Impact of Continuous Flow Left Ventricular Assist Device on Heart Transplant Candidates: A Multi-State Survival Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Design
2.3. LVAD Management
2.4. Heart Transplant Management
2.5. Statistical Analysis
3. Results
3.1. Patient Population
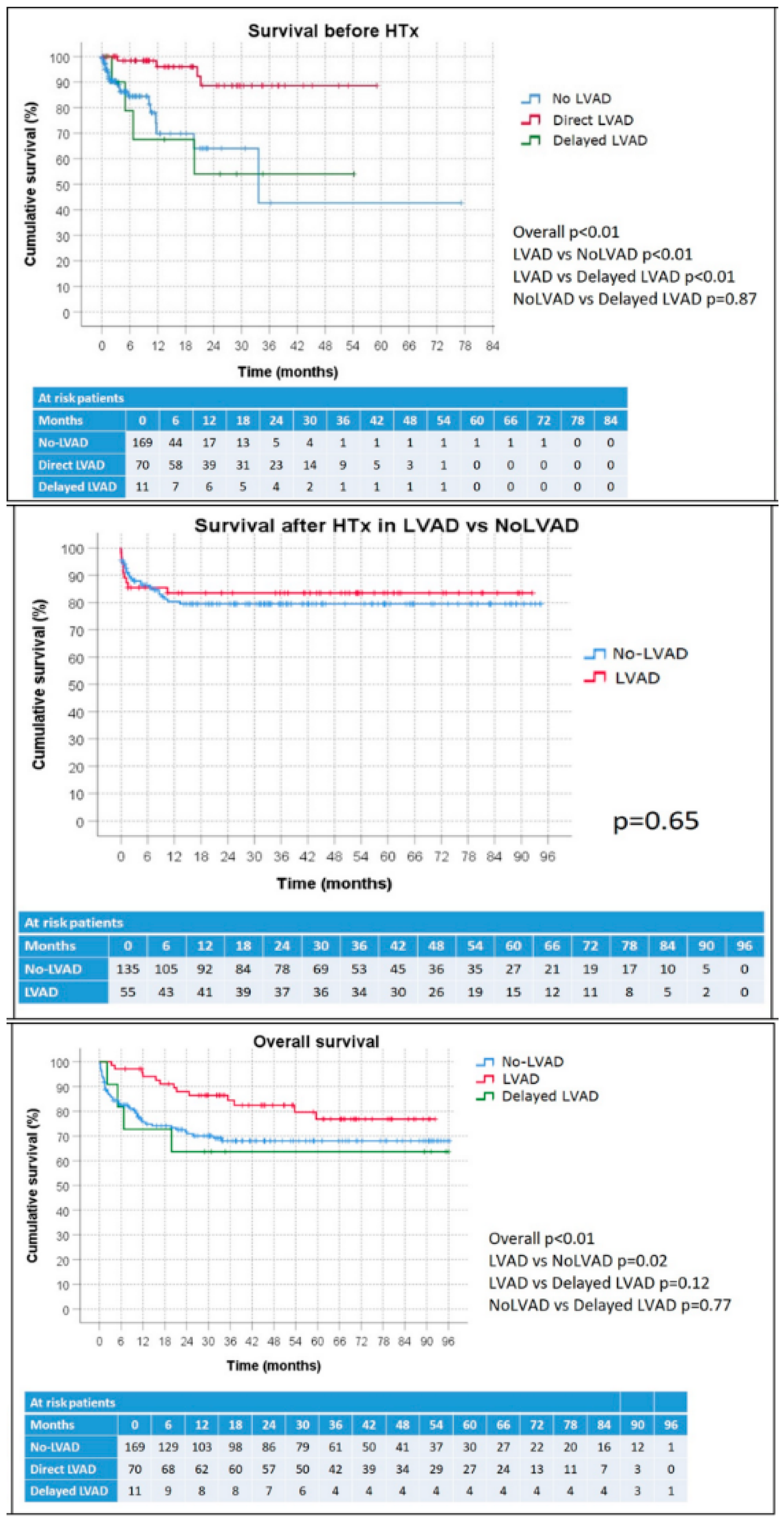
3.2. Survival before HTx
3.3. Outcomes after HTx
3.4. Acute and Chronic Rejection Rate
3.5. Overall Survival Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. ESC Scientific Document Group. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Metra, M.; Lund, L.H.; Milicic, D.; Costanzo, M.R.; Filippatos, G.; Gustafsson, F.; Tsui, S.; Barge-Caballero, E.; De Jonge, N.; et al. Advanced heart failure: A position statement of the Heart Failure Association of the European Society of Cardiology: Advanced heart failure: HFA position statement. Eur. J. Heart Fail. 2018, 20, 1505–1535. [Google Scholar] [CrossRef] [PubMed]
- Khush, K.K.; Potena, L.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.; Hsich, E.; Sadavarte, A.; Singh, T.P.; Zuckermann, A.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: 37th adult heart transplantation report—2020; focus on deceased donor characteristics. J. Heart Lung Transpl. 2020, 39, 1003–1015. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Mahr, C.; Mokadam, N.A.; Pal, J.; Smith, J.W.; Dardas, T.F. The Benefit of Donor-Recipient Matching for Patients Undergoing Heart Transplantation. J. Am. Coll. Cardiol. 2017, 69, 1707–1714. [Google Scholar] [CrossRef]
- De By, T.M.; Mohacsi, P.; Gummert, J.; Gahl, B.; Zittermann, A.; Krabatsch, T.; Gustafsson, F.; Leprince, P.; Meyns, B.; Netuka, I.; et al. The European Registry for Patients with Mechanical Circulatory Support (EUROMACS) of the European Association for Cardio-Thoracic Surgery (EACTS): Second report. Eur. J. Cardiothorac. Surg. 2018, 53, 309–316. [Google Scholar] [CrossRef]
- Goldstein, D.J.; Meyns, B.; Xie, R.; Cowger, J.; Pettit, S.; Nakatani, T.; Netuka, I.; Shaw, S.; Yanase, M.; Kirklin, J.K. Third Annual Report from the ISHLT Mechanically Assisted Circulatory Support Registry: A comparison of centrifugal and axial continuous flow left ventricular assist devices. J. Heart Lung Transpl. 2019, 38, 352–363. [Google Scholar] [CrossRef]
