Outcomes of a Standardized, High-Caloric, Inpatient Re-Alimentation Treatment Protocol in 120 Severely Malnourished Adolescents with Anorexia Nervosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
- Years of age, 12–20;
- Primary diagnosis of AN (any type);
- BMI < 15 kg/m2;
- Minimum inpatient treatment duration of 4 weeks (28 days);
- Availability of laboratory results at baseline and 28 days, plus at least two complete blood work results from the three weekly intervals between these dates.
2.2. Setting, Re-Alimentation Protocol and Clinical Routine
2.3. Evaluation of Clinical Status during Intital Re-Alimentation
- Critically low serum electrolyte concentrations:
- ○
- Potassium < 2.5 mmol/L;
- ○
- Phosphate < 0.32 mmol/L;
- ○
- Magnesium < 0.50 mmol/L.
- Peripheral edema or acute circulatory fluid overload.
- Disturbance of organ function, including respiratory failure, cardiac failure and pulmonary edema.
2.4. Statistical Analysis
3. Results
3.1. Characterization of the Study Population Including Medical Details, Comorbidities and Medications
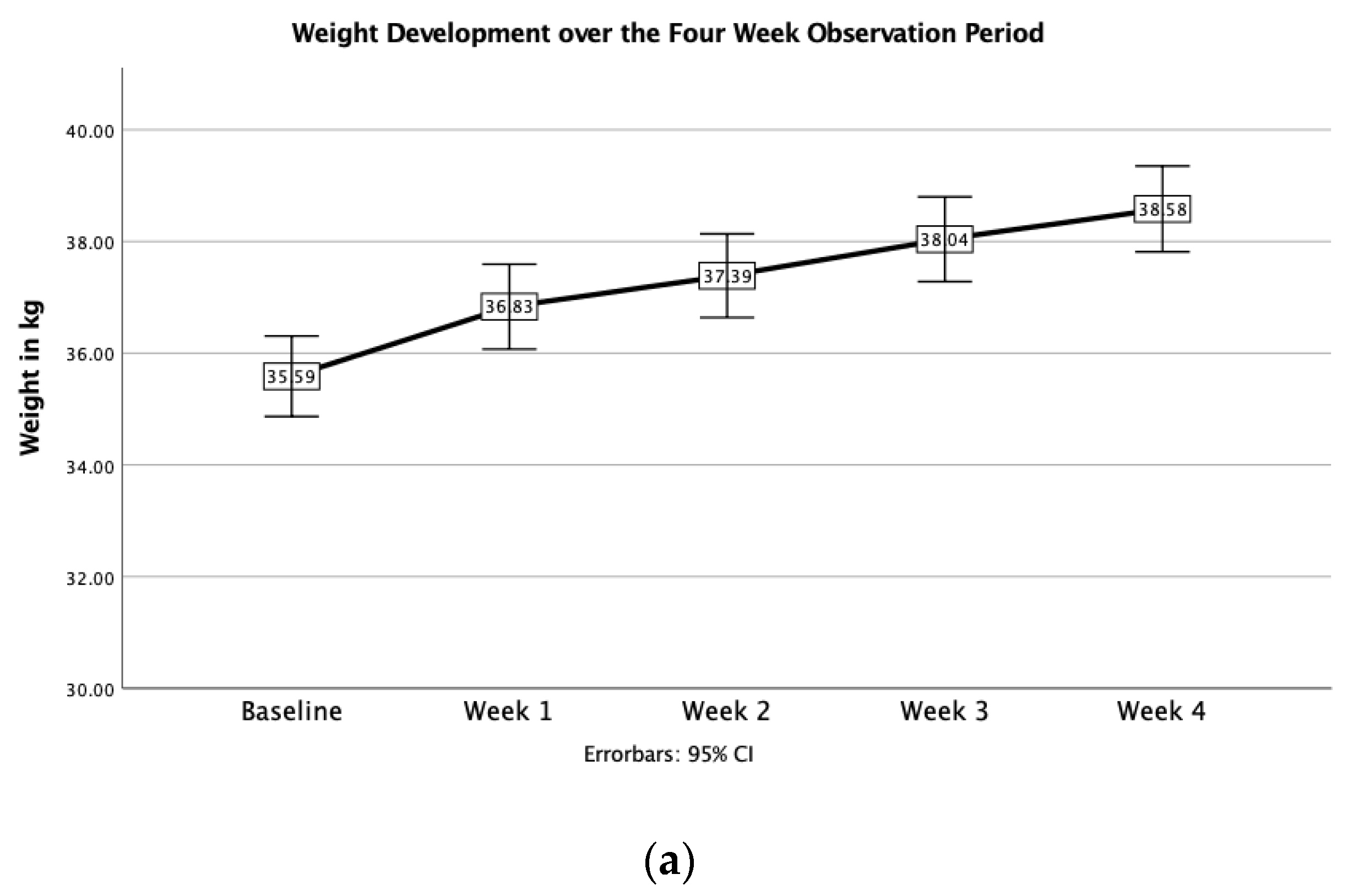
3.2. Changes in Body Weight
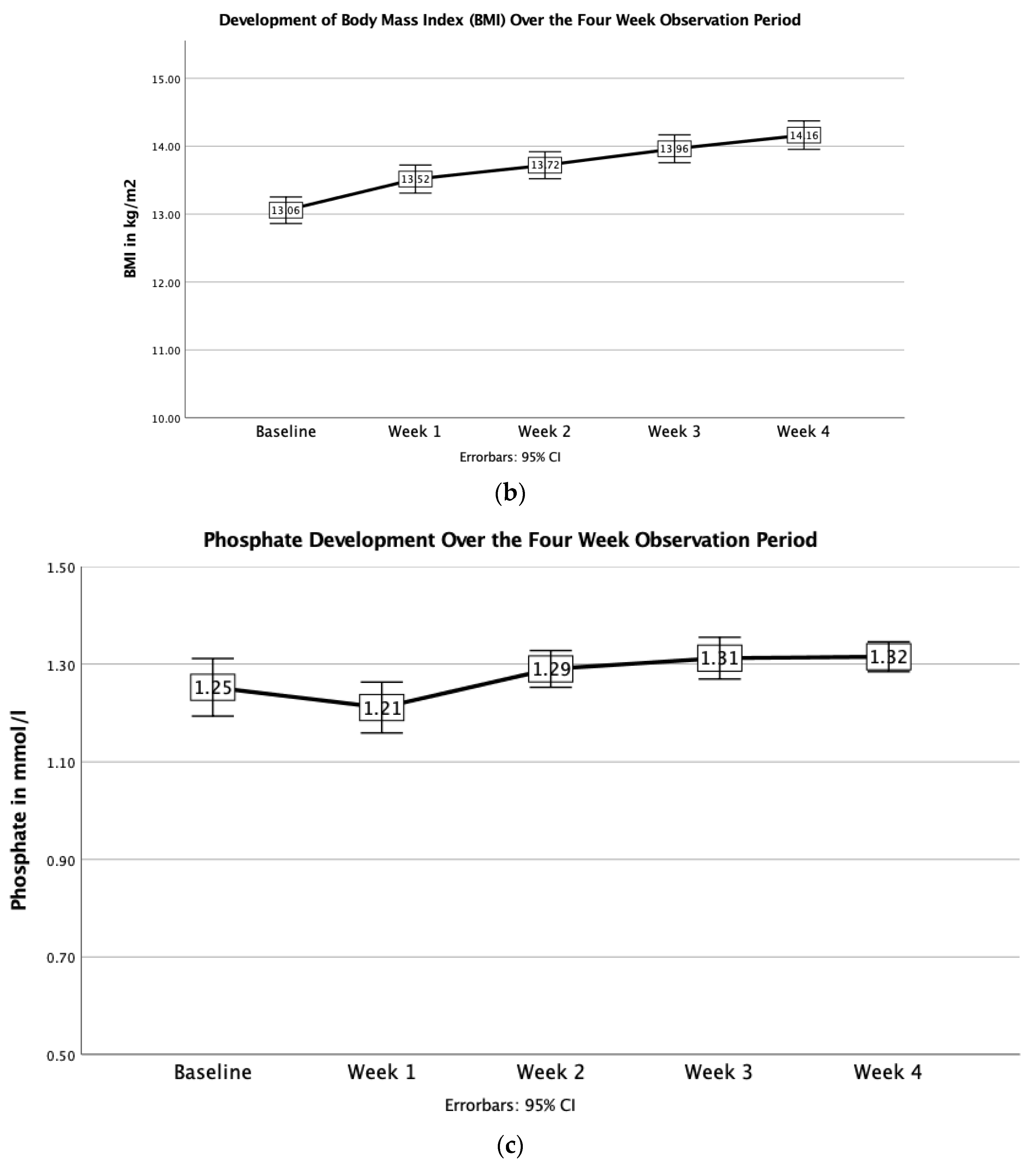
3.3. Changes in Blood Parameters
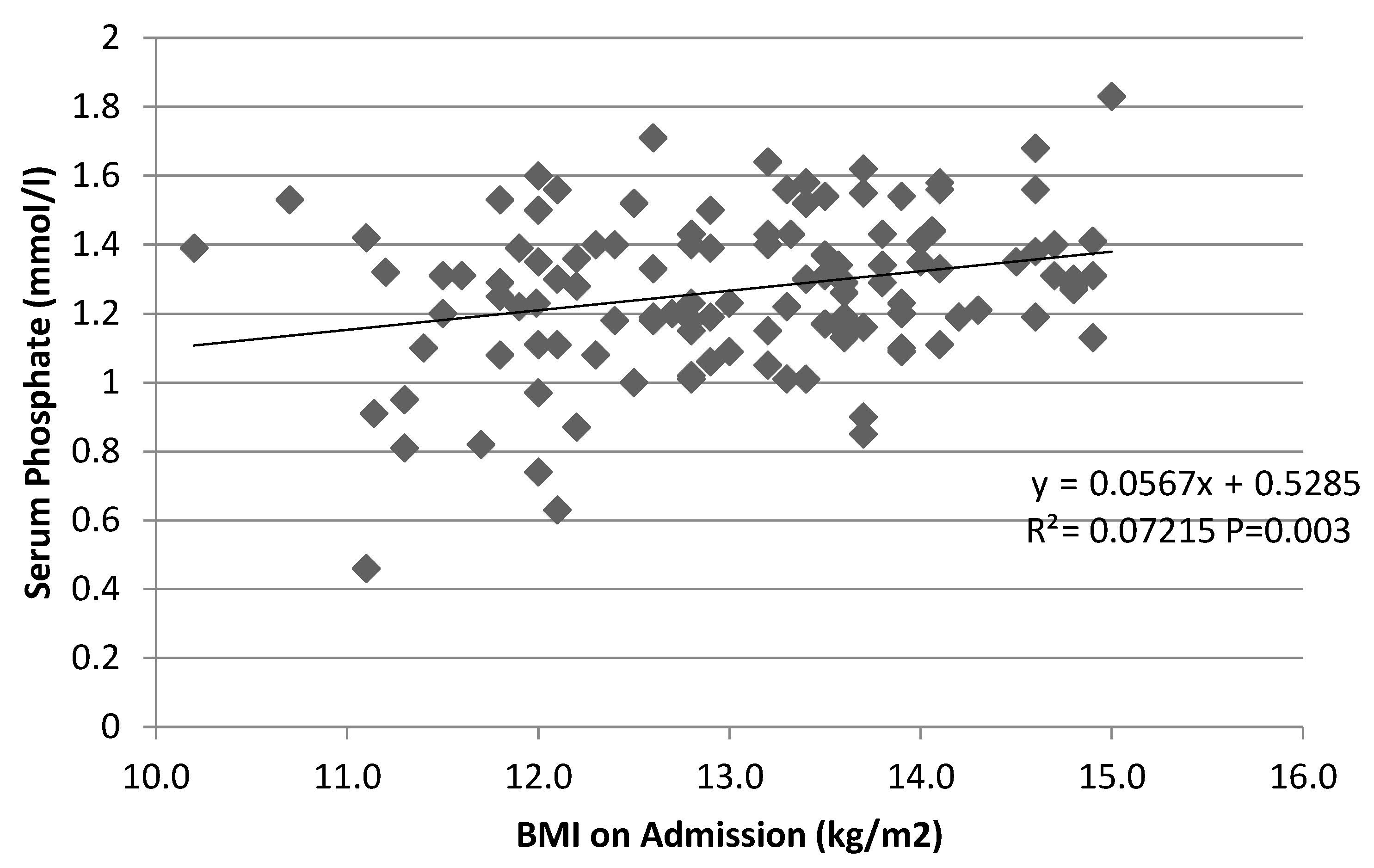
3.4. Potential Correlates of Phosphate Levels
4. Discussion
4.1. Energy Intake and Weight Gain
4.2. High-Caloric Re-Alimentation and Occurrence of Hypophosphatemia or Refeeding Syndrome
4.3. High Variability in Supplementation Practices
4.4. Correlation between Hypophosphatemia and Degree of Malnutrition
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisher, M.; Simpser, E.; Schneider, M. Hypophosphatemia secondary to oral refeeding in anorexia nervosa. Int. J. Eat. Disord. 2000, 28, 181–187. [Google Scholar] [CrossRef]
- Katzman, D.K.; Garber, A.K.; Kohn, M.; Golden, N.H. Refeeding hypophosphatemia in hospitalized adolescents with anorexia nervosa: A position statement of the Society for Adolescent Health and Medicine. J. Adolesc. Health 2014, 55, 455–457. [Google Scholar]
- Kells, M.; Gregas, M.; Wolfe, B.E.; Garber, A.K.; Kelly-Weeder, S. Factors associated with refeeding hypophosphatemia in adolescents and young adults hospitalized with anorexia nervosa. Nutr. Clin. Pract. 2021, 37, 470–478. [Google Scholar] [CrossRef] [PubMed]
