Increased Markers of Oxidative Stress and Positive Correlation Low-Grade Inflammation with Positive Symptoms in the First Episode of Schizophrenia in Drug-Naïve Patients
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Szymona, K.; Dudzińska, E.; Karakuła-Juchnowicz, H.; Gil-Kulik, P.; Chomik, P.; Świstowska, M.; Gałaszkiewicz, J.; Kocki, J. Analysis of the expression of BAX, BCL2, BIRC6, CASP3, CASP9 apoptosis genes during the first episode of schizophrenia. Psychiatr. Pol. 2019, 53, 1293–1303, (In English, Polish). [Google Scholar] [CrossRef]
- Patel, K.R.; Cherian, J.; Gohil, K.; Atkinson, D. Schizophrenia: Overview and treatment options. Pharm. Ther. 2014, 39, 638–645. [Google Scholar]
- Mahadik, S.P.; Pillai, A.; Joshi, S.; Foster, A. Prevention of oxidative stress-mediated neuropathology and improved clinical outcome by adjunctive use of a combination of antioxidants and omega-3 fatty acids in schizophrenia. Int. Rev. Psychiatry 2006, 18, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Maas, D.; Vallès, A.; Martens, G. Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia. Transl. Psychiatry 2017, 7, e1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bošković, M.; Vovk, T.; Kores Plesničar, B.; Grabnar, I. Oxidative stress in schizophrenia. Curr. Neuropharmacol. 2011, 9, 301–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micó, J.A.; Rojas-Corrales, M.O.; Gibert-Rahola, J.; Parellada, M.; Moreno, D.; Fraguas, D.; Graell, M.; Gil, J.; Irazusta, J.; Castro-Fornieles, J.; et al. Reduced antioxidant defense in early onset first-episode psychosis: A case-control study. BMC Psychiatry 2011, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Dietrich-Muszalska, A.; Kolodziejczyk-Czepas, J.; Nowak, P. Comparative Study of the Effects of Atypical Antipsychotic Drugs on Plasma and Urine Biomarkers of Oxidative Stress in Schizophrenic Patients. Neuropsychiatr. Dis. Treat. 2021, 17, 555–565. [Google Scholar] [CrossRef]