- Teuteberg, J.J.; Cleveland, J.C., Jr.; Cowger, J.; Higgins, R.S.; Goldstein, D.J.; Keebler, M.; Kirklin, J.K.; Myers, S.L.; Salerno, C.T.; Stehlik, J.; et al. The Society of Thoracic Surgeons Intermacs 2019 Annual Report: The Changing Landscape of Devices and Indications. Ann. Thorac. Surg. 2020, 109, 649–660. [Google Scholar] [CrossRef]
- Starling, R.C.; Estep, J.D.; Horstmanshof, D.A.; Milano, C.A.; Selzman, C.H.; Shah, K.B.; Estep, J.D. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: The ROADMAP Study 2-Year Results. JACC Heart Fail. 2017, 5, 518–527. [Google Scholar] [CrossRef]
- Ambardekar, A.V.; Kittleson, M.M.; Palardy, M.; Mountis, M.M.; Forde-McLean, R.C.; DeVore, A.D.; Pamboukian, S.V.; Thibodeau, J.; Teuteberg, J.J.; Cadaret, L.; et al. Outcomes with ambulatory advanced heart failure from the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) Registry. J. Heart Lung Transpl. 2018, 38, 408–417. [Google Scholar] [CrossRef]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 international society for heart lung transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transpl. 2016, 35, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Potapov, E.V.; Antonides, C.; Crespo-Leiro, M.G.; Combes, A.; Färber, G.; Hannan, M.M.; Kukucka, M.; De Jonge, N.; Loforte, A.; Lund, L.H.; et al. 2019 EACTS Expert Consensus on long-term mechanical circulatory support. Eur. J. Cardio-Thorac. Surg. 2019, 56, 230–270. [Google Scholar] [CrossRef] [PubMed]
- Kirklin, J.K.; Pagani, F.D.; Goldstein, D.J.; John, R.; Rogers, J.G.; Atluri, P.; Arabia, F.A.; Cheung, A.; Holman, W.; Hoopes, C.; et al. American Association for Thoracic Surgery/International Society for Heart and Lung Transplantation guidelines on selected topics in mechanical circulatory support. J. Heart Lung Transpl. 2020, 39, 187–219. [Google Scholar] [CrossRef] [PubMed]
- Carrozzini, M.; Bejko, J.; Guariento, A.; Rubino, M.; Bianco, R.; Tarzia, V.; Gregori, D.; Bottio, T.; Gerosa, G. Minimally Invasive Implantation of Continuous Flow Left Ventricular Assist Devices: The Evolution of Surgical Techniques in a Single-Center Experience: Minimally Invasive Implantation of Continuous Flow LVADS. Artif. Organs 2019, 43, e41–e52. [Google Scholar] [CrossRef]