- De Vries, M.C.; Koekkoek, W.A.C.; Opdam, M.H.; van Blokland, D.; van Zanten, A.R. Nutritional assessment of critically ill patients: Validation of the modified NUTRIC score. Eur. J. Clin. Nutr. 2018, 72, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Gourley, D.R. The role of adenosine triphosphate in the transport of phosphate in the human erythrocyte. Arch. Biochem. Biophys. 1952, 40, 1–12. [Google Scholar] [CrossRef]
- Mehanna, H.M.; Moledina, J.; Travis, J. Refeeding syndrome: What it is, and how to prevent and treat it. BMJ 2008, 336, 1495–1498. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, G.; Nicholls, D. Refeeding hypophosphatemia in adolescents with anorexia nervosa: A systematic review. Nutr. Clin. Pract. 2013, 28, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Bell, C.; Bulik, C.; Clayton, P.; Crow, S.; Davis, D.M.; DeMaso, D.R.; Dogin, J.; Fairburn, C.G.; Fink, A.H.; Fisher, M.; et al. Practice guideline for the treatment of patients with eating disorders (revision). Am. J. Psychiatr. 2000, 157, 1–39. [Google Scholar]
- American Dietetic Association. Position of the American Dietetic Association: Nutrition intervention in the treatment of anorexia nervosa, bulimia nervosa, and other eating disorders. J. Am. Diet. Assoc. 2006, 106, 2073–2082. [Google Scholar] [CrossRef]
- Garber, A.K.; Michihata, N.; Hetnal, K.; Shafer, M.A.; Moscicki, A.B. A prospective examination of weight gain in hospitalized adolescents with anorexia nervosa on a recommended refeeding protocol. J. Adolesc. Health 2012, 50, 24–29. [Google Scholar] [CrossRef] [Green Version]
- NICE. Eating Disorders: Core Interventions in the Treatment and Management of Anorexia Nervosa, Bulimia Nervosa, and Related Eating Disorders; National Collaborating Centre for Mental Health: London, UK, 2004. [Google Scholar]
- Garber, A.K.; Mauldin, K.; Michihata, N.; Buckelew, S.M.; Shafer, M.A.; Moscicki, A.B. Higher calorie diets increase rate of weight gain and shorten hospital stay in hospitalized adolescents with anorexia nervosa. J. Adolesc. Health 2013, 53, 579–584. [Google Scholar] [CrossRef] [Green Version]
