Potential Therapeutic Effects of Long-Term Stem Cell Administration: Impact on the Gene Profile and Kidney Function of PKD/Mhm (Cy/+) Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Protocols
2.2. Characterization of ABCB5+ and ASC Cells
2.3. Tissue Analysis
2.3.1. RNA Isolation and Sequencing
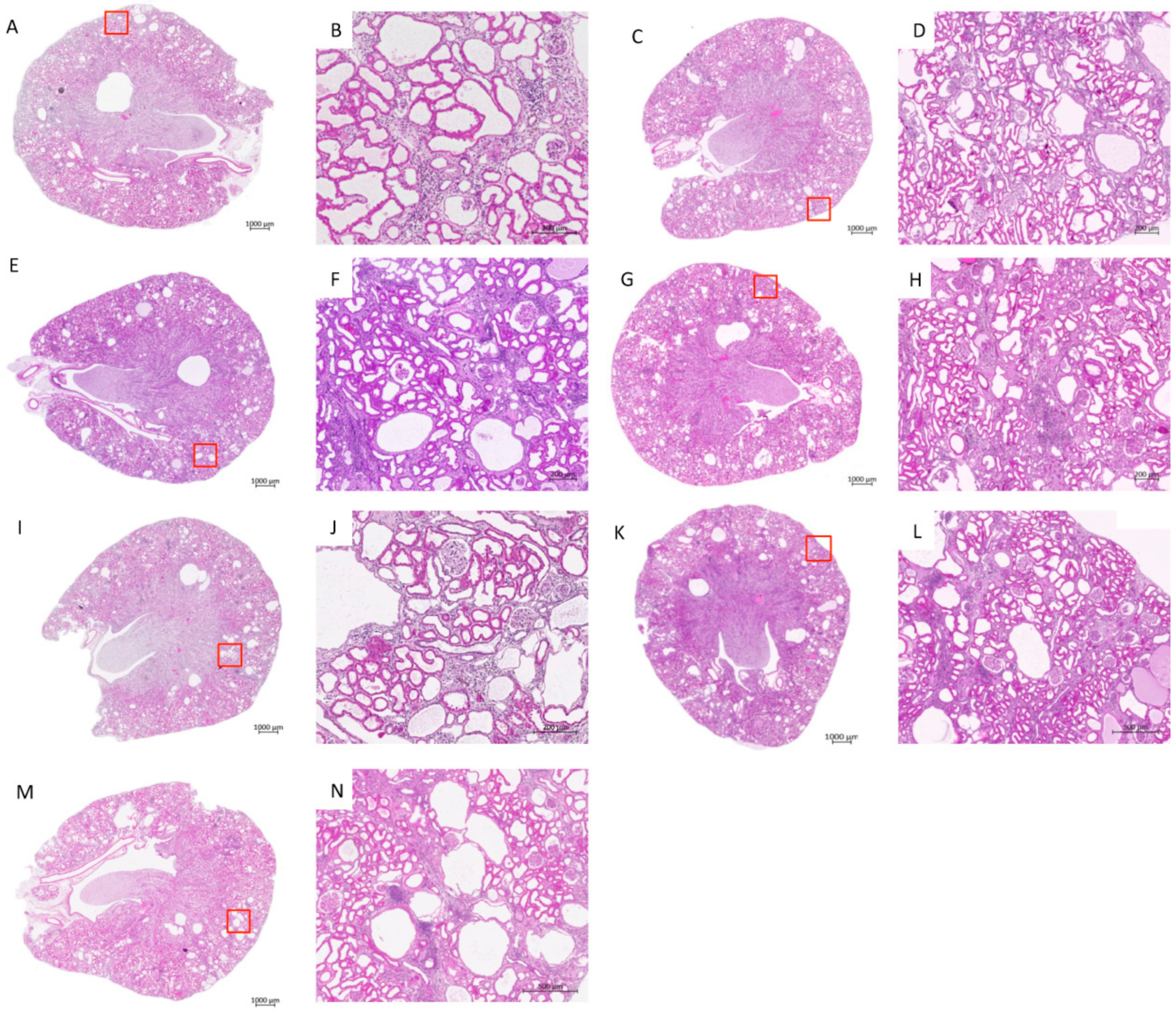
2.3.2. Histology
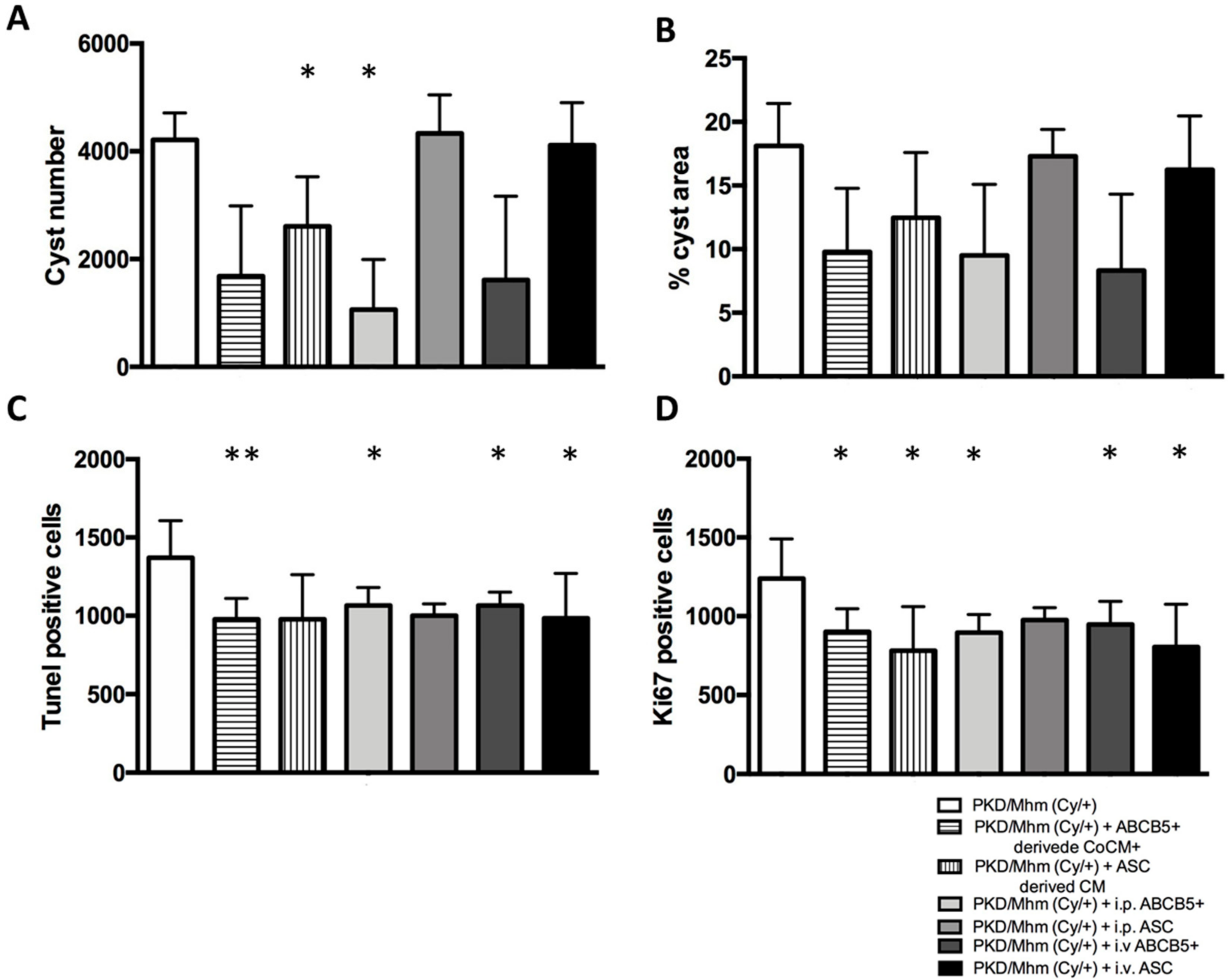
2.3.3. Proliferation and Apoptosis Assay
2.4. Kidney Function
2.4.1. Biochemistry Analyses
2.4.2. Transcutaneous GFR Measurement
2.5. Statistical Analysis
3. Results
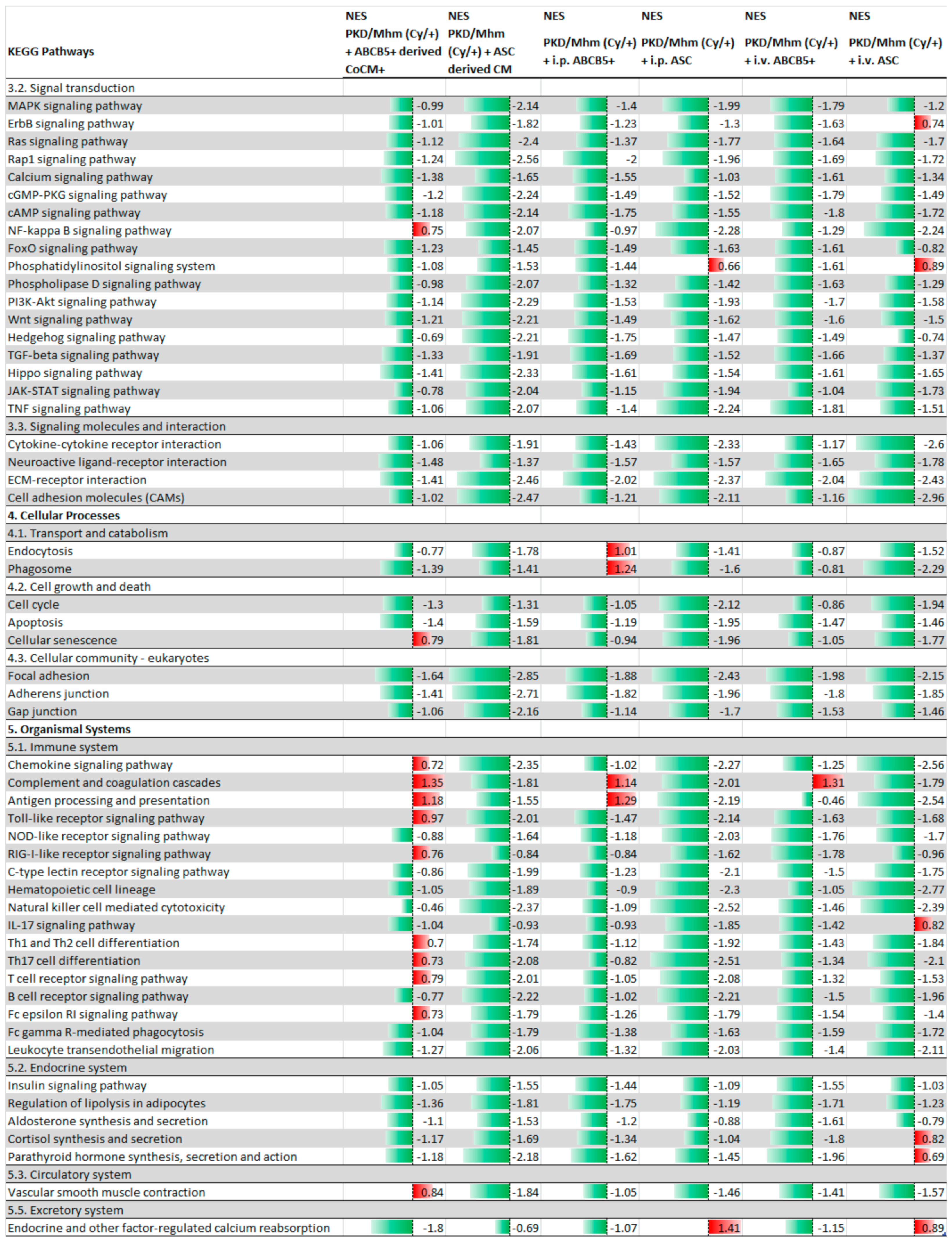
3.1. Treatment Effects on Gene Expression
3.2. Treatment Effects on Histology
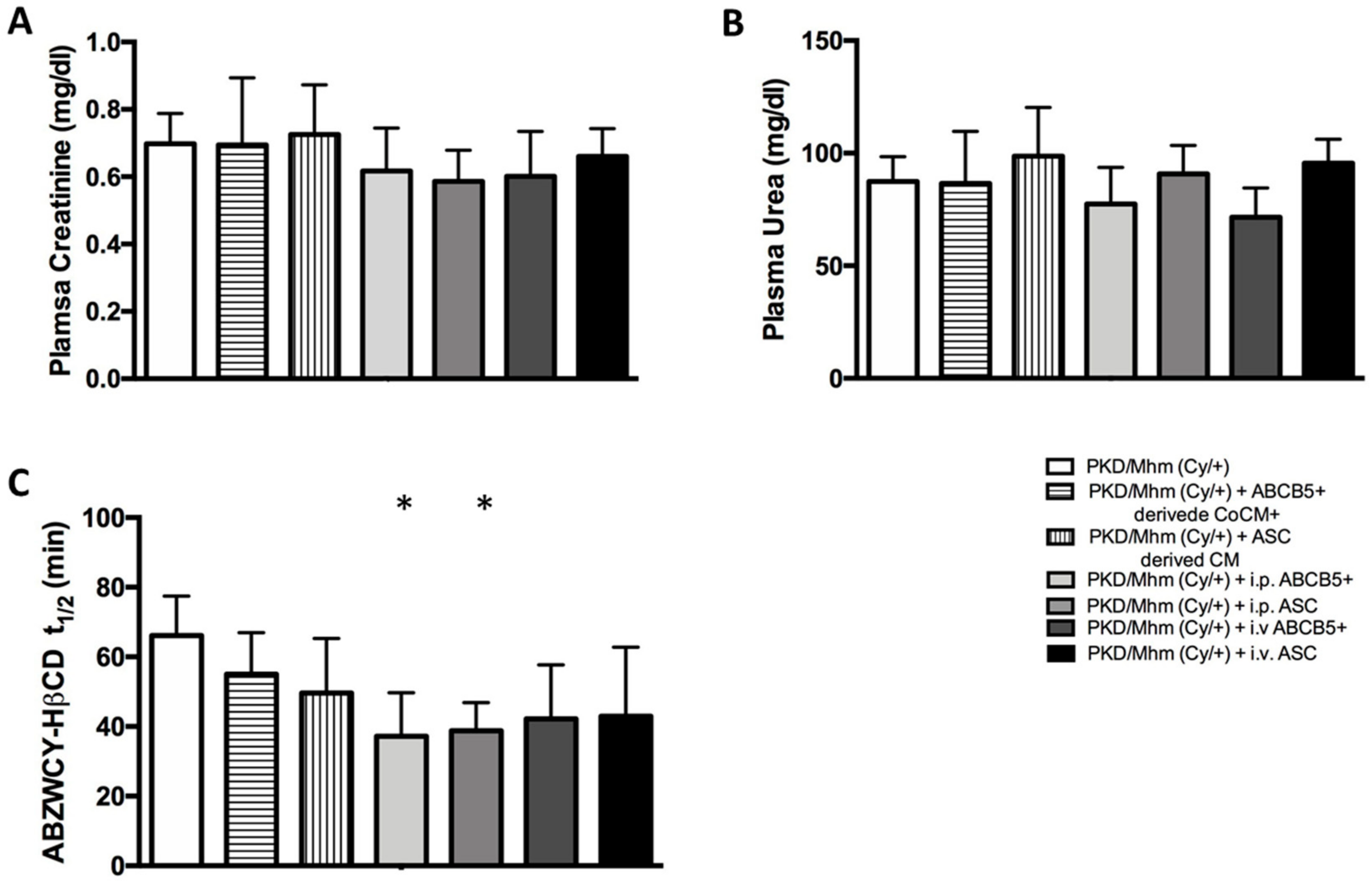
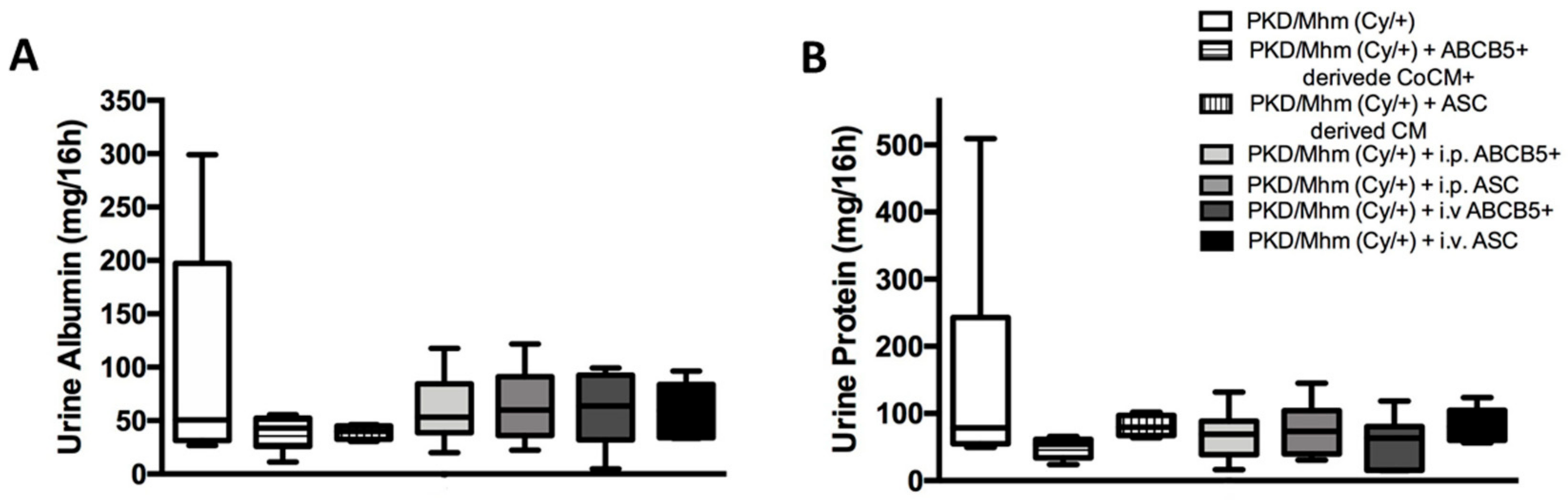
3.3. Treatment Effects on Glomerular Function
3.3.1. Plasma Biochemistry
3.3.2. Transcutaneous GFR Measurement
3.3.3. Urine Biochemistry
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, P.D.; Goilav, B. Cystic disease of the kidney. Ann. Rev. Pathol. 2007, 2, 341–368. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Guay-Woodford, L.M.; Harris, P.C.; Horie, S.; Peters, D.J.M.; Torres, V.E. Polycystic kidney disease. Nat. Rev. Dis. Primers 2018, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Idowu, J.; Home, T.; Patel, N.; Magenheimer, B.; Tran, P.V.; Maser, R.L.; Ward, C.J.; Calvet, J.P.; Wallace, D.P.; Sharma, M. Aberrant regulation of Notch3 signaling pathway in polycystic kidney disease. Sci. Rep. 2018, 8, 3340. [Google Scholar] [CrossRef] [Green Version]
- Bae, K.T.; Zhu, F.; Chapman, A.B.; Torres, V.E.; Grantham, J.J.; Guay-Woodford, L.M.; Baumgarten, D.A.; King, B.F., Jr.; Wetzel, L.H.; Kenney, P.J.; et al. Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin. J. Am. Soc. Nephrol. 2006, 1, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Bichet, D.; Peters, D.; Patel, A.J.; Delmas, P.; Honore, E. Cardiovascular polycystins: Insights from autosomal dominant polycystic kidney disease and transgenic animal models. Trends Cardiovasc Med. 2006, 16, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Dell, K.M. The spectrum of polycystic kidney disease in children. Adv. Chronic Kidney Dis. 2011, 18, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Hughes, J.; Ward, C.J.; Peral, B.; Aspinwall, R.; Clark, K.; San Millán, J.L.; Gamble, V.; Harris, P.C. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat. Genet. 1995, 10, 151–160. [Google Scholar] [CrossRef]
- Mochizuki, T.; Wu, G.; Hayashi, T.; Xenophontos, S.L.; Veldhuisen, B.; Saris, J.J.; Reynolds, D.M.; Cai, Y.; Gabow, P.A.; Pierides, A.; et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science 1996, 272, 1339–1342. [Google Scholar] [CrossRef]