- Carrozzini, M.; Bejko, J.; Gerosa, G.; Bottio, T. Bilateral mini-thoracotomy approach for minimally invasive implantation of HeartMate 3. Artif. Organs 2019, 43, 593–595. [Google Scholar] [CrossRef]
- Bottio, T.; Bejko, J.; Falasco, G.; Bortolussi, G.; Gallo, M.; Tarzia, V.; Gerosa, G. Less-invasive off-pump ventricular assist device implantation in regional paravertebral analgesia. J. Artif. Organs 2014, 17, 275–277. [Google Scholar] [CrossRef]
- Caraffa, R.; Bagozzi, L.; Fiocco, A.; Bifulco, O.; Nadali, M.; Ponzoni, M.; Carrozzini, M.; Toscano, G.; Fraiese, A.P.; Metra, M.; et al. Coronavirus disease 2019 (COVID-19) in the heart transplant population: A single-centre experience. Eur. J. Cardio-Thorac. Surg. 2020, 58, 899–906. [Google Scholar] [CrossRef]
- Bottio, T.; Bagozzi, L.; Fiocco, A.; Nadali, M.; Caraffa, R.; Bifulco, O.; Ponzoni, M.; Lombardi, C.M.; Metra, M.; Russo, C.F.; et al. COVID-19 in Heart Transplant Recipients. JACC Heart Fail. 2021, 9, 52–61. [Google Scholar] [CrossRef]
- Stewart, S.; Winters, G.L.; Fishbein, M.C.; Tazelaar, H.D.; Kobashigawa, J.; Abrams, J.; Andersen, C.B.; Angelini, A.; Berry, G.J.; Burke, M.M.; et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J. Heart Lung Transpl. 2005, 24, 1710–1720. [Google Scholar] [CrossRef]
- Berry, G.J.; Burke, M.M.; Andersen, C.; Bruneval, P.; Fedrigo, M.; Fishbein, M.C.; Goddard, M.; Hammond, E.H.; Leone, O.; Marboe, C.; et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J. Heart Lung Transpl. 2013, 32, 1147–1162. [Google Scholar] [CrossRef]
- Mehra, M.R.; Crespo-Leiro, M.G.; Dipchand, A.; Ensminger, S.M.; Hiemann, N.E.; Kobashigawa, J.A.; Madsen, J.; Parameshwar, J.; Starling, R.C.; Uber, P.A.; et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J. Heart Lung Transpl. 2010, 29, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Jackson, C.H. Multi-State Models for Panel Data: The msm Package for R. J. Stat. Softw. 2011, 38, 1–28. [Google Scholar] [CrossRef]
