Survival Impact of Residual Cancer Cells in Intraoperative Peritoneal Washes following Radical Hysterectomy for Cervical Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection and Clinical Follow-Up
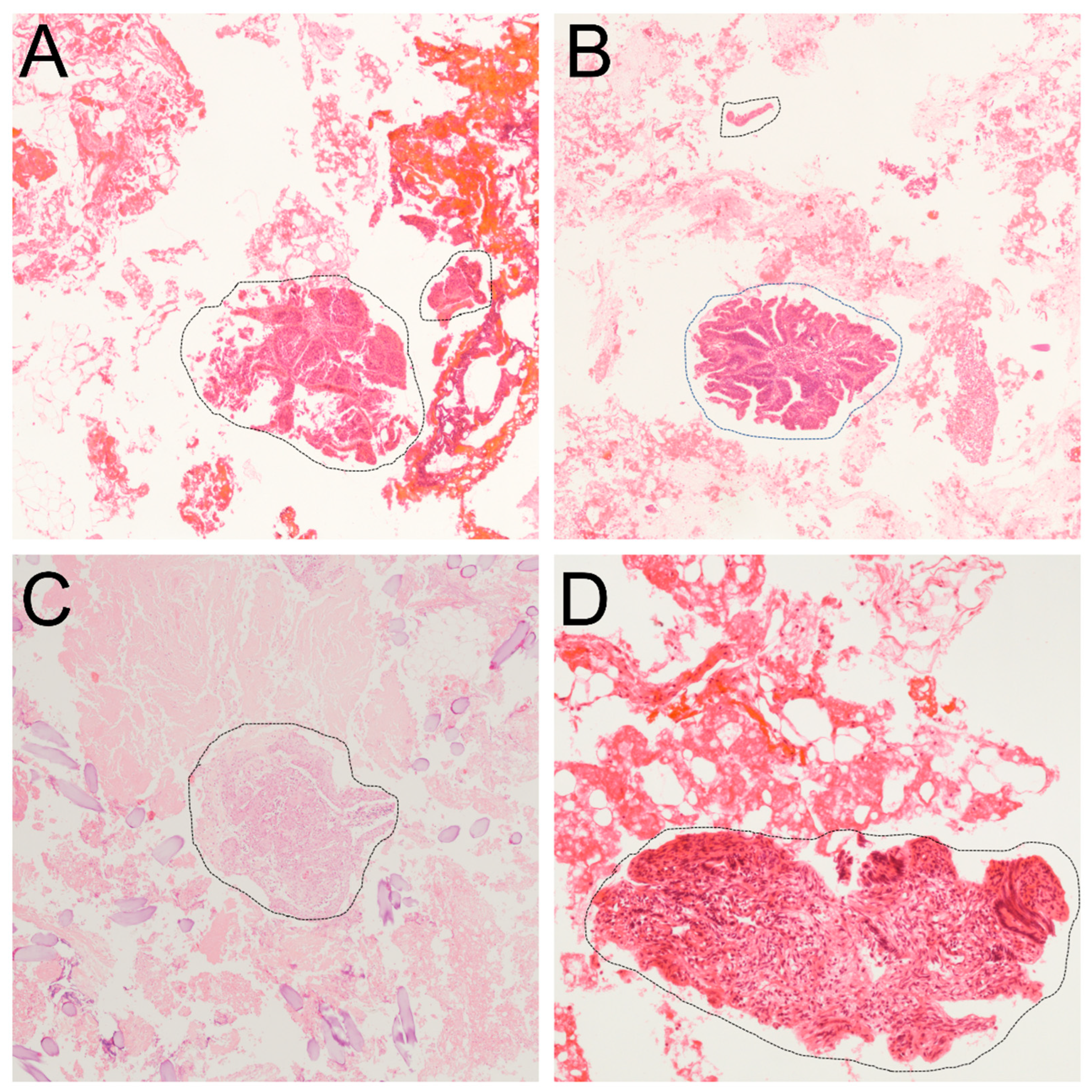
2.2. Histopathological Evaluation of RCCs
2.3. Statistical Analysis
3. Results
3.1. Clinicopathologic Characteristics
3.2. Treatment Outcomes
3.3. Survival Analyses
3.4. Clinicopathologic Variables Associated with Positive RCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wright, J.D.; Herzog, T.J.; Neugut, A.I.; Burke, W.M.; Lu, Y.S.; Lewin, S.N.; Hershman, D.L. Comparative effectiveness of minimally invasive and abdominal radical hysterectomy for cervical cancer. J. Gynecol. Oncol. 2012, 127, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Geetha, P.; Nair, M.K. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: A systematic review. J. Minimal Access Surg. 2012, 8, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Chiva, L.; Zanagnolo, V.; Querleu, D.; Martin-Calvo, N.; Arévalo-Serrano, J.; Căpîlna, M.E.; Fagotti, A.; Kucukmetin, A.; Mom, C.; Chakalova, G.; et al. An international European cohort observational study comparing minimally invasive surgery versus open abdominal radical hysterectomy in patients with stage IB1 cervical cancer. Int. J. Gynecol. Cancer 2020, 30, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Hewett, P.J.; Thomas, W.M.; King, G.; Eaton, M. Intraperitoneal cell movement during abdominal carbon dioxide insufflation and laparoscopy. An in vivo model. Dis. Colon Rectum 1996, 39 (Suppl. S10), S62–S66. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, C.A.; Wenger, F.A.; Ordemann, J.; Gutt, C.; Sabat, R.; Müller, J.M. Experimental study of the effect of intra-abdominal pressure during laparoscopy on tumour growth and port site metastasis. Br. J. Surg. 1998, 85, 1419–1422. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Hussein, A.A.; Ma, Y.; Azabdaftari, G.; Ahmed, Y.; Wong, L.P.; Hu, Q.; Luo, W.; Cranwell, V.N.; Bunch, B.L.; et al. Accurate Quantification of Residual Cancer Cells in Pelvic Washing Reveals Association with Cancer Reccurrence following Robot-Assisted Radical Cystectomy. J. Urol. 2019, 201, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Halder, K.; Khan, V.A.; Sodhani, P. Cell block as an adjunct to conventional Papanicolaou smear for diagnosis of cervical cancer in resource-limited settings. Cytopathology 2007, 18, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Pritschet, L.; Powell, D.; Horne, Z. Marginally Significant Effects as Evidence for Hypotheses: Changing Attitudes over Four Decades. Psychol. Sci. 2016, 27, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Nica, A.; Kim, S.R.; Gien, L.T.; Covens, A.; Bernardini, M.Q.; Bouchard-Fortier, G.; Kupets, R.; May, T.; Vicus, D.; Laframboise, S.; et al. Survival after minimally invasive surgery in early cervical cancer: Is the intra-uterine manipulator to blame? Int. J. Gynecol. Cancer 2020, 30, 1864–1870. [Google Scholar] [CrossRef]
- Kong, T.W.; Chang, S.J.; Piao, X.; Paek, J.; Lee, Y.; Lee, E.J.; Chun, M.; Ryu, H.S. Patterns of recurrence and survival after abdominal versus laparoscopic/robotic radical hysterectomy in patients with early cervical cancer. J. Obstet. Gynaecol. Res. 2016, 42, 77–86. [Google Scholar] [CrossRef]
- Yang, J.; Mead-Harvey, C.; Polen-De, C.; Magtibay, P.; Butler, K.; Cliby, W.; Langstraat, C.; Dinh, T.; Chen, L.; Magrina, J. Survival outcomes in patients with cervical cancer treated with open versus robotic radical hysterectomy: Our surgical pathology interrogation. Gynecol. Oncol. 2020, 159, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Chong, G.O.; Lee, Y.H.; Lee, H.J.; Hong, D.G.; Lee, Y.S. Comparison of the Long-Term Oncological Outcomes Between the Initial Learning Period of Robotic and the Experienced Period of Laparoscopic Radical Hysterectomy for Early-Stage Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Klapdor, R.; Hertel, H.; Hillemanns, P.; Röttger, M.; Soergel, P.; Kuehnle, E.; Jentschke, M. Peritoneal contamination with ICG-stained cervical secretion as surrogate for potential cervical cancer tumor cell dissemination: A proof-of-principle study for laparoscopic hysterectomy. Acta Obstet. Gynecol. Scand. 