- Thong, K.M.; Ong, A.C.M. The natural history of autosomal dominant polycystic kidney disease: 30-year experience from a single centre. QJM 2013, 106, 639–646. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, F.; Waldherr, R.; Kutt, R.; Brandis, M. The nephronophthisis complex: Clinical and genetic aspects. Clin. Investig. 1992, 70, 802–808. [Google Scholar] [CrossRef]
- Hildebrandt, F.; Zhou, W. Nephronophthisis-associated ciliopathies. J. Am. Soc. Nephrol. 2007, 18, 1855–1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, S.; Molinari, E.; Raman, S.; Sayer, J.A. Many genes-one disease? Genetics of nephronophthisis (NPHP) and NPHP-associated disorders. Front. Pediatr. 2017, 5, 287. [Google Scholar] [CrossRef] [PubMed]
- Couser, W.G.; Remuzzi, G.; Mendis, S.; Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011, 80, 1258–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoolwerth, A.C.; Engelgau, M.M.; Hostetter, T.H.; Rufo, K.H.; Chianchiano, D.; McClellan, W.M.; Warnock, D.G.; Vinicor, F. Chronic kidney disease: A public health problem that needs a public health action plan. Prev. Chronic Dis. 2006, 3, A57. [Google Scholar]
- Courivaud, C.; Roubiou, C.; Delabrousse, E.; Bresson-Vautrin, C.; Chalopin, J.M.; Ducloux, D. Polycystic kidney size and outcomes on peritoneal dialysis: Comparison with haemodialysis. Clin. Kidney J. 2014, 7, 138–143. [Google Scholar] [CrossRef]
- Spithoven, E.M.; Kramer, A.; Meijer, E.; Orskov, B.; Wanner, C.; Abad, J.M.; Aresté, N.; de la Torre, R.A.; Caskey, F.; Couchoud, C.; et al. Renal replacement therapy for autosomal dominant polycystic kidney disease (ADPKD) in Europe: Prevalence and survival—An analysis of data from the ERA-EDTA Registry. Nephrol. Dial. Transplant. 2014, 29 (Suppl. S4), iv15–iv25. [Google Scholar] [CrossRef] [Green Version]
- Gretz, N.; Hocker, A.; Baur, S.; Lasserre, J.J.; Bachmann, S.; Waldherr, R.; Strauch, M. Rat models of polycystic kidney disease. Contrib. Nephrol. 1992, 97, 35–46. [Google Scholar]
- Gretz, N.; Kränzlin, B.; Pey, R.; Schieren, G.; Bach, J.; Obermüller, N.; Ceccherini, I.; Klöting, I.; Rohmeiss, P.; Bachmann, S.; et al. Rat models of autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 1996, 11 (Suppl. S6), 46–51. [Google Scholar] [CrossRef] [Green Version]
- Kaspareit-Rittinghausen, J.; Deerberg, F.; Rapp, K.G.; Wcislo, A. A new rat model for polycystic kidney disease of humans. Transplant. Proc. 1990, 22, 2582–2583. [Google Scholar]
- Torremans, A.; Marescau, B.; Kranzlin, B.; Gretz, N.; Billiouw, J.M.; Vanholder, R.; De Smet, R.; Bouwman, K.; Brouns, R.; De Deyn, P.P. Biochemical validation of a rat model for polycystic kidney disease: Comparison of guanidino compound profile with the human condition. Kidney Int. 2006, 69, 2003–2012. [Google Scholar] [CrossRef]