- Truby, L.K.; Rogers, J.G. Advanced Heart Failure. JACC Heart Fail. 2020, 8, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Donal, E.; Kraigher-Krainer, E.; Vasan, R.S. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2011, 13, 18–28. [Google Scholar] [CrossRef]
- Taghavi, S.; Jayarajan, S.N.; Komaroff, E.; Mangi, A.A. Continuous flow left ventricular assist device technology has influenced wait times and affected donor allocation in cardiac transplantation. J. Thorac. Cardiovasc. Surg. 2014, 147, 1966–1971. [Google Scholar] [CrossRef][Green Version]
- Trivedi, J.R.; Cheng, A.; Singh, R.; Williams, M.L.; Slaughter, M.S. Survival on the heart transplant waiting list: Impact of continuous flow left ventricular assist device as bridge to transplant. Ann. Thorac. Surg. 2014, 98, 830–834. [Google Scholar] [CrossRef]
- Truby, L.K.; Garan, A.R.; Givens, R.C.; Takeda, K.; Takayama, H.; Trinh, P.N.; Yuzefpolskaya, M.; Farr, M.A.; Naka, Y.; Colombo, P.C.; et al. Ventricular Assist Device Utilization in Heart Transplant Candidates: Nationwide Variability and Impact on Waitlist Outcomes. Circ. Heart Fail. 2018, 11, e004586. [Google Scholar] [CrossRef]
- Khush, K.K.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.; Hsich, E.; Meiser, B.; Potena, L.; Robinson, A.; Rossano, J.W.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report–2019; focus theme: Donor and recipient size match. J. Heart Lung Transpl. 2019, 38, 1056–1066. [Google Scholar] [CrossRef]
- Nestorovic, E.M.; Grupper, A.; Joyce, L.D.; Milic, N.M.; Stulak, J.M.; Edwards, B.S.; Pereira, N.L.; Daly, R.C.; Kushwaha, S.S. Effect of Pretransplant Continuous-Flow Left Ventricular Assist Devices on Cellular and Antibody-Mediated Rejection and Subsequent Allograft Outcomes. Am. J. Cardiol. 2017, 119, 452–456. [Google Scholar] [CrossRef]
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| n (%) or Mean ± SD | p Value | HR | Lower CL | Upper CL | p Value | |
| Treatment received | 0.01 | <0.01 | ||||
| No-LVAD | 169 (68%) | Direct LVAD vs. No-LVAD: 0.010 | 0.002 | 0.057 | <0.01 | |
| Direct LVAD | 70 (28%) | Delayed LVAD vs. No-LVAD: 0.132 | 0.024 | 0.738 | 0.021 | |
| Delayed LVAD | 1 (4%) | |||||
| Age (years) | 54 ± 13 | 0.820 | ||||
| Female sex | 51 (20%) | 0.809 | ||||
