (n = 18) | p | Multivariate Analysis |
|---|---|---|---|---|
| n (%) or Median (IQR) | n (%) or Median (IQR) | |||
| List Status | 0.02 | |||
| 2B | 23 (50.0%) | 5 (27.8%) | ||
| 2A | 12 (26.1%) | 9 (50.0%) | ||
| 1 | 3 (6.5%) | 4 (22.2%) | ||
| HU | 8 (17.4%) | 0 (0.0%) | ||
| Status 1 + HU | 11 (23.9%) | 4 (22.2%) | 0.9 | |
| Donor Characteristics | Marginal Donors w/o PGF (n = 46) | Marginal Donors with PGF (n = 18) | p | Multivariate Analysis |
| n (%) or Median (IQR) | n (%) or Median (IQR) | |||
| Inotropic support | 30 (65.2%) | 16 (88.9%) | 0.06 | OR 6.50 (1.06–39.22; p = 0.04) |
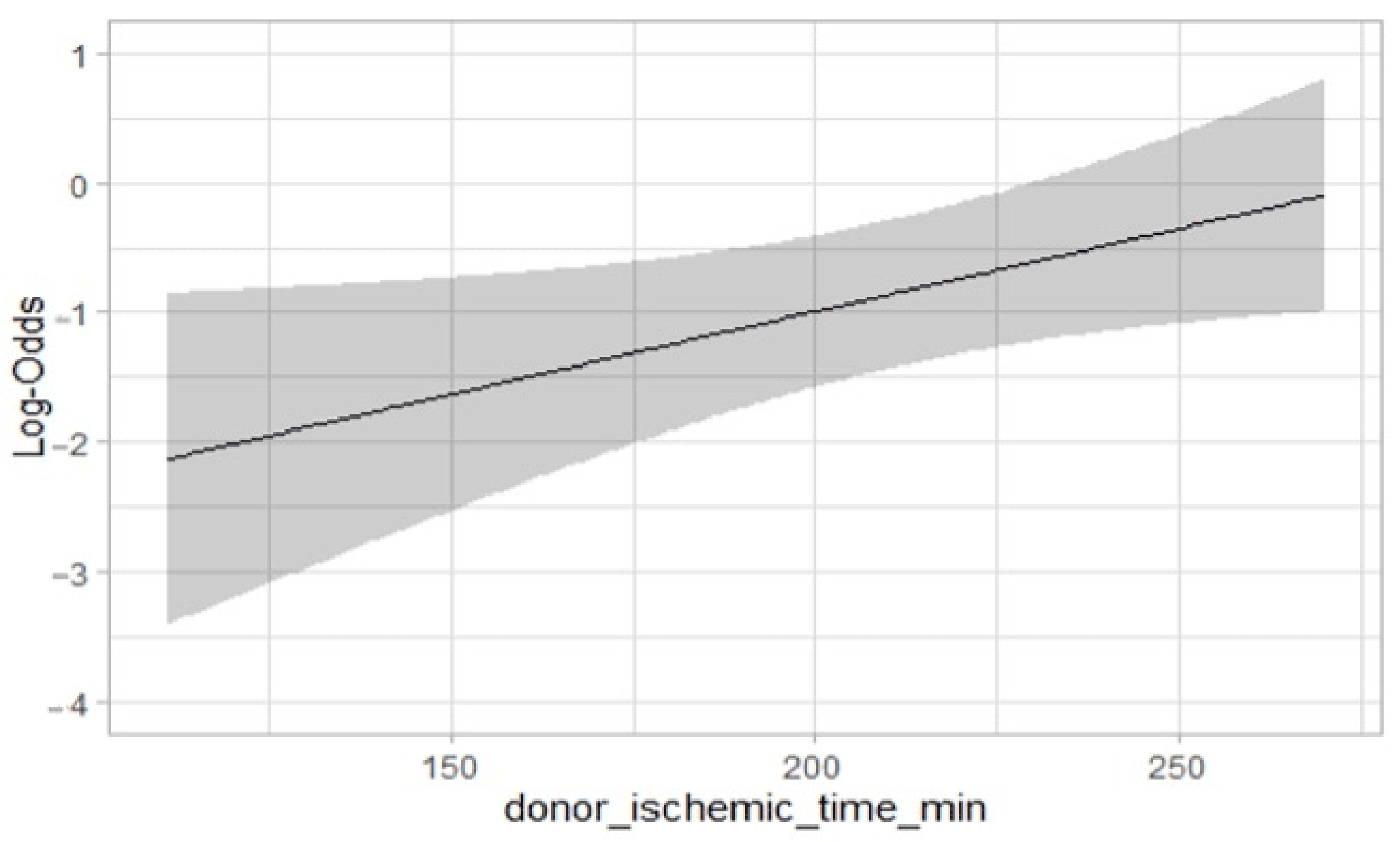
| Cold ischemic time (minutes) | 193.0 (147.3–228.5) | 227.5 (180.0–246.0) | 0.02 | OR 1.01 (1.00–1.02; p = 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bifulco, O.; Bottio, T.; Caraffa, R.; Carrozzini, M.; Guariento, A.; Bejko, J.; Fedrigo, M.; Castellani, C.; Toscano, G.; Lorenzoni, G.; et al. Marginal versus Standard Donors in Heart Transplantation: Proper Selection Means Heart Transplant Benefit. J. Clin. Med. 2022, 11, 2665. https://doi.org/10.3390/jcm11092665
Bifulco O, Bottio T, Caraffa R, Carrozzini M, Guariento A, Bejko J, Fedrigo M, Castellani C, Toscano G, Lorenzoni G, et al. Marginal versus Standard Donors in Heart Transplantation: Proper Selection Means Heart Transplant Benefit. Journal of Clinical Medicine. 2022; 11(9):2665. https://doi.org/10.3390/jcm11092665
Chicago/Turabian StyleBifulco, Olimpia, Tomaso Bottio, Raphael Caraffa, Massimiliano Carrozzini, Alvise Guariento, Jonida Bejko, Marny Fedrigo, Chiara Castellani, Giuseppe Toscano, Giulia Lorenzoni, and et al. 2022. "Marginal versus Standard Donors in Heart Transplantation: Proper Selection Means Heart Transplant Benefit" Journal of Clinical Medicine 11, no. 9: 2665. https://doi.org/10.3390/jcm11092665
APA StyleBifulco, O., Bottio, T., Caraffa, R., Carrozzini, M., Guariento, A., Bejko, J., Fedrigo, M., Castellani, C., Toscano, G., Lorenzoni, G., Tarzia, V., Gregori, D., Cardillo, M., Puoti, F., Feltrin, G., Angelini, A., & Gerosa, G. (2022). Marginal versus Standard Donors in Heart Transplantation: Proper Selection Means Heart Transplant Benefit. Journal of Clinical Medicine, 11(9), 2665. https://doi.org/10.3390/jcm11092665