- Bihoreau, M.-T.; Ceccherini, I.; Browne, J.; Kränzlin, B.; Romeo, G.; Lathrop, G.M.; James, M.R.; Gretz, N. Location of the first genetic locus, PKDr1, controlling autosomal dominant polycystic kidney disease in Han:SPRD cy/+ rat. Hum. Mol. Genet. 1997, 6, 609–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, J.H.; Bihoreau, M.T.; Hoffmann, S.; Kranzlin, B.; Tychinskaya, I.; Obermuller, N.; Podlich, D.; Boehn, S.N.; Kaisaki, P.J.; Megel, N.; et al. Missense mutation in sterile alpha motif of novel protein SamCystin is associated with polycystic kidney disease in (cy/+) rat. J. Am. Soc. Nephrol. 2005, 16, 3517–3526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoff, S.; Halbritter, J.; Epting, D.; Frank, V.; Nguyen, T.M.; van Reeuwijk, J.; Boehlke, C.; Schell, C.; Yasunaga, T.; Helmstadter, M.; et al. ANKS6 is a central component of a nephronophthisis module linking NEK8 to INVS and NPHP3. Nat. Genet. 2013, 45, 951–956. [Google Scholar] [CrossRef]
- Fang, B.; Guo, J.; Hao, C.; Guo, R.; Qian, S.; Li, W.; Jia, X. Whole-exome sequencing identifies a novel compound heterozygous mutation of ANKS6 gene in a Chinese nephronophthisis patient. Clin. Chim Acta 2019, 501, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Taskiran, E.Z.; Korkmaz, E.; Gucer, S.; Kosukcu, C.; Kaymaz, F.; Koyunlar, C.; Bryda, E.C.; Chaki, M.; Lu, D.; Vadnagara, K.; et al. Mutations in ANKS6 cause a nephronophthisis-like phenotype with ESRD. J. Am. Soc. Nephrol. 2014, 25, 1653–1661. [Google Scholar] [CrossRef] [Green Version]
- Torres Crigna, A.; Daniele, C.; Gamez, C.; Medina Balbuena, S.; Pastene, D.O.; Nardozi, D.; Brenna, C.; Yard, B.; Gretz, N.; Bieback, K. Stem/stromal cells for treatment of kidney injuries with focus on preclinical models. Front. Med. 2018, 5, 179. [Google Scholar] [CrossRef]
- Li, X.W.; Feng, L.X.; Zhu, X.J.; Liu, Q.; Wang, H.S.; Wu, X.; Yan, P.; Duan, X.J.; Xiao, Y.Q.; Cheng, W.; et al. Human umbilical cord blood mononuclear cells protect against renal tubulointerstitial fibrosis in cisplatin-treated rats. Biomed. Pharmacother. 2020, 121, 109310. [Google Scholar] [CrossRef]
- Feng, L.X.; Zhao, F.; Liu, Q.; Peng, J.C.; Duan, X.J.; Yan, P.; Wu, X.; Wang, H.S.; Deng, Y.H.; Duan, S.B. Role of Nrf2 in Lipopolysaccharide-induced acute kidney injury: Protection by human umbilical cord blood mononuclear cells. Oxid. Med. Cell Longev. 2020, 2020, 6123459. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Kocaoemer, A.; Kern, S.; Kluter, H.; Bieback, K. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells 2007, 25, 1270–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreher, L.; Elvers-Hornung, S.; Brinkmann, I.; Huck, V.; Henschler, R.; Gloe, T.; Kluter, H.; Bieback, K. Cultivation in human serum reduces adipose tissue-derived mesenchymal stromal cell adhesion to laminin and endothelium and reduces capillary entrapment. Stem Cells Dev. 2013, 22, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Tappenbeck, N.; Schroder, H.M.; Niebergall-Roth, E.; Hassinger, F.; Dehio, U.; Dieter, K.; Kraft, K.; Kerstan, A.; Esterlechner, J.; Frank, N.Y.; et al. In vivo safety profile and biodistribution of GMP-manufactured human skin-derived ABCB5-positive mesenchymal stromal cells for use in clinical trials. Cytotherapy 2019, 21, 546–560. [Google Scholar] [CrossRef] [PubMed]