| BSA (m2) | 1.8 ± 0.2 | 0.661 | ||||
| Blood group | 0.013 | |||||
| A | 101 (41%) | |||||
| B | 28 (11%) | |||||
| AB | 8 (3%) | |||||
| 0 | 110 (45%) | |||||
| Cardiac diagnosis | 0.599 | |||||
| Primary dilated | 9 (39%) | |||||
| Ischemic | 109 (44%) | |||||
| Other cardiomyopathy | 44 (18%) | |||||
| Acute onset | 48 (19%) | 0.036 | ||||
| Dyslipidemia | 95 (38%) | 0.149 | ||||
| Hypertension | 95 (38%) | 0.173 | ||||
| Diabetes | 55 (22%) | 0.011 | 2.930 | 1.149 | 7.470 | 0.024 |
| Peripheral artery disease | 15 (6%) | 0.512 | ||||
| COPD | 25 (10%) | 0.517 | ||||
| ICD/CRTD | 202 (81%) | 0.707 | ||||
| Previous cerebral event | 43 (17%) | 0.343 | ||||
| Previous smoking | 94 (38%) | 0.188 | ||||
| INTERMACS profile | ||||||
| 1 | 45 (18%) | 0.049 | ||||
| 2 | 6 (2%) | |||||
| 3 | 52 (21%) | |||||
| 4 | 147 (59%) | |||||
| Waiting list status (at entry) | 0.079 | <0.01 | ||||
| 2B | 120 (48%) | 2Avs2B: 12.439 | 12,439 | 3160 | <0.01 | |
| 2A | 97 (39%) | 1vs2B: 1241.170 | 1,241,170 | 60,164 | <0.01 | |
| 1 | 5 (2%) | HUvs2B: 52.953 | 52,953 | 8419 | <0.01 | |
| HU | 28 (11%) | |||||
| Peak-VO2 (mL/kg/min) | 11.5 ± 2.7 | 0.760 | ||||
| Cardiac index (L/min/m2) | 2.1 ± 0.6 | 0.875 | ||||
| SPAP (mmHg) | 40.4 ± 15 | 0.998 | ||||
| PVR (WU) | 2.3 ± 2.0 | 0.792 | ||||
| PLTs (×109/L) | 216 ± 76 | 0.917 | ||||
| Bilirubin (µmol/L) | 21 ± 14 | 0.148 | ||||
| C-reactive protein (mg/L) | 24 ± 42 | <0.01 | ||||
| GFR (mL/min/m2) | 68 ± 26 | 0.112 | ||||
| ICU stay | 81 (32%) | 0.343 | ||||
| Inotropic infusion | 95 (42%) | 0.877 | ||||
| Mechanical ventilation | 7 (3%) | <0.01 | ||||
| CRRT | 10 (4%) | <0.01 | ||||
| IABP | 5 (2%) | 0.493 | ||||
| Temporary MCS | 49 (20%) | <0.01 | ||||
| Time before HTx (months) | 10 ± 12 | 0.266 | ||||
| All Patients (n = 190) | No-LVAD (n = 135) | LVAD (n = 55) | p-Value | |
|---|---|---|---|---|
| Age | 54 ± 14 | 56 ± 13 | 50 ± 16 | 0.019 |
| Female sex | 43 (23%) | 35 (26%) | 8 (15%) | 0.089 |
| BSA (m2) | 1.8 ± 0.2 | 2 ± 0 | 1.8 ± 0.5 | |
| Blood group | 0.643 | |||
| A | 83 (44%) | 59 (44%) | 24 (45%) | |
| B | 27 (15%) | 22 (16%) | 5 (9%) | |
| AB | 8 (4%) | 6 (4%) | 2 (4%) | |
| 0 | 70 (37%) | 48 (36%) | 22 (42%) | |
