Relationships between Plasma Pyrophosphate, Vascular Calcification and Clinical Severity in Patients Affected by Pseudoxanthoma Elasticum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Committee Approval
2.2. Vascular Calcification Scoring
2.3. Plasma Pyrophosphate
2.4. Plasma Alkaline Phosphatase (ALP) Activity
2.5. Statistical Analyses
3. Results
3.1. PXE Patients Population
3.2. Assessment of PPi in Men and Women in PXE Patients
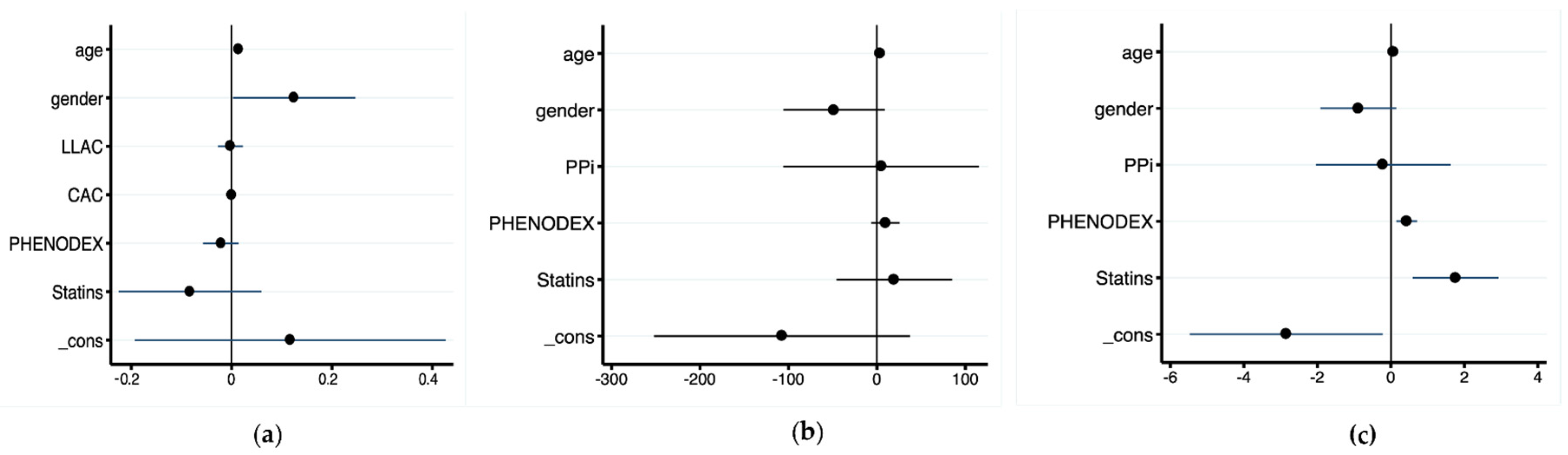
3.3. Relationships between PPi Level, Calcification Scores, and Disease Severity
3.4. Statin-Treated PXE Patients
4. Discussion
4.1. Plama PPi Level in PXE
4.2. PPi and Arterial Calcification
4.3. Duration of Exposure to A Low Plasma Level of PPi Is A Major Determinant of Arterial Calcification and Clinical Severity in PXE
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shimada, B.K.; Pomozi, V.; Zoll, J.; Kuo, S.; Martin, L.; Le Saux, O. ABCC6, Pyrophosphate and Ectopic Calcification: Therapeutic Solutions. Int. J. Mol. Sci. 2021, 22, 4555. [Google Scholar] [CrossRef] [PubMed]
- Plomp, A.S.; Toonstra, J.; Bergen, A.A.B.; van Dijk, M.R.; de Jong, P.T.V.M. Proposal for Updating the Pseudoxanthoma Elasticum Classification System and a Review of the Clinical Findings. Am. J. Med. Genet. A 2010, 152A, 1049–1058. [Google Scholar] [CrossRef]
- Pfendner, E.G.; Vanakker, O.M.; Terry, S.F.; Vourthis, S.; McAndrew, P.E.; McClain, M.R.; Fratta, S.; Marais, A.-S.; Hariri, S.; Coucke, P.J.; et al. Mutation Detection in the ABCC6 Gene and Genotype Phenotype Analysis in a Large International Case Series Affected by Pseudoxanthoma Elasticum. J. Med. Genet. 2007, 44, 621–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of Coronary Artery Calcium Using Ultrafast Computed Tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Leftheriotis, G.; Kauffenstein, G.; Hamel, J.F.; Abraham, P.; Le Saux, O.; Willoteaux, S.; Henrion, D.; Martin, L. The Contribution of Arterial Calcification to Peripheral Arterial Disease in Pseudoxanthoma Elasticum. PLoS ONE 2014, 9, e96003. [Google Scholar] [CrossRef] [Green Version]
