Efficacy of Tildrakizumab for the Treatment of Difficult-to-Treat Areas: Scalp, Nail, Palmoplantar and Genital Psoriasis
Abstract
:1. Introduction
2. Methods
2.1. Patients and Study Design
2.2. Outcome Measures
2.3. Safety
2.4. Statistical Analysis
3. Results
3.1. Patient Demographics and Clinical Characteristics
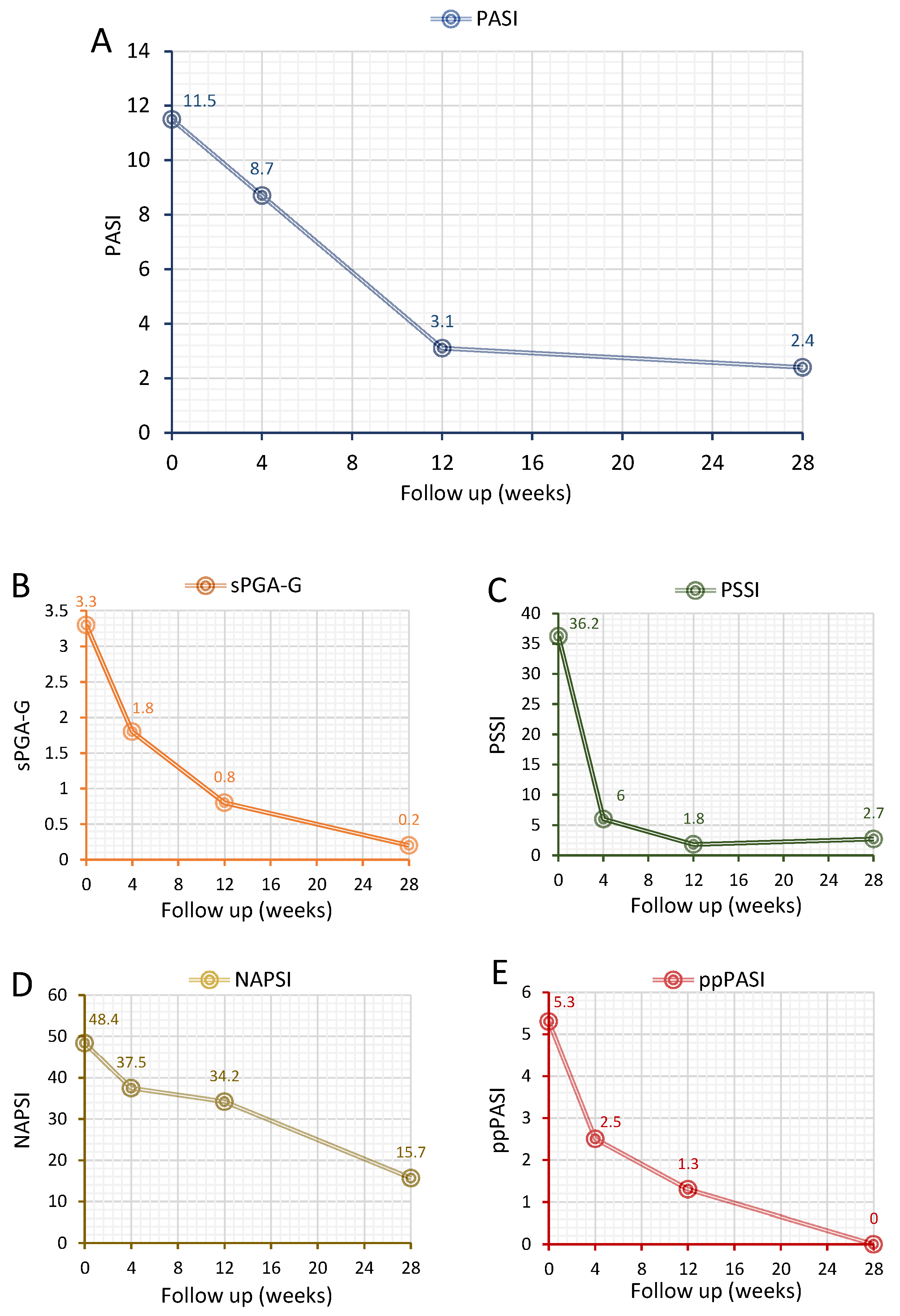
3.2. Treatment Response
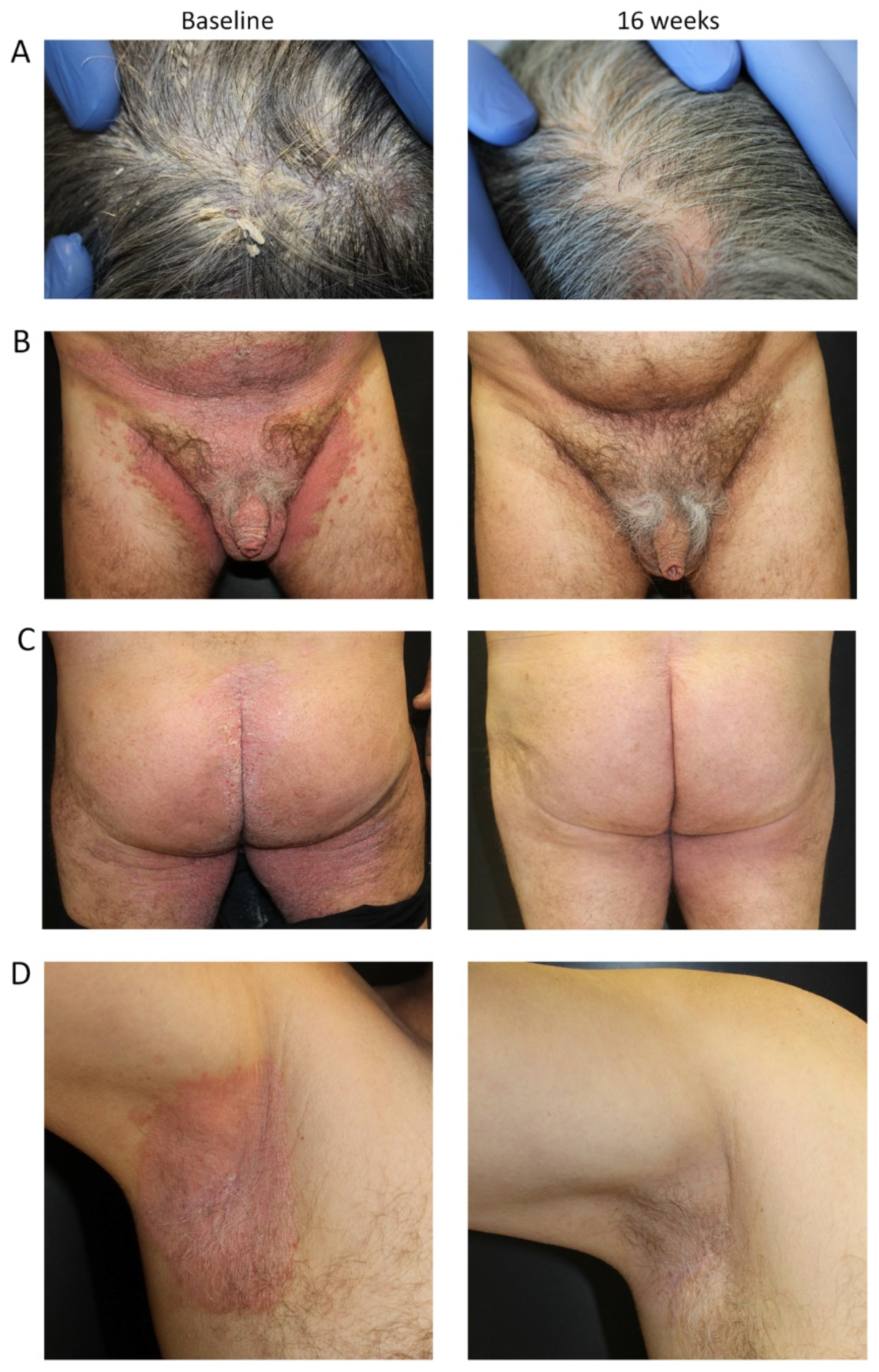
3.3. Representative Cases Showing Improvement in Difficult-to-Treat Areas after Tildrakizumab
3.3.1. Case #1
3.3.2. Case #2
3.3.3. Case #3
3.4. Safety
4. Discussion
5. Study Limitations and Strengths
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M. Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team Global Epidemiology of Psoriasis: A systematic review of incidence and prevalence. J. Invest. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Lewis-Beck, C.; Abouzaid, S.; Xie, L.; Baser, O.; Kim, E. Analysis of the relationship between psoriasis symptom severity and quality of life, work productivity, and activity impairment among patients with moderate-to-severe psoriasis using structural equation modeling. Patient Prefer. Adherence 2013, 7, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsabal, M.; Ly, S.; Sbidian, E.; Moyal-Barracco, M.; Dauendorffer, J.-N.; Dupin, N.; Richard, M.A.; Chosidow, O.; Beylot-Barry, M. GENIPSO: A French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br. J. Dermatol. 2019, 180, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Sommer, R.; Kirsten, N.; Danckworth, A.; Radtke, M.A.; Reich, K.; Thaci, D.; Boehncke, W.H.; Langenbruch, A.; Mrowietz, U. Topology of psoriasis in routine care: Results from high-resolution analysis of 2009 patients. Br. J. Dermatol. 2019, 181, 358–365. [Google Scholar] [CrossRef]