| Cardiac diagnosis | 0.062 | |||
| Primary dilated | 75 (40%) | 50 (37%) | 25 (46%) | |
| Ischemic | 82 (43%) | 56 (42%) | 26 (47%) | |
| Other cardiomyopathy | 33 (17%) | 29 (22%) | 4 (7%) | |
| Dyslipidemia | 67 (35%) | 49 (36%) | 18 (33%) | 0.641 |
| Hypertension | 72 (38%) | 51 (38%) | 21 (38%) | 0.958 |
| Diabetes | 31 (16%) | 21 (16%) | 10 (18%) | 0.657 |
| Peripheral artery disease | 11 (6%) | 9 (7%) | 2 (4%) | 0.515 |
| COPD | 19 (10%) | 14 (10%) | 5 (9%) | 0.790 |
| ICD/CRTD | 148 (78%) | 109 (81%) | 39 (71%) | 0.139 |
| Previous cerebral event | 35 (18%) | 25 (19%) | 10 (18%) | 0.957 |
| Previous smoking | 69 (36%) | 45 (33%) | 24 (44%) | 0.180 |
| Last waiting list status | <0.01 | |||
| 2B | 74 (39%) | 74 (55%) | 0 (0%) | |
| 2A | 60 (32%) | 17 (13%) | 43 (78%) | |
| 1 | 10 (5%) | 7 (5%) | 3 (6%) | |
| HU | 46 (24%) | 37 (27%) | 9 (16%) | |
| Peak-VO2 (mL/kg/min) | 11.7 ± 2.4 | 12.2 ± 2.5 | 11.8 ± 2.3 | 0.560 |
| Cardiac index (L/min/m2) | 2.1 ± 0.6 | 2.5 ± 2.8 | 2.0 ± 1.0 | 0.018 |
| SPAP (mmHg) | 37 ± 15 | 39 ± 16 | 33 ± 11 | 0.010 |
| PLTs (×109/L) | 211 ± 83 | 207 ± 86 | 221 ± 74 | 0.275 |
| Bilirubin (µmol/L) | 25 ± 38 | 37 ± 58 | 23 ± 26 | 0.330 |
| C-reactive protein (mg/L) | 38 ± 66 | 68 ± 85 | 45 ± 61 | 0.250 |
| GFR (ml/min/m2) | 70 ± 32 | 63 ± 30 | 84 ± 30 | <0.01 |
| ICU stay | 61 (32%) | 51 (38%) | 10 (18%) | <0.01 |
| Inotropic infusion | 61 (32%) | 54 (41%) | 7 (13%) | <0.01 |
| Mechanical ventilation | 7 (4%) | 6 (5%) | 1 (2%) | 0.676 |
| CRRT | 9 (5%) | 9 (7%) | 0 (0%) | 0.061 |
| IABP | 1 (1%) | 1 (1%) | 0 (0%) | 1.000 |
| Temporary MCS | 38 (20%) | 37 (27%) | 1 (2%) | <0.01 |
| Time before HTx (months) | 8 ± 10 | 4 ± 6 | 16 ± 14 | <0.01 |
| Donor age | 45 ± 17 | 46 ± 16 | 43 ± 19 | 0.210 |
| Female to male | 34 (25%) | 22 (23%) | 12 (27%) | 0.623 |
| Donor inotropic infusion | 132 (71%) | 91 (70%) | 41 (76%) | 0.377 |
| Donor cardiac arrest | 37 (20%) | 25 (19%) | 12 (22%) | 0.688 |
| Donor smoker | 47 (25%) | 34 (26%) | 13 (24%) | 0.740 |
| Donor diabetes | 4 (2%) | 2 (2%) | 2 (4%) | 0.583 |
| Donor cold ischemia time | 204 ± 59 | 202 ± 60 | 209 ± 56 | 0.483 |
| All Patients (n = 190) | No-LVAD (n = 135) | LVAD (n = 55) | p-Value | |