- Schatton, T.; Yang, J.; Kleffel, S.; Uehara, M.; Barthel, S.R.; Schlapbach, C.; Zhan, Q.; Dudeney, S.; Mueller, H.; Lee, N.; et al. ABCB5 identifies immunoregulatory dermal cells. Cell Rep. 2015, 12, 1564–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vander Beken, S.; de Vries, J.C.; Meier-Schiesser, B.; Meyer, P.; Jiang, D.; Sindrilaru, A.; Ferreira, F.F.; Hainzl, A.; Schatz, S.; Muschhammer, J.; et al. Newly defined ATP-binding cassette subfamily B member 5 positive dermal mesenchymal stem cells promote healing of chronic iron-overload wounds via secretion of interleukin-1 receptor antagonist. Stem Cells 2019, 37, 1057–1074. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Inker, L.A. GFR as the “gold standard”: Estimated, measured, and true. Am. J. Kidney Dis. 2016, 67, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Daniele, C.; Nardozi, D.; Torelli, A.; Khan, A.U.M.; Gretz, N. Transcutaneous measurement of glomerular filtration rate in rodents. Methods Mol. Biol. 2020, 2067, 129–137. [Google Scholar]
- Huang, J.; Weinfurter, S.; Daniele, C.; Perciaccante, R.; Federica, R.; Della Ciana, L.; Pill, J.; Gretz, N. Zwitterionic near infrared fluorescent agents for noninvasive real-time transcutaneous assessment of kidney function. Chem. Sci. 2017, 8, 2652–2660. [Google Scholar] [CrossRef] [Green Version]
- Owen, W.F., Jr. Patterns of care for patients with chronic kidney disease in the United States: Dying for improvement. J. Am. Soc. Nephrol. 2003, 14 (Suppl. S2), S76–S80. [Google Scholar] [CrossRef] [Green Version]
- Cloutier, M.; Manceur, A.M.; Guerin, A.; Aigbogun, M.S.; Oberdhan, D.; Gauthier-Loiselle, M. The societal economic burden of autosomal dominant polycystic kidney disease in the United States. BMC Health Serv. Res. 2020, 20, 126. [Google Scholar] [CrossRef] [Green Version]
- Cowley, B.D., Jr.; Gudapaty, S.; Kraybill, A.L.; Barash, B.D.; Harding, M.A.; Calvet, J.P.; Gattone, V.H., 2nd. Autosomal-dominant polycystic kidney disease in the rat. Kidney Int. 1993, 43, 522–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauer, S.; Urbschat, A.; Gretz, N.; Hoffmann, S.C.; Kranzlin, B.; Geiger, H.; Obermuller, N. Kidney injury molecule-1 is specifically expressed in cystically-transformed proximal tubules of the PKD/Mhm (cy/+) rat model of polycystic kidney disease. Int. J. Mol. Sci. 2016, 17, 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbash, I.M.; Chouraqui, P.; Baron, J.; Feinberg, M.S.; Etzion, S.; Tessone, A.; Miller, L.; Guetta, E.; Zipori, D.; Kedes, L.H.; et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation 2003, 108, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, A. Mesenchymal stem cell delivery routes and fate. Int. J. Stem Cells 2008, 1, 1–7. [Google Scholar] [CrossRef]