2019, 98, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Zoli, M.; Marucci, G.; Milanese, L.; Bonfatti, R.; Sturiale, C.; Ernesto, P.; Frank, G.; Mazzatenta, D. Suction Filter in Endoscopic Endonasal Surgery: A Technical Note. World Neurosurg. 2016, 95, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.; Russo, L.; Ulugoel, S.; Freire Dos Santos, R.; Breuer, E.; Gupta, A.; Lehmann, K. Peritoneal Metastasis: Current Status and Treatment Options. Cancers 2021, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Osman, M. The role of neoadjuvant chemotherapy in the management of locally advanced cervix cancer: A systematic review. Oncol. Rev. 2014, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, V.; Schiavi, M.C.; Ruscito, I.; Visentin, V.S.; Palaia, I.; Marchetti, C.; Fischetti, M.; Monti, M.; Muzii, L.; Benedetti Panici, P. Effects of Neoadjuvant Chemotherapy Plus Radical Surgery as Front Line Treatment Strategy in Patients Affected by FIGO Stage III Cervical Cancer. Ann. Surg. Oncol. 2016, 23 (Suppl. S5), 841–849. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Sardi, J.E.; Katsumata, N.; Ryu, H.S.; Nam, J.H.; Chung, H.H.; Park, N.H.; Song, Y.S.; Behtash, N.; Kamura, T.; et al. Efficacy of neoadjuvant chemotherapy in patients with FIGO stage IB1 to IIA cervical cancer: An international collaborative meta-analysis. Eur. J. Surg. Oncol. 2013, 39, 115–124. [Google Scholar] [CrossRef] [PubMed]
| Variables | All (n = 229) | No Recurrence (n = 194) | Recurrence (n = 35) | p Value |
|---|---|---|---|---|
| Age (years) | 49.99 ± 10.30 | 50.28 ± 10.14 | 48.40 ± 11.17 | 0.3218 |
| FIGO stage (n, %) | 0.0335 | |||
| IB1 | 162 (70.7) | 143 (73.7) | 19 (54.3) | |
| IB2 | 33 (14.4) | 25 (12.9) | 8 (22.9) | |
| IIA1 | 8 (3.5) | 5 (2.6) | 3 (8.6) | |
| IIA2 | 4 (1.7) | 2 (1.0) | 2 (5.7) | |
| IIB | 22 (9.6) | 19 (9.8) | 3 (8.6) | |
| Histology (n, %) | 0.0361 | |||
| SCC | 153 (66.8) | 135 (69.6) | 18 (51.4) | |
| AC/ASC | 76 (33.2) | 59 (30.4) | 17 (48.6) | |
| Tumor size (cm) | 2.23 ± 1.75 | 2.08 ± 1.74 | 3.07 ± 1.57 | 0.0019 |
| Lymphovascular invasion (n, %) | 81 (35.4) | 66 (34.0) | 15 (42.9) | 0.3153 |
| Deep stromal invasion (n, %) | 164 (71.6) | 130 (67.0) | 34 (97.1) | 0.0003 |
| Parametrial invasion (n, %) | 42 (18.3) | 31 (16.0) | 11 (31.4) | 0.0301 |