- Egeberg, A.; See, K.; Garrelts, A.; Burge, R. Epidemiology of psoriasis in hard-to-treat body locations: Data from the Danish skin cohort. BMC Dermatol. 2020, 20, 3. [Google Scholar] [CrossRef]
- Merola, J.F.; Li, T.; Li, W.-Q.; Cho, E.; Qureshi, A.A. Prevalence of psoriasis phenotypes among men and women in the USA. Clin. Exp. Dermatol. 2016, 41, 486–489. [Google Scholar] [CrossRef]
- Jiaravuthisan, M.M.; Sasseville, D.; Vender, R.B.; Murphy, F.; Muhn, C.Y. Psoriasis of the nail: Anatomy, pathology, clinical presentation, and a review of the literature on therapy. J. Am. Acad. Dermatol. 2007, 57, 1–27. [Google Scholar] [CrossRef]
- Engin, B.; Aşkın, Ö.; Tüzün, Y. Palmoplantar psoriasis. Clin. Dermatol. 2017, 35, 19–27. [Google Scholar] [CrossRef]
- Meeuwis, K.A.P.; Potts Bleakman, A.; Van De Kerkhof, P.C.M.; Dutronc, Y.; Henneges, C.; Kornberg, L.J.; Menter, A. Prevalence of genital psoriasis in patients with psoriasis. J. Dermatol. Treat. 2018, 29, 754–760. [Google Scholar] [CrossRef]
- Chan, C.S.; Voorhees, A.S.V.; Lebwohl, M.G.; Korman, N.J.; Young, M.; Bebo, B.F.; Kalb, R.E.; Hsu, S. Treatment of severe scalp psoriasis: From the medical board of the national psoriasis foundation. J. Am. Acad. Dermatol. 2009, 60, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Beck, K.M.; Sanchez, I.M.; Koo, J.; Liao, W. The impact of genital psoriasis on quality of life: A systematic review. Psoriasis Targets Ther. 2018, 8, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.J.; Mosca, M.L.; Hadeler, E.K.; Brownstone, N.D.; Bhutani, T.; Liao, W.J. Genital and inverse/intertriginous psoriasis: An updated review of therapies and recommendations for practical management. Dermatol. Ther. 2021, 11, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Van De Kerkhof, P.C.; Franssen, M.E. Psoriasis of the scalp. Diagnosis and management. Am. J. Clin. Dermatol. 