- Li, H.; Guo, Z.; Jiang, X.; Zhu, H.; Li, X.; Mao, N. Mesenchymal stem cells alter migratory property of T and dendritic cells to delay the development of murine lethal acute graft-versus-host disease. Stem Cells 2008, 26, 2531–2541. [Google Scholar] [CrossRef]
- Schrepfer, S.; Deuse, T.; Reichenspurner, H.; Fischbein, M.P.; Robbins, R.C.; Pelletier, M.P. Stem cell transplantation: The lung barrier. Transplant. Proc. 2007, 39, 573–576. [Google Scholar] [CrossRef]
- Padovano, V.; Podrini, C.; Boletta, A.; Caplan, M.J. Metabolism and mitochondria in polycystic kidney disease research and therapy. Nat. Rev. Nephrol. 2018, 14, 678–687. [Google Scholar] [CrossRef]
- Lunt, S.Y.; Vander Heiden, M.G. Aerobic glycolysis: Meeting the metabolic requirements of cell proliferation. Ann. Rev. Cell Dev. Biol. 2011, 27, 441–464. [Google Scholar] [CrossRef] [Green Version]
- Podrini, C.; Cassina, L.; Boletta, A. Metabolic reprogramming and the role of mitochondria in polycystic kidney disease. Cell. Signal. 2020, 67, 109495. [Google Scholar] [CrossRef]
- Podrini, C.; Rowe, I.; Pagliarini, R.; Costa, A.S.H.; Chiaravalli, M.; Di Meo, I.; Kim, H.; Distefano, G.; Tiranti, V.; Qian, F.; et al. Dissection of metabolic reprogramming in polycystic kidney disease reveals coordinated rewiring of bioenergetic pathways. Commun. Biol. 2018, 1, 194. [Google Scholar] [CrossRef] [Green Version]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchholz, B.; Schley, G.; Faria, D.; Kroening, S.; Willam, C.; Schreiber, R.; Klanke, B.; Burzlaff, N.; Jantsch, J.; Kunzelmann, K.; et al. Hypoxia-inducible factor-1α causes renal cyst expansion through calcium-activated chloride secretion. J. Am. Soc. Nephrol. 2014, 25, 465–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, R.; Yuan, Y.; Huang, S.; Zhang, A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am. J. Physiol. Renal. Physiol. 2014, 306, F367–F378. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-W.; Lu, X.-Y.; You, Y.; Sun, H.; Liu, X.-Y.; Ai, J.-Z.; Tan, R.-Z.; Chen, T.-L.; Chen, M.-Z.; Wang, H.-L.; et al. Comparative proteomic analysis suggests that mitochondria are involved in autosomal recessive polycystic kidney disease. Proteomics 2012, 12, 2556–2570. [Google Scholar] [CrossRef]
- Nowak, K.L.; Hopp, K. Metabolic reprogramming in autosomal dominant polycystic kidney disease: Evidence and therapeutic potential. Clin. J. Am. Soc. Nephrol. 2020, 15, 577–584. [Google Scholar] [CrossRef]
- Lakhia, R.; Yheskel, M.; Flaten, A.; Quittner-Strom, E.B.; Holland, W.L.; Patel, V. PPARalpha agonist fenofibrate enhances fatty acid beta-oxidation and attenuates polycystic kidney and liver disease in mice. Am. J. Physiol. Renal. Physiol. 2018, 314, F122–F131. [Google Scholar] [CrossRef] [PubMed]