- Campens, L.; Vanakker, O.M.; Trachet, B.; Segers, P.; Leroy, B.P.; De Zaeytijd, J.; Voet, D.; De Paepe, A.; De Backer, T.; De Backer, J. Characterization of Cardiovascular Involvement in Pseudoxanthoma Elasticum Families. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2646–2652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trip, M.D.; Smulders, Y.M.; Wegman, J.J.; Hu, X.; Boer, J.M.A.; ten Brink, J.B.; Zwinderman, A.H.; Kastelein, J.J.P.; Feskens, E.J.M.; Bergen, A.A.B. Frequent Mutation in the ABCC6 Gene (R1141X) Is Associated With a Strong Increase in the Prevalence of Coronary Artery Disease. Circulation 2002, 106, 773–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, R.S.; Kucukosmanoglu, A.; de Haas, M.; Sapthu, S.; Otero, J.A.; Hegman, I.E.; Bergen, A.A.; Gorgels, T.G.; Borst, P.; van de Wetering, K. ABCC6 Prevents Ectopic Mineralization Seen in Pseudoxanthoma Elasticum by Inducing Cellular Nucleotide Release. Proc. Natl. Acad. Sci. USA 2013, 110, 20206–20211. [Google Scholar] [CrossRef] [Green Version]
- Jansen, R.S.; Duijst, S.; Mahakena, S.; Sommer, D.; Szeri, F.; Váradi, A.; Plomp, A.; Bergen, A.A.; Oude Elferink, R.P.J.; Borst, P.; et al. ABCC6-Mediated ATP Secretion by the Liver Is the Main Source of the Mineralization Inhibitor Inorganic Pyrophosphate in the Systemic Circulation-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1985–1989. [Google Scholar] [CrossRef] [Green Version]
- Pomozi, V.; Brampton, C.; Szeri, F.; Dedinszki, D.; Kozák, E.; van de Wetering, K.; Hopkins, H.; Martin, L.; Váradi, A.; Le Saux, O. Functional Rescue of ABCC6 Deficiency by 4-Phenylbutyrate Therapy Reduces Dystrophic Calcification in Abcc6−/− Mice. J. Investig. Dermatol. 2017, 137, 595–602. [Google Scholar] [CrossRef] [Green Version]
- Letavernier, E.; Kauffenstein, G.; Huguet, L.; Navasiolava, N.; Bouderlique, E.; Tang, E.; Delaitre, L.; Bazin, D.; de Frutos, M.; Gay, C.; et al. ABCC6 Deficiency Promotes Development of Randall Plaque. J. Am. Soc. Nephrol. JASN 2018, 29, 2337–2347. [Google Scholar] [CrossRef] [Green Version]
- Pomozi, V.; Brampton, C.; van de Wetering, K.; Zoll, J.; Calio, B.; Pham, K.; Owens, J.B.; Marh, J.; Moisyadi, S.; Váradi, A.; et al. Pyrophosphate Supplementation Prevents Chronic and Acute Calcification in ABCC6-Deficient Mice. Am. J. Pathol. 2017, 187, 1258–1272. [Google Scholar] [CrossRef] [Green Version]