- Golden, N.H.; Keane-Miller, C.; Sainani, K.L.; Kapphahn, C.J. Higher caloric intake in hospitalized adolescents with anorexia nervosa is associated with reduced length of stay and no increased rate of refeeding syndrome. J. Adolesc. Health 2013, 53, 573–578. [Google Scholar] [CrossRef]
- Pettersson, C.; Tubic, B.; Svedlund, A.; Magnusson, P.; Ellegård, L.; Swolin-Eide, D.; Forslund, H.B. Description of an intensive nutrition therapy in hospitalized adolescents with anorexia nervosa. Eat. Behav. 2016, 21, 172–178. [Google Scholar] [CrossRef]
- Koerner, T.; Haas, V.; Heese, J.; Karacic, M.; Ngo, E.; Correll, C.U.; Voderholzer, U.; Cuntz, U. Outcomes of an Accelerated Inpatient Refeeding Protocol in 103 Extremely Underweight Adults with Anorexia Nervosa at a Specialized Clinic in Prien, Germany. J. Clin. Med. 2020, 9, 1535. [Google Scholar] [CrossRef]
- Garber, A.K.; Cheng, J.; Accurso, E.C.; Adams, S.H.; Buckelew, S.M.; Kapphahn, C.J.; Kreiter, A.; Le Grange, D.; Machen, V.I.; Moscicki, A.B.; et al. Short-term Outcomes of the Study of Refeeding to Optimize Inpatient Gains for Patients With Anorexia Nervosa: A Multicenter Randomized Clinical Trial. JAMA Pediatr. 2020, 175, 19–27. [Google Scholar] [CrossRef]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M.; Group, A.M.W.; Force, A.M.T. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: Characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J. Acad. Nutr. Diet. 2012, 36, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Parker, E.K.; Faruquie, S.S.; Anderson, G.; Gomes, L.; Kennedy, A.; Wearne, C.M.; Kohn, M.R.; Clarke, S.D. Higher Caloric Refeeding Is Safe in Hospitalised Adolescent Patients with Restrictive Eating Disorders. J. Nutr. Metab. 2016, 2016, 5168978. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.; Lesser, J.; Brandenburg, B.; Lesser, A.; Cici, J.; Juenneman, R.; Beadle, A.; Eckhardt, S.; Lantz, E.; Lock, J.; et al. Outcomes of an inpatient refeeding protocol in youth with Anorexia Nervosa and atypical Anorexia Nervosa at Children’s Hospitals and Clinics of Minnesota. J. Eat. Disord. 2016, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Kromeyer-Hausschild, K. Perzentile für den Body-mass-Index für das Kindes- und Jugend-alter unter Heranziehung ver-schiedener deutscher Stichproben. Mon. Kinderheilkd. 2001, 149, 807–818. [Google Scholar] [CrossRef] [Green Version]