- Caruso, G.; Grasso, M.; Fidilio, A.; Tascedda, F.; Drago, F.; Caraci, F. Antioxidant Properties of Second-Generation Antipsychotics: Focus on Microglia. Pharmaceuticals 2020, 13, 457. [Google Scholar] [CrossRef]
- Ognik, K.; Wertelecki, T. Effect of different vitamin E sources and levels on selected oxidative status indices in blood and tissues as well as on rearing performance of slaughter turkey hens. J. Appl. Poult. Res. 2012, 21, 259–271. [Google Scholar] [CrossRef]
- Buettner, G.R. Superoxide dismutase in redox biology: The roles of superoxide and hydrogen peroxide. Anticancer Agents Med. Chem. 2011, 11, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Hendouei, N.; Farnia, S.; Mohseni, F.; Salehi, A.; Bagheri, M.; Shadfar, F.; Barzegar, F.; Hoseini, S.D.; Charati, J.Y.; Shaki, F. Alterations in oxidative stress markers and its correlation with clinical findings in schizophrenic patients consuming perphenazine, clozapine and risperidone. Biomed. Pharmacother. 2018, 103, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.J.; Rogers, J.C.; Katshu, M.Z.U.H.; Liddle, P.F.; Upthegrove, R. Oxidative Stress and the Pathophysiology and Symptom Profile of Schizophrenia Spectrum Disorders. Front. Psychiatry 2021, 12, 703452. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Litago, F.; Seco, J.; Echevarría, E.; Martínez-Cengotitabengoa, M.; Gil, J.; Irazusta, J.; González-Pinto, A.M. Adaptive response in the antioxidant defence system in the course and outcome in first-episode schizophrenia patients: A 12-months follow-up study. Psychiatry Res. 2012, 200, 218–222. [Google Scholar] [CrossRef]
- Đorđević, V.V.; Lazarević, D.; Ćosić, V.; Knežević, M.; Đorđević, V.B. Age-related changes of superoxide dismutase activity in patients with schizophrenia. Vojnosanit. Pregl. 2017, 74, 31–37. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.Y.; Wang, H.; Tang, W.; Xia, Y.; Zhang, F.; Liu, J.; Fu, Y.; Hu, J.; Chen, Y.; et al. Elevated plasma superoxide dismutase in first-episode and drug naive patients with schizophrenia: Inverse association with positive symptoms. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 36, 34–38. [Google Scholar] [CrossRef]