- Dedinszki, D.; Szeri, F.; Kozák, E.; Pomozi, V.; Tőkési, N.; Mezei, T.R.; Merczel, K.; Letavernier, E.; Tang, E.; Le Saux, O.; et al. Oral Administration of Pyrophosphate Inhibits Connective Tissue Calcification. EMBO Mol. Med. 2017, 9, 1463–1470. [Google Scholar] [CrossRef]
- Pomozi, V.; Julian, C.B.; Zoll, J.; Pham, K.; Kuo, S.; Tőkési, N.; Martin, L.; Váradi, A.; Le Saux, O. Dietary Pyrophosphate Modulates Calcification in a Mouse Model of Pseudoxanthoma Elasticum: Implication for Treatment of Patients. J. Investig. Dermatol. 2019, 139, 1082–1088. [Google Scholar] [CrossRef]
- Le Corre, Y.; Le Saux, O.; Froeliger, F.; Libouban, H.; Kauffenstein, G.; Willoteaux, S.; Leftheriotis, G.; Martin, L. Quantification of the Calcification Phenotype of Abcc6-Deficient Mice with Microcomputed Tomography. Am. J. Pathol. 2012, 180, 2208–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurain, A.; Rubera, I.; Duranton, C.; Rutsch, F.; Nitschke, Y.; Ray, E.; Vido, S.; Sicard, A.; Lefthériotis, G.; Favre, G. Alkaline Phosphatases Account for Low Plasma Levels of Inorganic Pyrophosphate in Chronic Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 586831. [Google Scholar] [CrossRef] [PubMed]
- Legrand, A.; Pujol, C.; Durand, C.M.; Mesnil, A.; Rubera, I.; Duranton, C.; Zuily, S.; Sousa, A.B.; Renaud, M.; Boucher, J.L.; et al. Pseudoxanthoma Elasticum Overlaps Hereditary Spastic Paraplegia Type 56. J. Intern. Med. 2021, 289, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tévar, A.M.; García-Fernández, M.; Murcia-Casas, B.; Rioja-Villodres, J.; Carrillo, J.L.; Camacho, M.; Van Gils, M.; Sánchez-Chaparro, M.A.; Vanakker, O.; Valdivielso, P. Plasma Inorganic Pyrophosphate and Alkaline Phosphatase in Patients with Pseudoxanthoma Elasticum. Ann. Transl. Med. 2019, 7, 798. [Google Scholar] [CrossRef]
- Bernhard, E.; Nitschke, Y.; Khursigara, G.; Sabbagh, Y.; Wang, Y.; Rutsch, F. A Reference Range for Plasma Levels of Inorganic Pyrophosphate in Children Using the ATP Sulfurylase Method. J. Clin. Endocrinol. Metab. 2022, 107, 109–118. [Google Scholar] [CrossRef]
- Bouderlique, E.; Tang, E.; Perez, J.; Coudert, A.; Bazin, D.; Verpont, M.-C.; Duranton, C.; Rubera, I.; Haymann, J.-P.; Leftheriotis, G.; et al. Vitamin D and Calcium Supplementation Accelerates Randall’s Plaque Formation in a Murine Model. Am. J. Pathol. 2019, 189, 2171–2180. [Google Scholar] [CrossRef]