- Hajarnis, S.; Lakhia, R.; Yheskel, M.; Williams, D.; Sorourian, M.; Liu, X.; Aboudehen, K.; Zhang, S.; Kersjes, K.; Galasso, R.; et al. microRNA-17 family promotes polycystic kidney disease progression through modulation of mitochondrial metabolism. Nat. Commun. 2017, 8, 14395. [Google Scholar] [CrossRef]
- Ishimoto, Y.; Inagi, R.; Yoshihara, D.; Kugita, M.; Nagao, S.; Shimizu, A.; Takeda, N.; Wake, M.; Honda, K.; Zhou, J.; et al. Mitochondrial abnormality facilitates cyst formation in autosomal dominant polycystic kidney disease. Mol. Cell Biol. 2017, 37, 00337-17. [Google Scholar] [CrossRef] [Green Version]
- Weimbs, T. Polycystic kidney disease and renal injury repair: Common pathways, fluid flow, and the function of polycystin-1. Am. J. Physiol. Renal Physiol. 2007, 293, F1423–F1432. [Google Scholar] [CrossRef] [Green Version]
- Formica, C.; Peters, D.J.M. Molecular pathways involved in injury-repair and ADPKD progression. Cell. Signal. 2020, 72, 109648. [Google Scholar] [CrossRef]
- Leonhard, W.N.; Zandbergen, M.; Veraar, K.; van den Berg, S.; van der Weerd, L.; Breuning, M.; de Heer, E.; Peters, D.J. Scattered deletion of PKD1 in kidneys causes a cystic snowball effect and recapitulates polycystic kidney disease. J. Am. Soc. Nephrol. 2015, 26, 1322–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margaria, J.P.; Campa, C.C.; De Santis, M.C.; Hirsch, E.; Franco, I. The PI3K/Akt/mTOR pathway in polycystic kidney disease: A complex interaction with polycystins and primary cilium. Cell. Signal. 2020, 66, 109468. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K pathway in human disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, P.C.; Torres, V.E. Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J. Clin. Invest. 2014, 124, 2315–2324. [Google Scholar] [CrossRef] [Green Version]
- De Santis, M.C.; Sala, V.; Martini, M.; Ferrero, G.B.; Hirsch, E. PI3K signaling in tissue hyper-proliferation: From overgrowth syndromes to kidney cysts. Cancers 2017, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Mangolini, A.; Bogo, M.; Durante, C.; Borgatti, M.; Gambari, R.; Harris, P.C.; Rizzuto, R.; Pinton, P.; Aguiari, G.; del Senno, L. NF-kappaB activation is required for apoptosis in fibrocystin/polyductin-depleted kidney epithelial cells. Apoptosis 2010, 15, 94–104. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-kappaB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Taglienti, M.; Cai, L.; Zhou, J.; Kreidberg, J.A. c-Met and NF-Œ∫B-dependent overexpression of Wnt7a and -7b and Pax2 promotes cystogenesis in polycystic kidney disease. J. Am. Soc. Nephrol. 2012, 23, 1309–1318. [Google Scholar] [CrossRef]
- Dweep, H.; Sticht, C.; Kharkar, A.; Pandey, P.; Gretz, N. Parallel analysis of mRNA and microRNA microarray profiles to explore functional regulatory patterns in polycystic kidney disease: Using PKD/Mhm rat model. PLoS ONE 2013, 8, e53780. [Google Scholar] [CrossRef] [Green Version]