2001, 2, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Ash, Z.R.; Tinazzi, I.; Gallego, C.C.; Kwok, C.; Wilson, C.; Goodfield, M.; Gisondi, P.; Tan, A.L.; Marzo-Ortega, H.; Emery, P.; et al. Psoriasis patients with nail disease have a greater magnitude of underlying systemic subclinical enthesopathy than those with normal nails. Ann. Rheum. Dis. 2012, 71, 553–556. [Google Scholar] [CrossRef]
- Lanna, C.; Galluzzi, C.; Zangrilli, A.; Bavetta, M.; Bianchi, L.; Campione, E. Psoriasis in difficult to treat areas: Treatment role in improving health-related quality of life and perception of the disease stigma. J. Dermatol. Treat. 2022, 33, 531–534. [Google Scholar] [CrossRef]
- Kassir, M.; Kircik, L.; Weinberg, J.; Fatima, F.; Yamauchi, P.; Lotti, T.; Wollina, U.; Grabbe, S.; Goldust, M. Treatment of nail psoriasis. J. Drugs Dermatol. 2022, 21, 146–150. [Google Scholar] [CrossRef]
- Raposo, I.; Torres, T. Palmoplantar psoriasis and palmoplantar pustulosis: Current treatment and future prospects. Am. J. Clin. Dermatol. 2016, 17, 349–358. [Google Scholar] [CrossRef]
- Gisondi, P.; Del Giglio, M.; Girolomoni, G. Treatment approaches to moderate to severe psoriasis. Int. J. Mol. Sci. 2017, 18, 2427. [Google Scholar] [CrossRef] [Green Version]
- Reich, K.; Sullivan, J.; Arenberger, P.; Jazayeri, S.; Mrowietz, U.; Augustin, M.; Elewski, B.; You, R.; Regnault, P.; Frueh, J.A. Secukinumab shows high and sustained efficacy in nail psoriasis: 2.5-year results from the randomized placebo-controlled TRANSFIGURE study. Br. J. Dermatol. 2021, 184, 425–436. [Google Scholar] [CrossRef]
- Wasel, N.; Thaçi, D.; French, L.E.; Conrad, C.; Dutronc, Y.; Gallo, G.; Berggren, L.; Lacour, J.