- Koek, W.N.H.; Campos-Obando, N.; van der Eerden, B.C.J.; de Rijke, Y.B.; Ikram, M.A.; Uitterlinden, A.G.; van Leeuwen, J.P.T.M.; Zillikens, M.C. Age-Dependent Sex Differences in Calcium and Phosphate Homeostasis. Endocr. Connect. 2021, 10, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Kranenburg, G.; de Jong, P.A.; Mali, W.P.; Attrach, M.; Visseren, F.L.J.; Spiering, W. Prevalence and Severity of Arterial Calcifications in Pseudoxanthoma Elasticum (PXE) Compared to Hospital Controls. Novel Insights into the Vascular Phenotype of PXE. Atherosclerosis 2017, 256, 7–14. [Google Scholar] [CrossRef]
- Boraldi, F.; Murro, V.; Lofaro, F.D.; Mucciolo, D.P.; Costa, S.; Pavese, L.; Quaglino, D. Phenotypic Features and Genetic Findings in a Cohort of Italian Pseudoxanthoma Elasticum Patients and Update of the Ophthalmologic Evaluation Score. J. Clin. Med. 2021, 10, 2710. [Google Scholar] [CrossRef]
- Ziegler, S.G.; Ferreira, C.R.; MacFarlane, E.G.; Riddle, R.C.; Tomlinson, R.E.; Chew, E.Y.; Martin, L.; Ma, C.-T.; Sergienko, E.; Pinkerton, A.B.; et al. Ectopic Calcification in Pseudoxanthoma Elasticum Responds to Inhibition of Tissue-Nonspecific Alkaline Phosphatase. Sci. Transl. Med. 2017, 9, eaal1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veiga-Lopez, A.; Sethuraman, V.; Navasiolava, N.; Makela, B.; Olomu, I.; Long, R.; van de Wetering, K.; Martin, L.; Aranyi, T.; Szeri, F. Plasma Inorganic Pyrophosphate Deficiency Links Multiparity to Cardiovascular Disease Risk. Front. Cell Dev. Biol. 2020, 8, 573727. [Google Scholar] [CrossRef]
- Bartstra, J.W.; de Jong, P.A.; Spiering, W. Accelerated Peripheral Vascular Aging in Pseudoxanthoma Elasticum—Proof of Concept for Arterial Calcification-Induced Cardiovascular Disease. Aging 2019, 11, 1062–1064. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, J.; Pinkerton, A.B.; Millan, J.L.; van Zelst, B.D.; Levine, M.A.; Sundberg, J.P.; Uitto, J. Inhibition of Tissue-Nonspecific Alkaline Phosphatase Attenuates Ectopic Mineralization in the Abcc6−/− Mouse Model of PXE but Not in the Enpp1 Mutant Mouse Models of GACI. J. Investig. Dermatol. 2019, 139, 360–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Kingman, J.; Sundberg, J.P.; Uitto, J.; Li, Q. Plasma PPi Deficiency Is the Major, but Not the Exclusive, Cause of Ectopic Mineralization in an Abcc6−/− Mouse Model of PXE. J. Investig. Dermatol. 