| Cardiopulmonary bypass time (min) | 209 ± 64 | 200 ± 62 | 228 ± 67 | <0.01 |
| ECMO | 44 (23%) | 29 (22%) | 15 (27%) | 0.420 |
| Mechanical ventilation (hours) | 83 ± 171 | 92 ± 187 | 62 ± 124 | 0.282 |
| CRRT | 67 (35%) | 51 (38%) | 16 (29%) | 0.242 |
| Documented Infection | 65 (35%) | 48 (36%) | 17 (31%) | 0.497 |
| Severe bleeding | 23 (12%) | 20 (15%) | 3 (6%) | 0.087 |
| Cerebral event | 22 (12%) | 15 (11%) | 17 (13%) | 0.765 |
| Ischemic stroke | 17 (9%) | 11 (8%) | 6 (11%) | 0.556 |
| Hemorrhagic stroke | 3 (2%) | 2 (2%) | 1 (2%) | 1.000 |
| Bowel ischemia | 10 (5%) | 6 (5%) | 4 (7%) | 0.482 |
| Major arrhythmia | 11 (6%) | 9 (7%) | 2 (4%) | 0.513 |
| Pericardial/pleural effusions | 39 (21%) | 35 (26%) | 4 (7%) | <0.01 |
| Tracheostomy | 19 (10%) | 18 (13%) | 1 (2%) | 0.015 |
| Hepatic or pancreatic complication | 13 (7%) | 11 (8%) | 2 (4%) | 0.352 |
| ICU stay (days) | 10 ± 13 | 11 ± 14 | 7 ± 6 | 0.01 |
| Hospital stay (days) | 42 ± 26 | 43 ± 29 | 38 ± 18 | 0.132 |
| Follow-up time (months) | 36 ± 29 | 34 ± 29 | 42 ± 30 | 0.08 |
| 30-day death | 16 (9%) | 10 (8%) | 6 (11%) | 0.449 |
| In-hospital death | 18 (13%) | 12 (13%) | 6 (13%) | 0.926 |
| Overall post-HTx death | 35 (18%) | 26 (19%) | 9 (16%) | 0.641 |
| Immunological findings | ||||
| All patients (n = 190) | No-LVAD (n = 135) | LVAD (n = 55) | p Value | |
| Baseline positive CDC | 9 (5%) | 7 (5%) | 2 (4%) | 1.000 |
| Baseline positive CDC >10% | 3 (2%) | 3 (2%) | 0 (0%) | 0.558 |
| Baseline positive Luminex class 1 | 23 (12%) | 18 (13%) | 5 (9%) | 0.472 |
| Baseline positive Luminex class 2 | 15 (8%) | 14 (10%) | 1 (2%) | 0.07 |
| Baseline positive Luminex class 1/2 | 30 (16%) | 24 (18%) | 6 (11%) | 0.232 |
| Donor specific antibody | 17 (16%) | 9 (12%) | 8 (24%) | 0.108 |
| Acute cellular rejection (any grade) | 153 (89%) | 107 (88%) | 46 (90%) | 0.736 |
| Acute cellular rejection (≥grade 2R) | 66 (38%) | 50 (41%) | 16 (31%) | 0.220 |
| Cellular rejection score 3 months | 0.46 ± 0.35 | 0.44 ± 0.36 | 0.50 ± 0.33 | 0.328 |
| Cellular rejection score 6 months | 0.47 ± 0.30 | 0.46 ± 0.31 | 0.51 ± 0.27 | 0.373 |
| Cellular rejection score 12 months | 0.46 ± 0.26 | 0.45 ± 0.28 | 0.48 ± 0.22 | 0.622 |