-P. Ixekizumab and Ustekinumab efficacy in nail psoriasis in patients with moderate-to-severe psoriasis: 52-week results from a phase 3, head-to-head study (IXORA-S). Dermatol. Ther. 2020, 10, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Thaçi, D.; Unnebrink, K.; Sundaram, M.; Sood, S.; Yamaguchi, Y. Adalimumab for the treatment of moderate to severe psoriasis: Subanalysis of effects on scalp and nails in the BELIEVE study. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E.; Okun, M.M.; Papp, K.; Baker, C.S.; Crowley, J.J.; Guillet, G.; Sundaram, M.; Poulin, Y.; Gu, Y.; Geng, Z.; et al. Adalimumab for nail psoriasis: Efficacy and safety from the first 26 weeks of a phase 3, randomized, placebo-controlled trial. J. Am. Acad. Dermatol. 2018, 78, 90–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, P.; Gordon, K.; Griffiths, C.E.M.; Wasfi, Y.; Randazzo, B.; Song, M.; Li, S.; Shen, Y.-K.; Blauvelt, A. Efficacy of Guselkumab compared with adalimumab and placebo for psoriasis in specific body regions: A secondary analysis of 2 randomized clinical trials. JAMA Dermatol. 2018, 154, 676–683. [Google Scholar] [CrossRef] [Green Version]
- Menter, A. Consistency of infliximab response in different body regions for treatment of moderate to severe psoriasis: Results from controlled clinical trials. J. Am. Acad. Dermatol. 2008, 58, AB120. [Google Scholar] [CrossRef]
- Kopp, T.; Riedl, E.; Bangert, C.; Bowman, E.; Greisenegger, E.K.; Horowitz, A.; Kittler, H.; Blumenschein, W.; Mcclanahan, T.; Marbury, T.; et al. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature 2015, 521, 222–226. [Google Scholar] [CrossRef]
- Papp, K.; Thaçi, D.; Reich, K.; Riedl, E.; Langley, R.G.; Krueger, J.G.; Gottlieb, A.B.; Nakagawa, H.; Bowman, E.P.; Mehta, A.; et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br. J. Dermatol. 2015, 173, 930–939. [Google Scholar] [CrossRef]
- Ilumetri, SmPC. Available online: https://www.ema.europa.eu/en/documents/product-information/ilumetri-epar-product-information_it.pdf (accessed on 23 March 2022).