- Marzola, E.; Nasser, J.A.; Hashim, S.A.; Shih, P.A.B.; Kaye, W.H. Nutritional rehabilitation in anorexia nervosa: Review of the literature and implications for treatment. BMC Psychiatry 2013, 13, 290. [Google Scholar] [CrossRef] [Green Version]
- Meule, A.; Schrambke, D.; Furst Loredo, A.; Schlegl, S.; Naab, S.; Voderholzer, U. Inpatient treatment of anorexia nervosa in adolescents: A 1-year follow-up study. Eur. Eat. Disord. Rev. 2021, 29, 165–177. [Google Scholar] [CrossRef]
- Rio, A.; Whelan, K.; Goff, L.; Reidlinger, D.P.; Smeeton, N. Occurrence of refeeding syndrome in adults started on artificial nutrition support: Prospective cohort study. BMJ Open 2013, 3, e002173. [Google Scholar] [CrossRef] [Green Version]
- Kohn, M.R.; Madden, S.; Clarke, S.D. Refeeding in anorexia nervosa: Increased safety and efficiency through understanding the pathophysiology of protein calorie malnutrition. Curr. Opin. Pediatr. 2011, 23, 390–394. [Google Scholar] [CrossRef]
- Madden, S.; Miskovic-Wheatley, J.; Clarke, S.; Touyz, S.; Hay, P.; Kohn, M.R. Outcomes of a rapid refeeding protocol in Adolescent Anorexia Nervosa. J. Eat. Disord. 2015, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Redgrave, G.W.; Coughlin, J.W.; Schreyer, C.C.; Martin, L.M.; Leonpacher, A.K.; Seide, M.; Verdi, A.M.; Pletch, A.; Guarda, A.S. Refeeding and weight restoration outcomes in anorexia nervosa: Challenging current guidelines. Int. J. Eat. Disord. 2015, 48, 866–873. [Google Scholar] [CrossRef]
- Leitner, M.; Burstein, B.; Agostino, H. Prophylactic Phosphate Supplementation for the Inpatient Treatment of Restrictive Eating Disorders. J. Adolesc. Health 2016, 58, 616–620. [Google Scholar] [CrossRef] [Green Version]
- Whitelaw, M.; Gilbertson, H.; Lam, P.Y.; Sawyer, S.M. Does aggressive refeeding in hospitalized adolescents with anorexia nervosa result in increased hypophosphatemia? J. Adolesc. Health 2010, 46, 577–582. [Google Scholar] [CrossRef]
- Proulx-Cabana, S.; Metras, M.E.; Taddeo, D.; Jamoulle, O.; Frappier, J.Y.; Stheneur, C. To Improve the Initial Inpatient Management of Adolescents Admitted with Severe Anorexia Nervosa: A Narrative Review and a Convenient Protocol. Nutrients 2022, 14, 229. [Google Scholar] [CrossRef]