2017, 137, 2336–2343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saremi, A.; Bahn, G.; Reaven, P.D. VADT Investigators Progression of Vascular Calcification Is Increased with Statin Use in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 2012, 35, 2390–2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puri, R.; Nicholls, S.J.; Shao, M.; Kataoka, Y.; Uno, K.; Kapadia, S.R.; Tuzcu, E.M.; Nissen, S.E. Impact of Statins on Serial Coronary Calcification during Atheroma Progression and Regression. J. Am. Coll. Cardiol. 2015, 65, 1273–1282. [Google Scholar] [CrossRef]
- Guo, H.; Li, Q.; Chou, D.W.; Uitto, J. Atorvastatin Counteracts Aberrant Soft Tissue Mineralization in a Mouse Model of Pseudoxanthoma Elasticum (Abcc6−/−). J. Mol. Med. Berl. Ger. 2013, 91, 1177–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brampton, C.; Pomozi, V.; Chen, L.-H.; Apana, A.; McCurdy, S.; Zoll, J.; Boisvert, W.A.; Lambert, G.; Henrion, D.; Blanchard, S.; et al. ABCC6 Deficiency Promotes Dyslipidemia and Atherosclerosis. Sci. Rep. 2021, 11, 3881. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Chinnaiyan, K.M. Annual Progression of Coronary Calcification in Trials of Preventive Therapies: A Systematic Review. Arch. Intern. Med. 2009, 169, 2064–2070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, C.R.; Hackbarth, M.E.; Ziegler, S.G.; Pan, K.S.; Roberts, M.S.; Rosing, D.R.; Whelpley, M.S.; Bryant, J.C.; Macnamara, E.F.; Wang, S.; et al. Prospective Phenotyping of Long-Term Survivors of Generalized Arterial Calcification of Infancy (GACI). Genet. Med. Off. J. Am. Coll. Med. Genet. 2021, 23, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, Y.; Yan, Y.; Buers, I.; Kintziger, K.; Askew, K.; Rutsch, F. ENPP1-Fc Prevents Neointima Formation in Generalized Arterial Calcification of Infancy through the Generation of AMP. Exp. Mol. Med. 2018, 50, 781–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Kingman, J.; van de Wetering, K.; Tannouri, S.; Sundberg, J.P.; Uitto, J. Abcc6 Knockout Rat Model Highlights the Role of Liver in PPi Homeostasis in Pseudoxanthoma Elasticum. J. Investig. Dermatol. 2017, 137, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