- Reich, K.; Papp, K.A.; Blauvelt, A.; Tyring, S.K.; Sinclair, R.; Thaçi, D.; Nograles, K.; Mehta, A.; Cichanowitz, N.; Li, Q.; et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (ReSURFACE 1 and ReSURFACE 2): Results from two randomised controlled, phase 3 trials. Lancet 2017, 390, 276–288. [Google Scholar] [CrossRef]
- Elewski, B.; Menter, A.; Crowley, J.; Tyring, S.; Zhao, Y.; Lowry, S.; Rozzo, S.; Mendelsohn, A.M.; Parno, J.; Gordon, K. Sustained and continuously improved efficacy of tildrakizumab in patients with moderate-to-severe plaque psoriasis. J. Dermatol. Treat. 2020, 31, 763–768. [Google Scholar] [CrossRef]
- Burlando, M.; Castelli, R.; Cozzani, E.; Parodi, A. Treatment of moderate-to-severe plaque psoriasis with tildrakizumab in the real-life setting. Drugs Context 2021, 10, 2–6. [Google Scholar] [CrossRef]
- Galán-Gutierrez, M.; Ruiz-Villaverde, R. Tildrakizumab: Short-term efficacy and safety in real clinical practice. Int. J. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Drerup, K.A.; Seemann, C.; Gerdes, S.; Mrowietz, U. Effective and safe treatment of psoriatic disease with the anti-il-23p19 biologic tildrakizumab: Results of a real-world prospective cohort study in nonselected patients. Dermatology 2021, 12, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Caldarola, G.; Galluzzo, M.; Bernardini, N.; Calabrese, L.; Grimaldi, M.; Moretta, G.; Pagnanelli, G.; Shumak, R.G.; Talamonti, M.; Tofani, L.; et al. Tildrakizumab in moderate-to-severe plaque psoriasis: A multicenter, retrospective, real-life study. Dermatol. Ther. 2022, e15488. [Google Scholar] [CrossRef]
- Simpson, K.; Low, Z.M.; Howard, A.; Kern, J.S. Successful management of treatment resistant nail psoriasis with tildrakizumab. Australas. J. Dermatol. 2021, 62, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Gisondi, P.; Altomare, G.; Ayala, F.; Bardazzi, F.; Bianchi, L.; Chiricozzi, A.; Costanzo, A.; Conti, A.; Dapavo, P.; De Simone, C.; et al. Italian guidelines on the systemic treatments of moderate-to-severe plaque psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 774–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merola, J.F.; Bleakman, A.P.; Gottlieb, A.B.; Menter, A.; Naegeli, A.N.; Bissonnette, R.; Guenther, L.; Sullivan, J.; Meeuwis, K.; See, K.; et al. The static physician’s global assessment of genitalia: A clinical outcome measure for the severity of genital psoriasis. J. Drugs Dermatol. 2017, 16, 793–799. [Google Scholar] [PubMed]
- Wozel, G.; Klein, E.; Mrowietz, U.; Reich, K.; Sebastian, M.; Streit, V. Scalp psoriasis. J. Dtsch. Dermatol. Ges 2011, 9, 70–74. [Google Scholar] [CrossRef]
- Rich, P.; Scher, R.K. Nail psoriasis severity index: A useful tool for evaluation of nail psoriasis. J. Am. Acad. Dermatol. 2003, 49, 206–212. [Google Scholar] [CrossRef]
- Bhushan, M.; Burden, A.; McElhone, K.; James, R.; Vanhoutte, F.; Griffiths, C. Oral liarozole in the treatment of palmoplantar pustular psoriasis: A randomized, double-blind, placebo-controlled study. Br. J. Dermatol. 2001, 145, 546–553. [Google Scholar] [CrossRef]
- Galluzzo, M.; Chiricozzi, A.; Cinotti, E.; Brunasso, G.; Congedo, M.; Esposito, M.; Franchi, C.; Malara, G.; Narcisi, A.; Piaserico, S.; et al. Tildrakizumab for treatment of moderate to severe psoriasis: An expert opinion of efficacy, safety, and use in special populations. Expert Opin. Biol. Ther. 2022, 22, 367–376. [Google Scholar] [CrossRef]
- Ryan, C.; Menter, A.; Guenther, L.; Blauvelt, A.; Bissonnette, R.; Meeuwis, K.; Sullivan, J.; Cather, J.C.; Yosipovitch, G.; Gottlieb, A.B.; et al. Efficacy and safety of ixekizumab in a randomized, double-blinded, placebo-controlled phase IIIb study of patients with moderate-to-severe genital psoriasis. Br. J. Dermatol. 2018, 179, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Janssen-Cilag, S.P.A. Observational Study on GUselkumab: Effectiveness and Impact on Quality of Life in NaïVE or Bio Experienced Patients with Regional (Facial and Genital) Psoriasis (GULLIVER Study). 2022. Available online: https://clinicaltrials.gov (accessed on 21 March 2022).