- Murdeshwar, A.; Heaton, P.A.; Vaina, C.L.; Paul, S.P. How Useful are Re-feeding Bloods for Paediatric Inpatients with Anorexia Nervosa? Mymensingh Med. J. 2022, 31, 258–262. [Google Scholar]
- Ornstein, R.M.; Golden, N.H.; Jacobson, M.S.; Shenker, I.R. Hypophosphatemia during nutritional rehabilitation in anorexia nervosa: Implications for refeeding and monitoring. J. Adolesc. Health 2003, 32, 83–88. [Google Scholar] [CrossRef]
| Study | Study Design | Follow-Up Duration (Weeks) | Patient Characteristics | Re-Alimentation Protocol | Weight Gain Outcome | Additional Outcomes |
|---|---|---|---|---|---|---|
| Garber et al., 2013 [12] | Quasi-experimental study design comparing lower- and higher-caloric re-alimentation protocols | 14.9 (±0.9) days | 56 adolescents with Anorexia Nervosa (AN) (age = 16.2 ± 0.3 years; baseline percent median body mass index (%mBMI) = 79.2% ± 1.5%) | Groups were split at a median of 1200 kcal refeeding initiation (1764 ± 60 kcal/d vs. 1093 ± 28 kcal/d) | (0.46 ± 0.04 vs. 0.26 ± 0.03% of median BMI (%mBMI)/day, p < 0.001). | No Refeeding Syndrome (RS), increased tendency to receive phosphate supplementation (12 vs. 8 participants, p = 0.0273) |
| Golden et al., 2013 [13] | Retrospective chart review comparing lower- vs. higher-calorie re-alimentation protocols | Youth between the ages of 10 and 21 (mean = 16.1 ± 2.3 years; female = 88%; %mBMI = 78.5 ± 8.3) | Lower-calorie group (n = 88) began at 1163 ± 107 kcal/d (range = 720–1320 kcal/d), Higher caloric realimentation (HCR) group started at 1557 ± 265 kcal/d (range = 1400–2800 kcal/d) | Reduced inpatient treatment duration (13.0 ± 7.3 days vs. 16.6 ± 9.0 days; p < 0.0001), no differences in the incidence of hypophosphatemia (38.90% vs. 25.8%, p = 0.49) | ||
| Pettersson et al., 2016 [14] | Observational study | 12 weeks | Youth and young adults aged 16–24 with (AN) (mean age = 19.9 ± 2.4 years; Body Mass Index (BMI) = 15.4 ± 0.94 kg/m2) | 3264 ± 196 kcal/d in week one, decreasing gradually during treatment to 2622 ± 331 kcal/d | 9.8 kg weight gain in 12 weeks, (0.82 kg per week) | |
| Koerner et al., 2020 [15] | Retrospective chart review | 4 weeks | Severely malnourished adults with AN (mean BMI = 11.5) | Initial energy intake of 2000 kcal/day | Mean total weight gain 4.2 kg in four weeks, (1.05 kg per week) | Normalization of laboratory parameters without any case of RS |