- Ta, M.H.; Harris, D.C.; Rangan, G.K. Role of interstitial inflammation in the pathogenesis of polycystic kidney disease. Nephrology 2013, 18, 317–330. [Google Scholar] [CrossRef]
- Ogborn, M.R.; Nitschmann, E.; Bankovic-Calic, N.; Weiler, H.A.; Fitzpatrick-Wong, S.; Aukema, H.M. Dietary conjugated linoleic acid reduces PGE2 release and interstitial injury in rat polycystic kidney disease. Kidney Int. 2003, 64, 1214–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somlo, S.; Ehrlich, B. Human disease: Calcium signaling in polycystic kidney disease. Curr. Biol. 2001, 11, R356–R360. [Google Scholar] [CrossRef] [Green Version]
- Goilav, B.; Satlin, L.M.; Wilson, P.D. Pathways of apoptosis in human autosomal recessive and autosomal dominant polycystic kidney diseases. Pediatr. Nephrol. 2008, 23, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S. Increased apoptosis and proliferative capacity are early events in cyst formation in autosomal-dominant, polycystic kidney disease. Sci. World J. 2007, 7, 1757–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rule, A.D. Understanding estimated glomerular filtration rate: Implications for identifying chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2007, 16, 242–249. [Google Scholar] [CrossRef]
- Tonomura, Y.; Morikawa, Y.; Takagi, S.; Torii, M.; Matsubara, M. Underestimation of urinary biomarker-to-creatinine ratio resulting from age-related gain in muscle mass in rats. Toxicology 2013, 303, 169–176. [Google Scholar] [CrossRef]
- Tögel, F.; Hu, Z.; Weiss, K.; Isaac, J.; Lange, C.; Westenfelder, C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am. J. Physiol. Renal Physiol. 2005, 289, F31–F42. [Google Scholar] [CrossRef] [Green Version]
- Mousaei Ghasroldasht, M.; Seok, J.; Park, H.S.; Liakath Ali, F.B.; Al-Hendy, A. Stem cell therapy: From idea to clinical practice. Int. J. Mol. Sci. 2022, 23, 2850. [Google Scholar] [CrossRef]
- Lee, J.M.; Jung, J.; Lee, H.J.; Jeong, S.J.; Cho, K.J.; Hwang, S.G.; Kim, G.J. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int. Immunopharmacol. 2012, 13, 219–224. [Google Scholar] [CrossRef]
- Riedl, J.; Pickett-Leonard, M.; Eide, C.; Kluth, M.A.; Ganss, C.; Frank, N.Y.; Frank, M.H.; Ebens, C.L.; Tolar, J. ABCB5+ dermal mesenchymal stromal cells with favorable skin homing and local immunomodulation for recessive dystrophic epidermolysis bullosa treatment. Stem Cells 2021, 39, 897–903. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Klomjit, N.; Conley, S.M.; Ostlie, M.M.; Jordan, K.L.; Lerman, A.; Lerman, L.O. Impaired immunomodulatory capacity in adipose tissue-derived mesenchymal stem/stromal cells isolated from obese patients. J. Cell Mol. Med. 2021, 25, 9051–9059. [Google Scholar] [CrossRef] [PubMed]
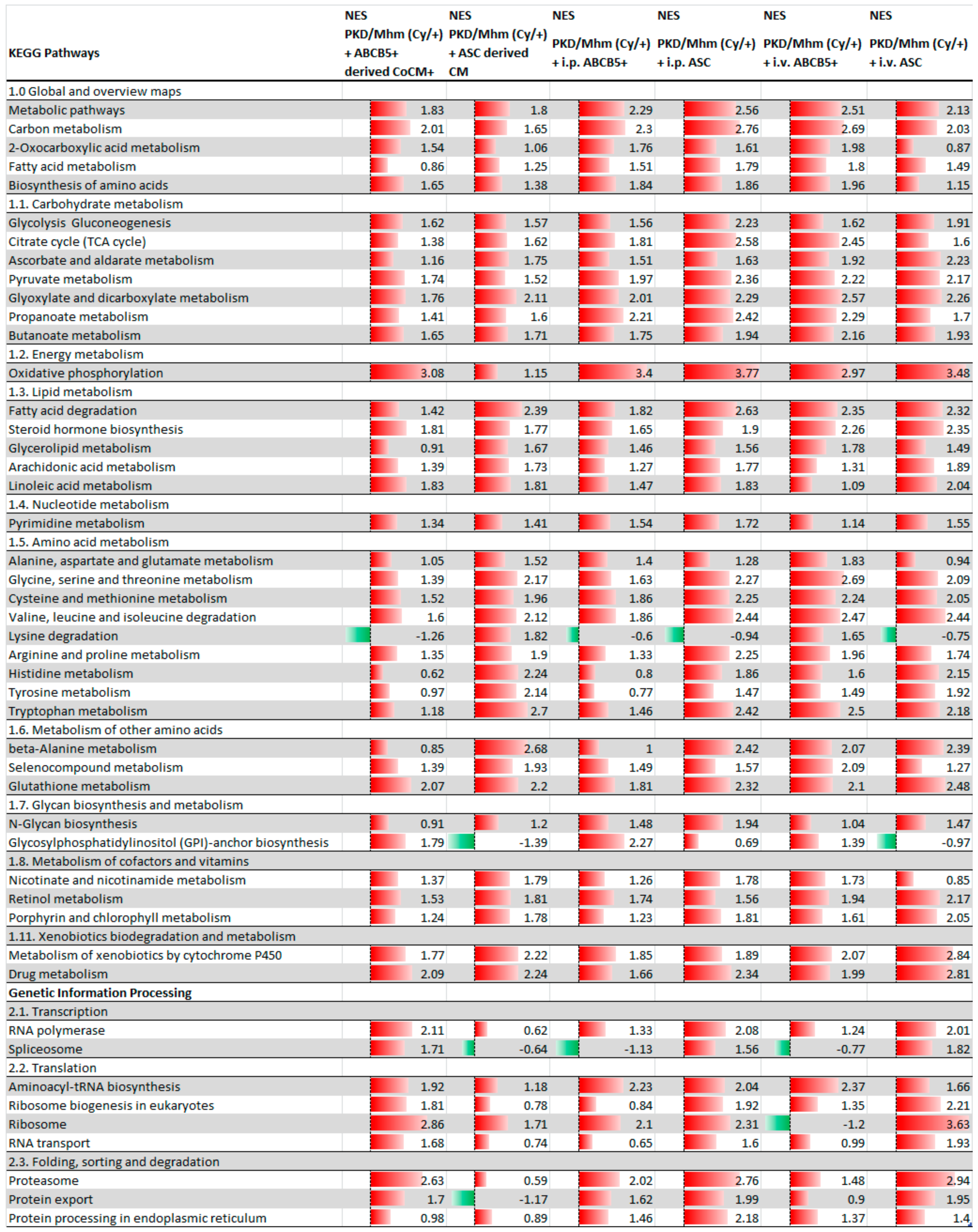