| Lymph node metastasis (n, %) | 43 (18.8) | 30 (15.5) | 13 (37.1) | 0.0026 |
| Residual cancer cells (n, %) | 19 (8.3) | 14 (7.2) | 5 (14.3) | 0.1638 |
| Preoperative LEEP (n, %) | 60 (26.2) | 58 (29.9) | 2 (5.7) | 0.0028 |
| Neoadjuvant chemotherapy (n, %) | 57 (24.9) | 43 (22.2) | 14 (40.0) | 0.025 |
| Adjuvant therapy (n, %) | 0.0026 | |||
| CCRT | 38 (16.6) | 26 (13.4) | 12 (34.3) | |
| Chemotherapy | 49 (21.4) | 42 (21.6) | 7 (20.0) | |
| Radiotherapy | 23 (10.0) | 17 (8.8) | 6 (17.1) | |
| Surgical approach | 0.1606 | |||
| Minimally invasive surgery | 208 (90.8) | 174 (89.7) | 34 (97.1) | |
| Open surgery | 21 (9.2) | 20 (10.3) | 1 (2.9) |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (years) ≤40 vs. >40 | 2.17 | 0.87–5.42 | 0.0980 | |||
| FIGO stage ≥IB2 vs. IB1 | 2.67 | 1.26–5.67 | 0.0105 | |||
| Histology AC/ASC vs. SCC | 2.12 | 1.04–4.32 | 0.0381 | |||
| Tumor size >2 cm vs. ≤2 cm | 2.14 | 1.10–4.16 | 0.0252 | |||
| Lymphovascular invasion | 1.46 | 0.72–2.93 | 0.2926 | |||
| Deep stromal invasion | 3.73 | 1.81–7.68 | 0.0003 | 13.32 | 1.81–98.27 | 0.0111 |
| Parametrial invasion | 3.10 | 1.26–7.65 | 0.0140 | |||
| Lymph node metastasis | 4.25 | 1.75–10.30 | 0.0014 | 2.00 | 1.01–3.99 | 0.0482 |
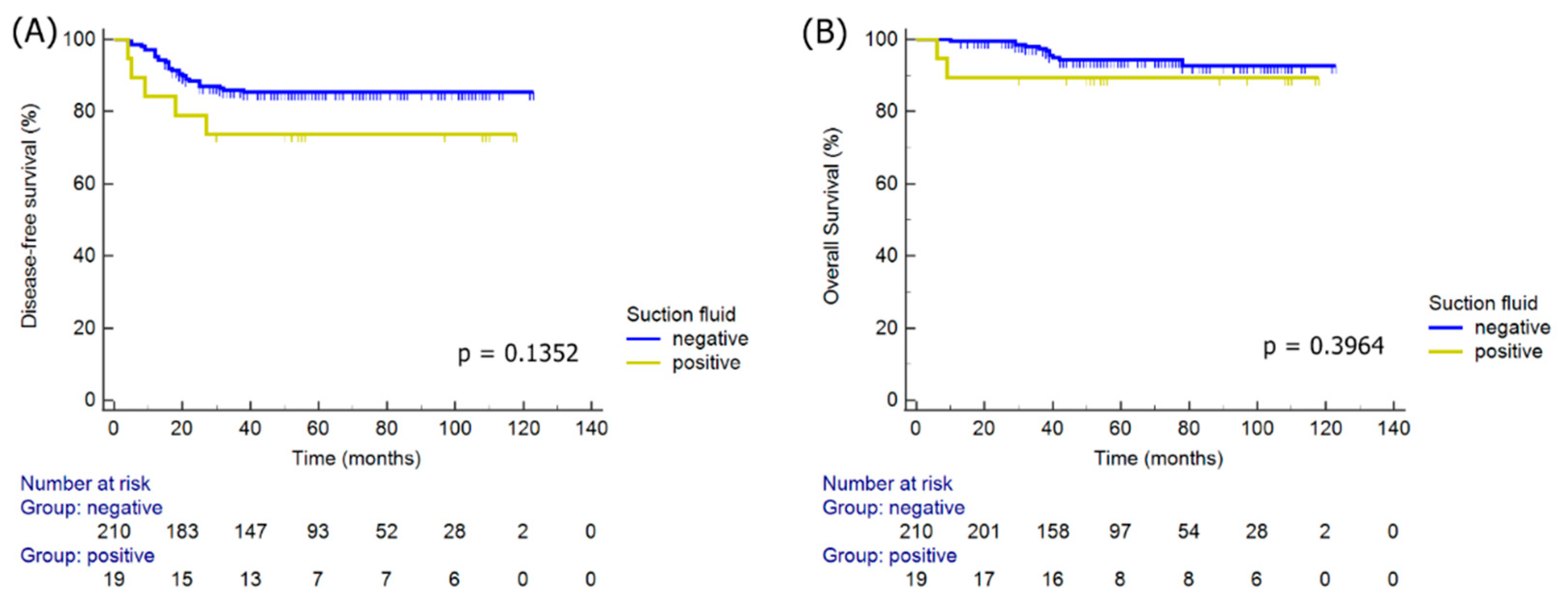
| Positive residual cancer cell | 2.60 | 0.74–9.11 | 0.1352 | |||
| Preoperative LEEP | 0.33 | 0.16–0.70 | 0.0036 | |||
| Neoadjuvant chemotherapy | 2.67 | 1.21–5.91 | 0.0150 | 2.34 | 1.89–4.61 | 0.0139 |
| Minimally invasive surgery | 2.15 | 0.68–6.79 | 0.1929 | |||
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Age (years) ≤40 vs. >40 | 7.70 | 1.82–32.59 | 0.0055 | 5.37 | 1.77–16.28 | 0.0030 |
| FIGO stage ≥IB2 vs. IB1 | 5.05 | 1.51–16.84 | 0.0084 | |||
| Histology AC/ASC vs. SCC | 1.90 | 0.59–6.11 | 0.2786 | |||
| Tumor size >2 cm vs. ≤2 cm | 5.34 | 1.80–15.86 | 0.0025 | 9.58 | 1.20–76.38 | 0.0329 |
| Lymphovascular invasion | 1.19 | 0.38–3.74 | 0.7647 | |||
| Deep stromal invasion | 3.01 | 0.93–9.75 | 0.0665 | |||
| Parametrial invasion | 7.77 | 1.83–32.96 | 0.0054 | 2.92 | 0.95–9.01 | 0.0623 |
| Lymph node metastasis | 6.54 | 1.60–26.80 | 0.0090 | |||
| Positive residual cancer cell | 2.30 | 0.34–15.81 | 0.3964 | |||