| Garber et al., 2020 [16] | Randomized controlled study | 12 months | 111 youth aged 12–24 years (mean = 16.4 ± 2.5 years; females = 91%) with AN and %mBMI ≥ 60% comparing higher-caloric and lower-caloric re-alimentation protocols | HCR: beginning at 2000 kcal/day and increasing by 200 kcal daily Lower-caloric re-alimentation: beginning at 1400 kcal/day and increasing by 200 kcal every other day | Significantly faster restoration of medical stability (hazard ratio = 1.67, 95% confidence interval (CI) = 1.10–2.53; p = 0 .01); shorter hospital stay: 4.0 days (95%CI = −6.1 to −1.9) for patients who received HCR; 5% developed hypophosphatemia |
| Included Patients | Excluded Patients | p | |
|---|---|---|---|
| n | 120 | 479 | |
| Age (years) | 17.3 ± 1.8 (13.4–20.8) | 16.6 ± 1.7 (12.9–20.9) | <0.001 |
| Weight (kg) | 35.6 ± 4.0 (25.2–44.0) | 37.6 ± 4.0 (27.0–51.7) | <0.001 |
| Height (cm) | 165 ± 6 (151–180) | 165 ± 7 (144–189) | 0.784 |
| Body Mass Index (BMI) (kg/m2) | 13.1 ± 1.1 (10.2–15.0) | 13.8 ± 0.9 (10.6–15.0) | <0.001 |
| BMI percentile (n = 71 included; n = 382 excluded) | 0.0 (0.0/0.0) | 0.0 (0.0/0.1) | <0.001 |
| (BMI-SDS) 1 | −5.6 ± 1.87 (−6.3/−3.9) | −4.0 ± 1.3 (−4.6/−3.2) | <0.001 |
| Duration of illness (months) | 35 ± 23 (15/48) | Not applicable (NA) | NA |
| Length of stay | 113 ± 48 (30–232) | 107 ±55 (71/139) | 0.247 |
| Diagnosis | n | Percentage |
|---|---|---|
| Anorexia Nervosa | ||
| Restrictive | 103 | 85.8 |
| Active | 16 | 13.3 |
| Atypical | 1 | 0.8 |
| Psychiatric Comorbidities | ||
| Depressive disorders | 66 | 55.0 |
| Moderate depressive episode (F32.1) | 40 | 33.3 |
| Recurrent depressive disorder, current episode severe (F33.2) | 8 | 6.7 |
| Severe depressive episode (F32.1) | 7 | 5.8 |
| Recurrent depressive disorder, current episode moderate (F33.1) | 6 | 5.0 |
| Mild depressive episode (F32.0) | 3 | 2.5 |
| Recurrent depressive disorder, currently in remission (F33.4) | 1 | 0.8 |
| Other recurrent depressive disorders (F33.8) | 1 | 0.8 |
| Obsessive compulsive disorders | 14 | 11.7 |
| Mixed obsessive compulsive disorder (F42.2) | 9 | 7.5 |
| Predominantly compulsive disorder (F42.1) | 4 | 3.3 |
| Other obsessive compulsive disorders (F42.8) | 1 | 0.8 |
| Anxiety disorders | 9 | 7.5 |