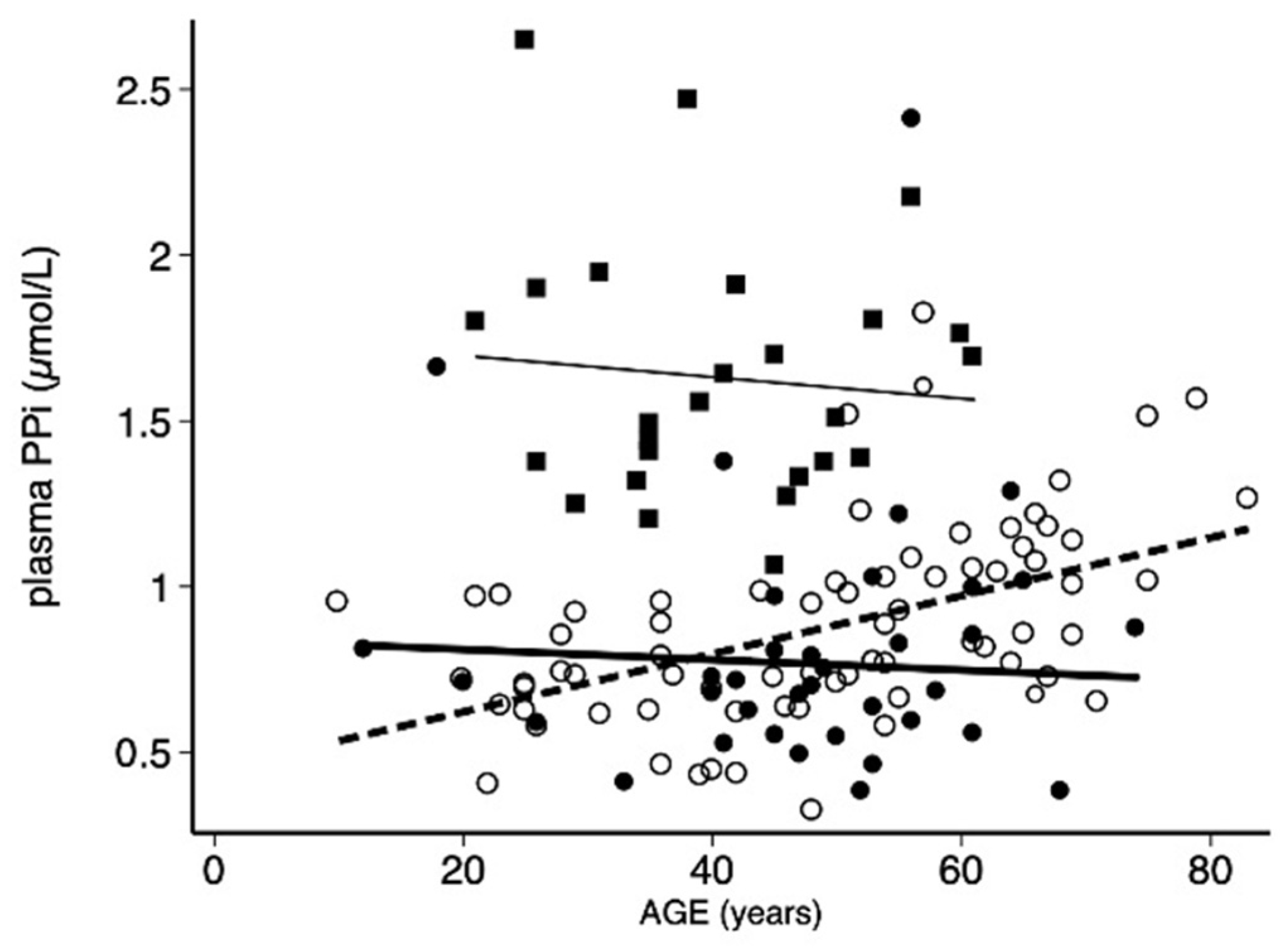
| Men, n = 35 | Women, n = 72 | All, n = 107 | p Value | ||||
|---|---|---|---|---|---|---|---|
| Age (years) | 47.7 | ±3.8 | 48.7 | ±16.7 | 48.4 | ±15.7 | 0.7542 |
| Body mass index (kg/m2) | 24.91 | (3.55) | 23.03 | (5.35) | 23.81 | (5.14) | 0.0756 |
| Total cholesterol (mmol/L) | 5.1 | (1.3) | 5.0 | (1.5) | 5.0 | (1.3) | 0.3729 |
| HDL cholesterol (mmol/L) | 1.3 | (0.6) | 1.4 | (0.5) | 1.4 | (0.4) | 0.0039 |
| GFR (mL/min/1.73 m2) | 115.5 | ±21.0 | 114.0 | 24.5 | 114.4 | 23.4 | 0.7589 |
| PPi (µmol/L) | 0.71 | (0.31) | 0.84 | (0.33) | 0.77 | (0.37) | 0.0299 |
| Pi (mmol/L) | 1.07 | ±0.21 | 1.17 | ±0.15 | 1.14 | ±0.18 | 0.0049 |
| PPi/Pi ratio | 0.66 | (0.28) | 0.73 | (0.27) | 0.7 | (0.28) | 0.3751 |
| ALP (UI/L) | 66 | (22) | 62 | (23) | 63 | (24) | 0.0649 |
| CAC (HU) | 36.3 | (129) | 2 | (90) | 7.5 | (90) | 0.0684 |
| LLAC (HU/mm) | 0.89 | (2.76) | 0.17 | (1.88) | 0.35 | (1.95) | 0.0452 |
| PHENODEX score | 5.9 | ±1.9 | 6.1 | ±1.9 | 6.0 | ±1.9 | 0.5846 |
| CVR (%) | 1 | (0) | 1 | (1) | 1 | (1) | 0.0500 |
| Normal PPi, n = 39 | Low PPi, n = 68 | All, n = 107 | p Value | ||||
|---|---|---|---|---|---|---|---|
| Age (years) | 57 | (18) | 46.5 | (18) | 50 | (22) | 0.0006 |
| Gender (W/M) | 34/9 | 43/28 | 0.0500 | ||||
| Body mass index (kg/m2) | 22.13 | (5.60) | 24.49 | (4.06) | 23.81 | (5.14) | 0.0675 |
| Total cholesterol (mmol/L) | 5.2 | (0.9) | 4.8 | (1.4) | 5.0 | (1.3) | 0.7998 |
| HDL cholesterol (mmol/L) | 1.5 | (0.8) | 1.4 | (0.5) | 1.4 | (0.4) | 0.0820 |
| PPi (µmol/L) | 1.05 | (0.25) | 0.69 | (0.19) | 0.77 | (0.37) | 9.2 × 10−18 |
| Pi (mmol/L) | 1.22 | (0.13) | 1.08 | (0.25) | 1.15 | (0.24) | 0.0009 |
| PPi/Pi ratio | 0.90 | (0.25) | 0.61 | (0.16) | 0.70 | (0.28) | 4.6 × 10−15 |
| ALP (UI/L) | 62 | (29) | 64 | (20) | 63 | (24) | 0.4754 |
| GFR (mL/min/1.73 m2) | 106.7 | ±23.6 | 118.9 | ±22.2 | 114.4 | ±23.4 | 0.0097 |
| CAC (HU) | 26.9 | (150) | 5.6 | (46.2) | 7.5 | (90) | 0.0872 |
| LLAC (HU/mm) | 0.66 | (3.16) | 0.32 | (1.84) | 0.35 | (1.95) | 0.5732 |
| PHENODEX score | 6.1 | ±1.9 | 6.0 | ±1.8 | 6.0 | ±1.9 | 0.8385 |
| CVR (%) | 1 | (1) | 1 | (0) | 1 | (1) | 0.5828 |
| No Statine, n = 86 | Statine, n = 21 | All, n = 107 | p Value | ||||
|---|---|---|---|---|---|---|---|
| Age (years) | 45.9 | ±15.8 | 58.5 | ±11.0 | 48.4 | ±15.7 | 0.0008 |