| Preoperative LEEP | 0.37 | 0.11–1.25 | 0.1092 | |||
| Neoadjuvant chemotherapy | 4.94 | 1.39–17.54 | 0.0134 | |||
| Minimally invasive surgery | 1.08 | 0.15–7.79 | 0.9362 | |||
| Variables | OR | 95% CI | p Value |
|---|---|---|---|
| Age (years) ≤40 vs. >40 | 0.95 | 0.21–4.24 | 0.9427 |
| FIGO stage ≥IB2 vs. IB1 | 2.46 | 0.65–9.34 | 0.1842 |
| Histology AC/ASC vs. SCC | 0.44 | 0.12–1.65 | 0.2222 |
| Tumor size >2 cm vs. ≤2 cm | 4.16 | 0.77–22.48 | 0.0981 |
| Lymphovascular invasion | 0.32 | 0.08–1.25 | 0.1013 |
| Deep stromal invasion | - | - | 0.9978 |
| Parametrial invasion | 3.28 | 0.85–12.63 | 0.0846 |
| Lymph node metastasis | 1.38 | 0.35–5.38 | 0.6450 |
| Preoperative LEEP | 0.90 | 0.14–5.70 | 0.9080 |
| Neoadjuvant chemotherapy | 0.22 | 0.05–0.99 | 0.0488 |
| Minimally invasive surgery | 0.72 | 0.17–2.96 | 0.6495 |
| Recurrence | 1.91 | 0.44–8.34 | 0.3875 |
| Death | 0.61 | 0.07–5.57 | 0.6574 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.M.; Chong, G.O.; Park, N.J.-Y.; Choi, Y.E.; Lee, J.; Lee, Y.H.; Hong, D.G.; Park, J.Y. Survival Impact of Residual Cancer Cells in Intraoperative Peritoneal Washes following Radical Hysterectomy for Cervical Cancer. J. Clin. Med. 2022, 11, 2659. https://doi.org/10.3390/jcm11092659
Kim JM, Chong GO, Park NJ-Y, Choi YE, Lee J, Lee YH, Hong DG, Park JY. Survival Impact of Residual Cancer Cells in Intraoperative Peritoneal Washes following Radical Hysterectomy for Cervical Cancer. Journal of Clinical Medicine. 2022; 11(9):2659. https://doi.org/10.3390/jcm11092659
Chicago/Turabian StyleKim, Jong Mi, Gun Oh Chong, Nora Jee-Young Park, Yeong Eun Choi, Juhun Lee, Yoon Hee Lee, Dae Gy Hong, and Ji Young Park. 2022. "Survival Impact of Residual Cancer Cells in Intraoperative Peritoneal Washes following Radical Hysterectomy for Cervical Cancer" Journal of Clinical Medicine 11, no. 9: 2659. https://doi.org/10.3390/jcm11092659
APA StyleKim, J. M., Chong, G. O., Park, N. J.-Y., Choi, Y. E., Lee, J., Lee, Y. H., Hong, D. G., & Park, J. Y. (2022). Survival Impact of Residual Cancer Cells in Intraoperative Peritoneal Washes following Radical Hysterectomy for Cervical Cancer. Journal of Clinical Medicine, 11(9), 2659. https://doi.org/10.3390/jcm11092659