| Social anxiety disorder (F40.1) | 6 | 5.0 |
| Specific, isolated phobia (F40.2) | 3 | 2.5 |
| Other psychiatric comorbidities | 6 | 5.0 |
| Post-traumatic stress disorder (F43.1) | 4 | 3.3 |
| Mixed anxiety and depressive disorder (F41.2) | 1 | 0.8 |
| Depersonalization-derealization syndrome (F48.1) | 1 | 0.8 |
| Medications at Baseline | ||
| None | 55 | 45.8 |
| Psychotropic medications | 32 | 26.7 |
| Antidepressants | 27 | 22.5 |
| Antipsychotics | 4 | 3.3 |
| Both | 1 | 0.8 |
| Non-psychotropic medications | 33 | 27.5 |
| Baseline | Day 7 | Day 14 | Day 21 | Day 28 | |
|---|---|---|---|---|---|
| Weight (kg) | 35.6 ± 4.0 | 36.8 ± 4.2 | 37.4 ± 4.2 | 38.0 ± 4.2 | 38.6 ± 4.2 |
| (25.2–44.0) | (27.0–45.5) | (28.0–46.7) | (28.5–49.6) | (29.6–50.6) | |
| Body mass index (BMI) (kg/m2) | 13.1 ± 1.1 | 13.5 ± 1.14 | 13.7 ± 1.1 | 14.0 ± 1.1 | 14.2 ± 1.2 |
| (10.2–15.0) | (11.0–16.3) | (11.1–16.7) | (11.3–17.2) | (11.1–17.5) | |
| percentage of median body mass index (%mBMI) | 62.1 ± 6.0 | 64.1 ± 6.5 | 65.0 ± 6.4 | 66.1 ± 6.7 | 66.9 ± 6.9 |
| p | <0.001 | <0.001 | <0.001 | <0.001 |
| CK | Phosphate | Hematocrit | Sodium | GOT | GPT | Leukocytes | Hemoglobin | Thrombocytes | |
|---|---|---|---|---|---|---|---|---|---|
| Cut-Off for Abnormality | >123 U/L | <1.00 mmol/L | <36% | <135 mmol/L | >35 U/L | >35 U/L | <3.50 g/L | <12 g/L | 200 g/L |
| At admission | 91.0 | 1.26 ± 0.26 | 39.9 | 145 ± 3 | 30.4 | 29.6 | 4.39 | 13.21 ± 1.47 | 242 |
| (52.5/139.5) | (0.46–1.83) | (36.6/42.1) | (131–151) | (24.5/39.4) | (20.7/53.5) | (3.47/5.68) | (8.00–16.00) | (211/292) | |
| Week 1 | 72.0 | 1.21 ± 0.28 | 37.6 ± 4.6 | 142.66 ± 2.92 | 24.3 | 29.0 | 4.24 | 12.45 ± 1.62 | 246 |
| (510/114.00) | (0.49–1.56) | (23.3–44.8) | (135.0–150.0) | (19.9/31.1) | (20.9/44.0) | (3.54/5.37) | (7.80–14.90) | (201/288) | |
| Week 2 | 69.0 | 1.29 ± 0.16 | 37.2 ± 4.2 | 142.2 ± 4.4 | 22.6 | 26.9 | 4.21 | 12.23 ± 1.49 | 256 |
| (49.0/94.0) | (0.90–1.84) | (20.3–44.1) | (131.0–170–0) | (19.0/28.4) | (19.4/37.7) | (3.51/5.62) | (6.40–14.50) | (221/309) | |
| Week 3 | 67.0 | 1.31 ± 0.18 | 37.6 ± 3.8 | 141.9 ± 2.80 | 22.8 | 23.6 | 4.56 ± 1.56 | 12.28 1.40 | 278 |
| (47.0/93.0) | (0.68–1.91) | (24.1–44.0) | (128.0–147.0) | (18.2/27.0) | (20.5/35.9) | (2.15–8.47) | (7.30–14.70) | (231/313) | |