- Bagel, J.; Lynde, C.; Tyring, S.; Kricorian, G.; Shi, Y.; Klekotka, P. Moderate to severe plaque psoriasis with scalp involvement: A randomized, double-blind, placebo-controlled study of etanercept. J. Am. Acad. Dermatol. 2012, 67, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.G.; Rich, P.; Menter, A.; Krueger, G.; Goldblum, O.; Dutronc, Y.; Zhu, B.; Wei, H.; Cameron, G.S.; Heffernan, M.P. Improvement of scalp and nail lesions with ixekizumab in a phase 2 trial in patients with chronic plaque psoriasis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Kircik, L.; Fowler, J.; Weiss, J.; Meng, X.; Guana, A.; Nyirady, J. Efficacy of secukinumab for moderate-to-severe head and neck psoriasis over 52 weeks: Pooled analysis of four phase 3 studies. Dermatol. Ther. 2016, 6, 627–638. [Google Scholar] [CrossRef] [Green Version]
- Papp, K.A.; Blauvelt, A.; Bukhalo, M.; Gooderham, M.; Krueger, J.G.; Lacour, J.-P.; Menter, A.; Philipp, S.; Sofen, H.; Tyring, S.; et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N. Engl. J. Med. 2017, 376, 1551–1560. [Google Scholar] [CrossRef]
- Menter, M.A.; Murakawa, G.J.; Glover, H.; Mendelsohn, A.M.; Parno, J.; Rozzo, S.J.; Davidson, D.; Gupta, A.K. Clearance of head and neck involvement in plaque psoriasis with tildrakizumab treatment in the phase 3 ReSURFACE 1 study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e803–e805. [Google Scholar] [CrossRef]
- Sun Pharmaceutical Industries Limited. A Multicenter, Randomized, Double Blind, Placebo Controlled Clinical Study to Assess the Efficacy and Safety of Tildrakizumab in the Treatment of Moderate to Severe Plaque Psoriasis of the Scalp; Sun Pharmaceutical Industries Limited: Mumbai, India, 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03897088 (accessed on 3 March 2022).
- Huang, I.-H.; Wu, P.-C.; Yang, T.-H.; Li, H.; Huang, Y.-T.; Cheng, Y.-C.; Kuo, P.-H.; Lee, Y.-H.; Huang, Y.-C.; Tu, Y.-K. Small molecule inhibitors and biologics in treating nail psoriasis: A systematic review and network meta-analysis. J. Am. Acad. Dermatol. 2021, 85, 135–143. [Google Scholar] [CrossRef]
- Galluzzo, M.; D’Adamio, S.; Chimenti, M.S.; Teoli, M.; Bianchi, L.; Talamonti, M. Successful treatment of psoriatic crumbly nails with ustekinumab. Dermatol. Ther. 2019, 32, e12914. [Google Scholar] [CrossRef]
- Ismail, F.F.; May, J.; Moi, J.; Sinclair, R. Clinical improvement in psoriatic nail disease and psoriatic arthritis with tildrakizumab treatment. Dermatol. Ther. 2020, 33, e13216. [Google Scholar] [CrossRef]
- Galluzzo, M.; Talamonti, M.; Atzori, L.; Bardazzi, F.; Campanati, A.; Di Cesare, A.; Diotallevi, F.; Flori, M.L.; Mugheddu, C.; Offidani, A.; et al. Secukinumab for the treatment of palmoplantar psoriasis: A 2-year, multicenter, real-life observational study. Expert Opin. Biol. Ther. 2022, 22, 547–554. [Google Scholar] [CrossRef]