| ABCB5+ Animal Group | ||
| Total analysed pathways | 304 | |
| +ABCB5+-derived CoCM+ | Significantly regulated pathways (adj. p-value < 0.05) | 185 |
| Significantly upregulated pathways (adj. p-value < 0.05) | 37 | |
| Significantly downregulated pathways (adj. p-value < 0.05) | 148 | |
| +i.p. ABCB5+ | Significantly regulated pathways (adj. p-value < 0.05) | 165 |
| Significantly upregulated pathways (adj. p-value < 0.05) | 46 | |
| Significantly downregulated pathways (adj. p-value < 0.05) | 119 | |
| +i.v. ABCB5+ | Significantly regulated pathways (adj. p-value < 0.05) | 142 |
| Significantly upregulated pathways (adj. p-value < 0.05) | 47 | |
| Significantly downregulated pathways (adj. p-value < 0.05) | 95 | |
| ASC Animal Group | ||
| Total analysed pathways | 305 | |
| +ASC-derived CM | Significantly regulated pathways (adj. p-value < 0.05) | 42 |
| Significantly upregulated pathways (adj. p-value < 0.05) | 31 | |
| Significantly downregulated pathways (adj. p-value < 0.05) | 11 | |
| +i.p. ASC | Significantly regulated pathways (adj. p-value < 0.05) | 101 |
| Significantly upregulated pathways (adj. p-value < 0.05) | 55 | |
| Significantly downregulated pathways (adj. p-value < 0.05) | 46 | |
| +i.v. ASC | Significantly regulated pathways (adj. p-value < 0.05) | 122 |
| Significantly upregulated pathways (adj. p-value < 0.05) | 48 | |
| Significantly downregulated pathways (adj. p-value < 0.05) | 74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardozi, D.; Palumbo, S.; Khan, A.u.M.; Sticht, C.; Bieback, K.; Sadeghi, S.; Kluth, M.A.; Keese, M.; Gretz, N. Potential Therapeutic Effects of Long-Term Stem Cell Administration: Impact on the Gene Profile and Kidney Function of PKD/Mhm (Cy/+) Rats. J. Clin. Med. 2022, 11, 2601. https://doi.org/10.3390/jcm11092601
Nardozi D, Palumbo S, Khan AuM, Sticht C, Bieback K, Sadeghi S, Kluth MA, Keese M, Gretz N. Potential Therapeutic Effects of Long-Term Stem Cell Administration: Impact on the Gene Profile and Kidney Function of PKD/Mhm (Cy/+) Rats. Journal of Clinical Medicine. 2022; 11(9):2601. https://doi.org/10.3390/jcm11092601
Chicago/Turabian StyleNardozi, Daniela, Stefania Palumbo, Arif ul Maula Khan, Carsten Sticht, Karen Bieback, Samar Sadeghi, Mark Andreas Kluth, Michael Keese, and Norbert Gretz. 2022. "Potential Therapeutic Effects of Long-Term Stem Cell Administration: Impact on the Gene Profile and Kidney Function of PKD/Mhm (Cy/+) Rats" Journal of Clinical Medicine 11, no. 9: 2601. https://doi.org/10.3390/jcm11092601
APA StyleNardozi, D., Palumbo, S., Khan, A. u. M., Sticht, C., Bieback, K., Sadeghi, S., Kluth, M. A., Keese, M., & Gretz, N. (2022). Potential Therapeutic Effects of Long-Term Stem Cell Administration: Impact on the Gene Profile and Kidney Function of PKD/Mhm (Cy/+) Rats. Journal of Clinical Medicine, 11(9), 2601. https://doi.org/10.3390/jcm11092601