| Week 4 | 66.0 | 1.33 ± 0.15 | 38.7 ± 3.0 | 142 ± 2 | 23.7 | 25.6 | 4.88 | 12.70 ± 1.15 | 278 ± 66 |
| (54.0/92.0) | (1.06–1.77) | (28.5–44.1) | (135–148) | (18.9/28.2) | (19.9/35.7) | (3.79/6.03) | (8.70–15.00) | (144–546) | |
| Abnormal at admission | 42/117 (35.9%) | 12/118 (10.2%) | 26/119 (21.8%) | 6/120 (5.0%) | 43/119 (36.1%) | 52/119 (43.7%) | 31/119 (26.0%) | 24/119 (20.2%) | 21/119 (17.6%) |
| Abnormal after 4 weeks | 18/117 (15.4%) | 0/117 (0%) | 22/118 (18.6%) | 2/120 (1.7 %) | 12/119 (10.0%) | 31/119 (26.0%) | 21/118 (17.5%) | 32/118 (27.1%) | 12/118 (10.2%) |
| p-value | <0.0011 | 0.002 | 0.020 | 0.086 | <0.001 | <0.001 | 0.017 | <0.001 | <0.001 |
| Normal–Normal (n) | Normal–Low (n) | Low–Normal (n) | Low–Low (n) | |
|---|---|---|---|---|
| Baseline–Week 1 | 101 | 7 | 8 | 4 |
| Week 1–Week 2 | 109 | 0 | 9 | 2 |
| Week 2–Week 3 | 116 | 2 | 1 | 1 |
| Week 3–Week 4 | 116 | 0 | 4 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalenbrook, S.; Naab, S.; Garber, A.K.; Correll, C.U.; Voderholzer, U.; Haas, V. Outcomes of a Standardized, High-Caloric, Inpatient Re-Alimentation Treatment Protocol in 120 Severely Malnourished Adolescents with Anorexia Nervosa. J. Clin. Med. 2022, 11, 2585. https://doi.org/10.3390/jcm11092585
Dalenbrook S, Naab S, Garber AK, Correll CU, Voderholzer U, Haas V. Outcomes of a Standardized, High-Caloric, Inpatient Re-Alimentation Treatment Protocol in 120 Severely Malnourished Adolescents with Anorexia Nervosa. Journal of Clinical Medicine. 2022; 11(9):2585. https://doi.org/10.3390/jcm11092585
Chicago/Turabian StyleDalenbrook, Sophia, Silke Naab, Andrea K. Garber, Christoph U. Correll, Ulrich Voderholzer, and Verena Haas. 2022. "Outcomes of a Standardized, High-Caloric, Inpatient Re-Alimentation Treatment Protocol in 120 Severely Malnourished Adolescents with Anorexia Nervosa" Journal of Clinical Medicine 11, no. 9: 2585. https://doi.org/10.3390/jcm11092585
APA StyleDalenbrook, S., Naab, S., Garber, A. K., Correll, C. U., Voderholzer, U., & Haas, V. (2022). Outcomes of a Standardized, High-Caloric, Inpatient Re-Alimentation Treatment Protocol in 120 Severely Malnourished Adolescents with Anorexia Nervosa. Journal of Clinical Medicine, 11(9), 2585. https://doi.org/10.3390/jcm11092585