- Reyazuddin, M.; Azmi, S.A.; Islam, N.; Rizvi, A. Oxidative stress and level of antioxidant enzymes in drug-naive schizophrenics. Indian J. Psychiatry 2014, 56, 344–349. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, L.; Fan, Y.; Lv, Q.; Wu, K.; Lang, X.; Li, Z.; Yi, Z.; Geng, D. Interaction between TNF-α and oxidative stress status in first-episode drug-naïve schizophrenia. Psychoneuroendocrinology 2020, 114, 104595. [Google Scholar] [CrossRef]
- Upthegrove, R.; Khandaker, G.M. Cytokines, Oxidative Stress and Cellular Markers of Inflammation in Schizophrenia. Curr. Top. Behav. Neurosci. 2020, 44, 49–66. [Google Scholar] [CrossRef]
- Zelzer, S.; Tatzber, F.; Herrmann, M.; Wonisch, W.; Rinnerhofer, S.; Kundi, M.; Obermayer-Pietsch, B.; Niedrist, T.; Cvirn, G.; Wultsch, G.; et al. Work Intensity, Low-Grade Inflammation, and Oxidative Status: A Comparison between Office and Slaughterhouse Workers. Oxid. Med. Cell. Longev. 2018, 2018, 2737563. [Google Scholar] [CrossRef] [Green Version]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Latheef, S.K.; Dadar, M.; Samad, H.A.; Munjal, A.; Khandia, R.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; et al. Biomarkers in Stress Related Diseases/Disorders: Diagnostic, Prognostic, and Therapeutic Values. Front. Mol. Biosci. 2019, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Bang, M. Anti-inflammatory Strategies for Schizophrenia: A Review of Evidence for Therapeutic Applications and Drug Repurposing. Clin. Psychopharmacol. Neurosci. 2020, 18, 10–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldsmith, D.R.; Rapaport, M.H. Inflammation and Negative Symptoms of Schizophrenia: Implications for Reward Processing and Motivational Deficits. Front. Psychiatry 2020, 11, 46. [Google Scholar] [CrossRef] [Green Version]