| Antibody mediated rejection | 13 (8%) | 13 (11%) | 0 (0%) | 0.011 |
| Chronic allograft vasculopathy | 3 (2%) | 3 (4%) | 0 (0%) | 0.276 |
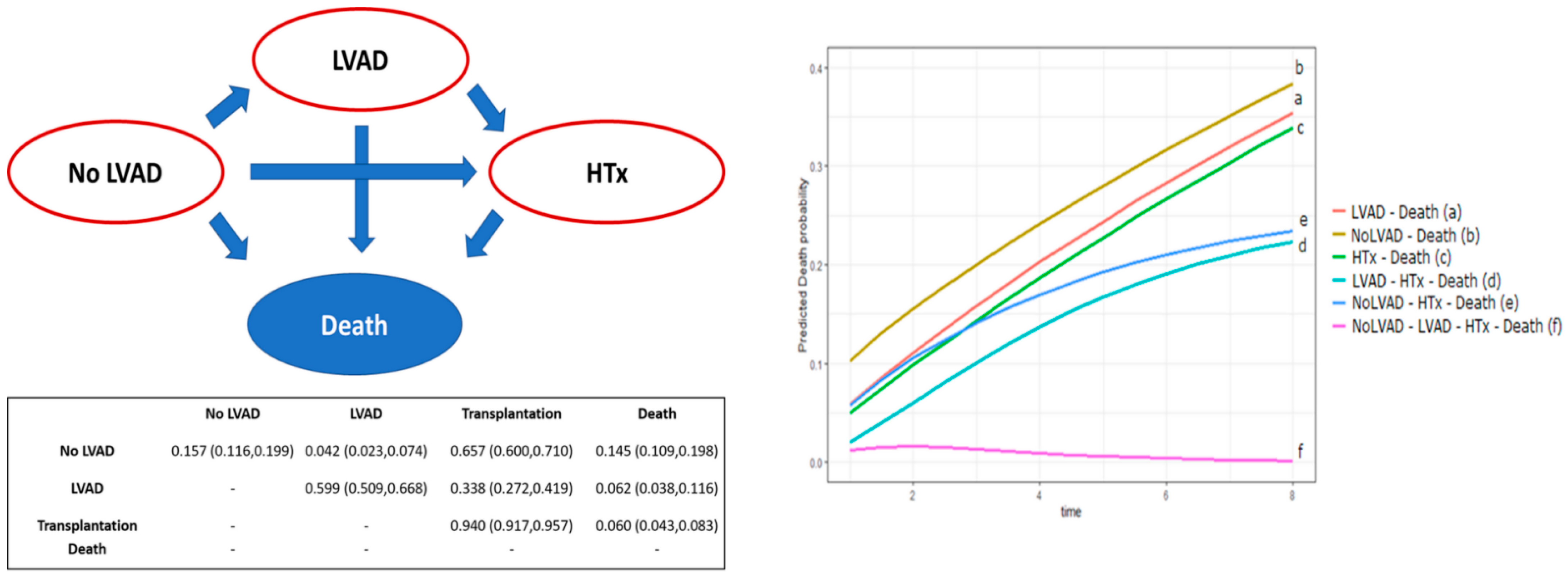
| LVAD–Death | No-LVAD–Death | HTx–Death | LVAD–HTx–Death | No-LVAD–HTx–Death | No-LVAD–LVAD–HTx–Death | |
|---|---|---|---|---|---|---|
| LVAD–Death | - | 0.038 | 0.217 | 0.032 | 0.221 | 0.006 |
| No-LVAD–Death | - | - | 0.022 | <0.001 | 0.033 | <0.001 |
| HTx–Death | - | - | - | 0.059 | 0.207 | 0.018 |
| LVAD–HTx–Death | - | - | - | - | 0.026 | 0.338 |
| No-LVAD–HTx–Death | - | - | - | - | - | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrozzini, M.; Bottio, T.; Caraffa, R.; Bejko, J.; Bifulco, O.; Guariento, A.; Lombardi, C.M.; Metra, M.; Azzolina, D.; Gregori, D.; et al. Impact of Continuous Flow Left Ventricular Assist Device on Heart Transplant Candidates: A Multi-State Survival Analysis. J. Clin. Med. 2022, 11, 3425. https://doi.org/10.3390/jcm11123425
Carrozzini M, Bottio T, Caraffa R, Bejko J, Bifulco O, Guariento A, Lombardi CM, Metra M, Azzolina D, Gregori D, et al. Impact of Continuous Flow Left Ventricular Assist Device on Heart Transplant Candidates: A Multi-State Survival Analysis. Journal of Clinical Medicine. 2022; 11(12):3425. https://doi.org/10.3390/jcm11123425
Chicago/Turabian StyleCarrozzini, Massimiliano, Tomaso Bottio, Raphael Caraffa, Jonida Bejko, Olimpia Bifulco, Alvise Guariento, Carlo Mario Lombardi, Marco Metra, Danila Azzolina, Dario Gregori, and et al. 2022. "Impact of Continuous Flow Left Ventricular Assist Device on Heart Transplant Candidates: A Multi-State Survival Analysis" Journal of Clinical Medicine 11, no. 12: 3425. https://doi.org/10.3390/jcm11123425
APA StyleCarrozzini, M., Bottio, T., Caraffa, R., Bejko, J., Bifulco, O., Guariento, A., Lombardi, C. M., Metra, M., Azzolina, D., Gregori, D., Fedrigo, M., Castellani, C., Tarzia, V., Toscano, G., Gambino, A., Jorgji, V., Ferrari, E., Angelini, A., & Gerosa, G. (2022). Impact of Continuous Flow Left Ventricular Assist Device on Heart Transplant Candidates: A Multi-State Survival Analysis. Journal of Clinical Medicine, 11(12), 3425. https://doi.org/10.3390/jcm11123425