| Body mass index (kg/m2) | 23.53 | (5.06) | 24.91 | (4.03) | 23.81 | (5.14) | 0.1404 |
| Total cholesterol (mmol/L) | 5.2 | (1.4) | 4.7 | (1.0) | 5.0 | (1.3) | 0.1115 |
| HDL cholesterol (mmol/L) | 1.4 | (0.4) | 1.4 | (0.5) | 1.4 | (0.4) | 0.4138 |
| PPi (µmol/L) | 0.77 | (0.36) | 0.80 | (0.33) | 0.77 | (0.37) | 0.4102 |
| Pi (mmol/L) | 1.14 | ±0.18 | 1.12 | ±0.18 | 1.14 | ±0.18 | 0.5921 |
| PPi/Pi ratio | 0.69 | (0.29) | 0.71 | (0.25) | 0.70 | (0.28) | 0.3252 |
| ALP (UI/L) | 62 | (25) | 64 | (22) | 63 | (24) | 0.9999 |
| CAC (HU) | 1 | (49) | 56.9 | (112) | 7.5 | (90) | 0.0037 |
| LLAC (HU/mm) | 0.19 | (1.43) | 1.90 | (7.47) | 0.35 | (1.95) | 0.0004 |
| PHENODEX score | 6 | (3) | 7 | (3) | 6 | (3) | 0.0062 |
| CVR (%) | 1 | (1) | 1 | (0) | 1 | (1) | 0.4050 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leftheriotis, G.; Navasiolava, N.; Clotaire, L.; Duranton, C.; Le Saux, O.; Bendahhou, S.; Laurain, A.; Rubera, I.; Martin, L. Relationships between Plasma Pyrophosphate, Vascular Calcification and Clinical Severity in Patients Affected by Pseudoxanthoma Elasticum. J. Clin. Med. 2022, 11, 2588. https://doi.org/10.3390/jcm11092588
Leftheriotis G, Navasiolava N, Clotaire L, Duranton C, Le Saux O, Bendahhou S, Laurain A, Rubera I, Martin L. Relationships between Plasma Pyrophosphate, Vascular Calcification and Clinical Severity in Patients Affected by Pseudoxanthoma Elasticum. Journal of Clinical Medicine. 2022; 11(9):2588. https://doi.org/10.3390/jcm11092588
Chicago/Turabian StyleLeftheriotis, Georges, Nastassia Navasiolava, Laetitia Clotaire, Christophe Duranton, Olivier Le Saux, Saïd Bendahhou, Audrey Laurain, Isabelle Rubera, and Ludovic Martin. 2022. "Relationships between Plasma Pyrophosphate, Vascular Calcification and Clinical Severity in Patients Affected by Pseudoxanthoma Elasticum" Journal of Clinical Medicine 11, no. 9: 2588. https://doi.org/10.3390/jcm11092588
APA StyleLeftheriotis, G., Navasiolava, N., Clotaire, L., Duranton, C., Le Saux, O., Bendahhou, S., Laurain, A., Rubera, I., & Martin, L. (2022). Relationships between Plasma Pyrophosphate, Vascular Calcification and Clinical Severity in Patients Affected by Pseudoxanthoma Elasticum. Journal of Clinical Medicine, 11(9), 2588. https://doi.org/10.3390/jcm11092588