- Sandre, M.K.; Rohekar, S. Psoriatic arthritis and nail changes: Exploring the relationship. Semin. Arthritis Rheum. 2014, 44, 162–169. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D. Enthesitis: An autoinflammatory lesion linking nail and joint involvement in psoriatic disease. J. Eur. Acad. Dermatol. Venereol. 2009, 23 (Suppl. 1), 9–13. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.L.; Grainger, A.J.; Tanner, S.F.; Emery, P.; McGonagle, D. A high-resolution magnetic resonance imaging study of distal interphalangeal joint arthropathy in psoriatic arthritis and osteoarthritis: Are they the same? Arthritis Rheum. 2006, 54, 1328–1333. [Google Scholar] [CrossRef]
- Thaci, D.; Piaserico, S.; Warren, R.B.; Gupta, A.K.; Cantrell, W.; Draelos, Z.; Foley, P.; Igarashi, A.; Langley, R.G.; Asahina, A.; et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: Pooled analyses of two randomized phase III clinical trials (ReSURFACE 1 and ReSURFACE 2). Br. J. Dermatol. 2021, 185, 323–334. [Google Scholar] [CrossRef]
- Bonifati, C.; Morrone, A.; Cristaudo, A.; Graceffa, D. Effectiveness of anti-interleukin 23 biologic drugs in psoriasis patients who failed anti-interleukin 17 regimens. A real-life experience. Dermatol. Ther. 2021, 34, e14584. [Google Scholar] [CrossRef] [PubMed]


| Clinical Characteristic | N = 18 |
|---|---|
| General | |
| Male gender, n (%) | 11 (61.1) |
| Age (years) | 49.1 ± 12.7 |
| BMI (kg/m2) | 27.3 ± 3.9 |
| Current cigarette smoker, n (%) | 12 (66.7) |
| Disease characteristics | |
| Age at disease onset | 34.8 ± 13.0 |
| Disease duration | 14.3 ± 11.9 |
| PASI at baseline | 11.5 ± 11.7 |
| Difficult-to-treat locations | |
| Genital | 7 (38.9) |
| Scalp | 6 (33.3) |
| Nails | 5 (27.6) |
| Palmar/plantar | 7 (38.9) |
| Biologic therapy, n, (%) | |
| Biologic naïve | 14 (77.8) |
| Previous biologic | 4 (22.2) |
| Comorbidities, n (%) | |
| Hypertension | 6 (33.3) |
| Obesity | 6 (33.3) |
| Diabetes mellitus | 3 (16.7) |
| Dyslipidemia | 2 (11.1) |
| Psoriatic arthritis | 2 (11.1) |
| Thyroid disorder | 1 (5.6) |
| HBV+/HCV+ | 1 (5.6) |
| QuantiFERON-TB Gold+ | 1 (5.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galluzzo, M.; Talamonti, M.; Cioni, A.; Maffei, V.; Shumak, R.G.; Tofani, L.; Bianchi, L.; Campione, E. Efficacy of Tildrakizumab for the Treatment of Difficult-to-Treat Areas: Scalp, Nail, Palmoplantar and Genital Psoriasis. J. Clin. Med. 2022, 11, 2631. https://doi.org/10.3390/jcm11092631
Galluzzo M, Talamonti M, Cioni A, Maffei V, Shumak RG, Tofani L, Bianchi L, Campione E. Efficacy of Tildrakizumab for the Treatment of Difficult-to-Treat Areas: Scalp, Nail, Palmoplantar and Genital Psoriasis. Journal of Clinical Medicine. 2022; 11(9):2631. https://doi.org/10.3390/jcm11092631
Chicago/Turabian StyleGalluzzo, Marco, Marina Talamonti, Arnaldo Cioni, Virginia Maffei, Ruslana Gaeta Shumak, Lorenzo Tofani, Luca Bianchi, and Elena Campione. 2022. "Efficacy of Tildrakizumab for the Treatment of Difficult-to-Treat Areas: Scalp, Nail, Palmoplantar and Genital Psoriasis" Journal of Clinical Medicine 11, no. 9: 2631. https://doi.org/10.3390/jcm11092631
APA StyleGalluzzo, M., Talamonti, M., Cioni, A., Maffei, V., Shumak, R. G., Tofani, L., Bianchi, L., & Campione, E. (2022). Efficacy of Tildrakizumab for the Treatment of Difficult-to-Treat Areas: Scalp, Nail, Palmoplantar and Genital Psoriasis. Journal of Clinical Medicine, 11(9), 2631. https://doi.org/10.3390/jcm11092631