- Ermakov, E.A.; Dmitrieva, E.M.; Parshukova, D.A.; Kazantseva, D.V.; Vasilieva, A.R.; Smirnova, L.P. Oxidative Stress-Related Mechanisms in Schizophrenia Pathogenesis and New Treatment Perspectives. Oxid. Med. Cell. Longev. 2021, 2021, 8881770. [Google Scholar] [CrossRef]
- Corsi-Zuelli, F.; Loureiro, C.M.; Shuhama, R.; Fachim, H.A.; Menezes, P.R.; Louzada-Junior, P.; Mondelli, V.; Del-Ben, C.M. Cytokine profile in first-episode psychosis, unaffected siblings and community-based controls: The effects of familial liability and childhood maltreatment. Psychol. Med. 2020, 50, 1139–1147. [Google Scholar] [CrossRef]

| Variables | Group = Control | Group = SCHI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | M | Me | SD | SEM | N | M | Me | SD | SEM | |
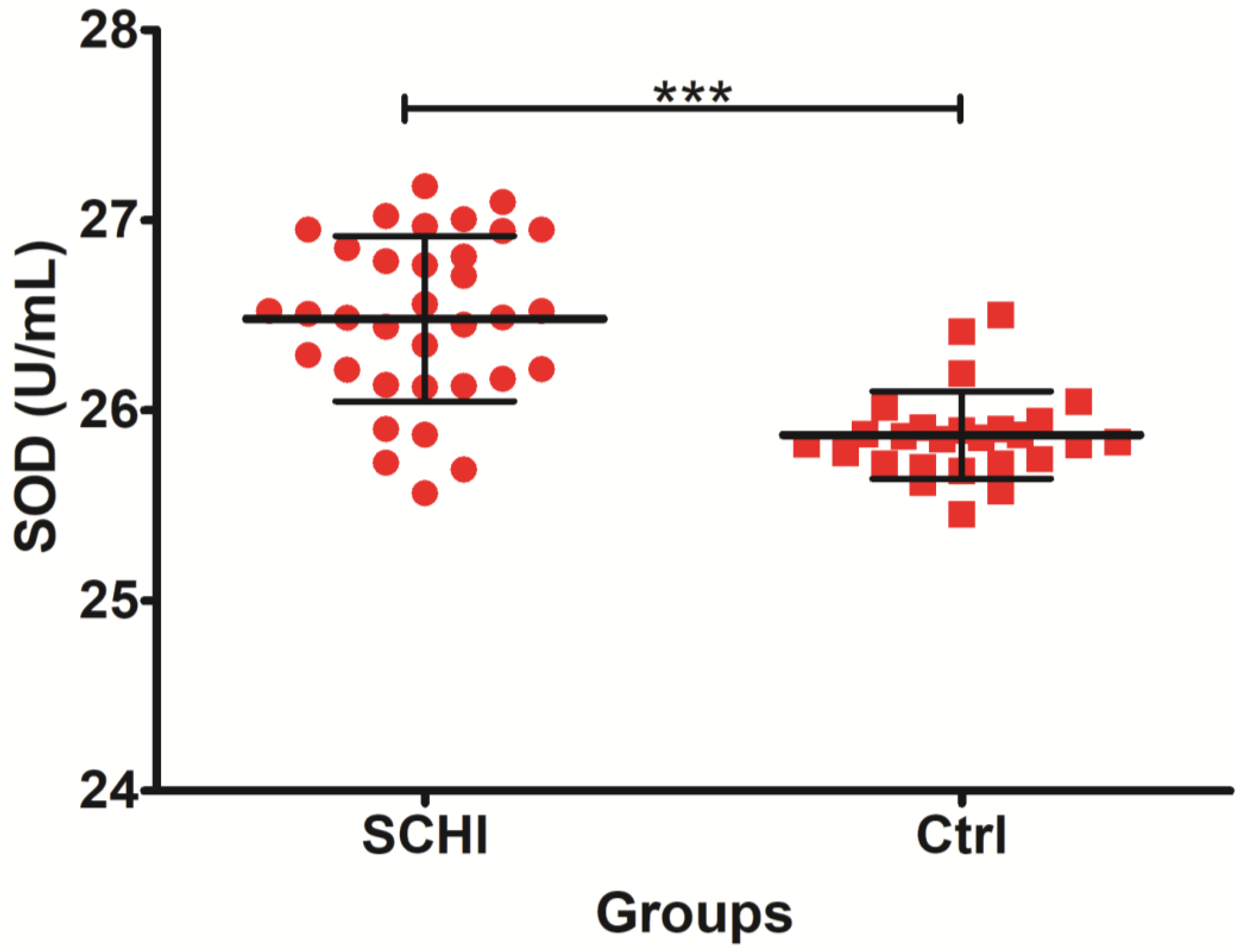
| SOD (U/mL) | 26 | 25.87 | 25.86 | 0.23 | 0.045 | 34 | 26.48 | 26.50 | 0.43 | 0.074 |
| CAT U/L | 26 | 49,778.96 | 48,505.75 | 23,232.02 | 4556.174 | 34 | 51,926.98 | 48,505.75 | 38,422.30 | 6589.370 |
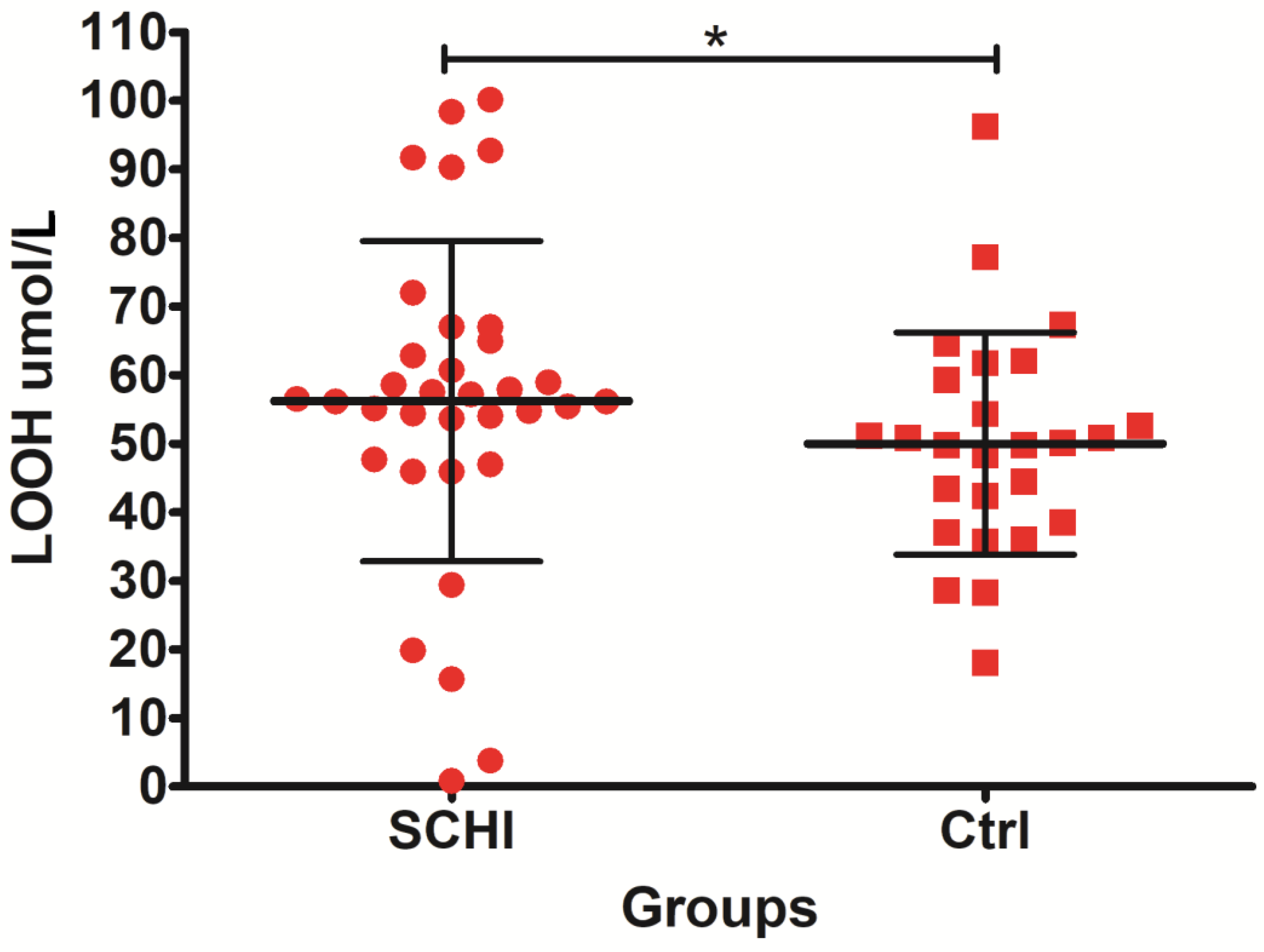
| LOOH µmol/L | 26 | 50.01 | 50.00 | 16.21 | 3.180 | 34 | 57.65 | 56.34 | 20.61 | 3.534 |
| MDA µmol/L | 26 | 0.54 | 0.51 | 0.34 | 0.068 | 34 | 0.66 | 0.64 | 0.23 | 0.040 |
| FRAP µmol/L | 26 | 16.63 | 17.14 | 7.98 | 1.566 | 34 | 18.86 | 19.30 | 7.22 | 1.238 |
| CRP mg/L | 26 | 4.85 | 4.85 | 0.55 | 0.11 | 29 * | 16.26 | 16.40 | 2.38 | 0.442 |
| U Mann-Witney Test | Sum of Rang Ctrl | Sum of Rang SCHI | U | Z | p |
|---|---|---|---|---|---|
| SOD (U/mL) | 460.00 | 1370.00 | 109.00 | −4.96757 | 0.000001 |
| CAT U/L | 829.50 | 1000.50 | 405.50 | 0.54449 | 0.586 |
| LOOH µmol/L | 660.50 | 1169.50 | 309.50 | −1.97658 | 0.048 |
| MDA µmol/L | 651.50 | 1178.50 | 300.50 | −2.11084 | 0.034 |
| FRAP µmol/L | 692.00 | 1138.00 | 341.00 | −1.50668 | 0.131 |
| CRP mg/L | 1160.00 | 325.00 | 139.00 | 6.288 | 0.000001 |
| Variables | PANSS P | PANSS N | PANSS G | PANSS T |
|---|---|---|---|---|
| SOD (U/mL) | −0.189 | −0.223 | −0.155 | −0.252 |
| CAT U/L | −0.128 | −0.079 | −0.396 | −0.240 |
| LOOH µmol/L | −0.171 | −0.168 | −0.214 | −0.254 |
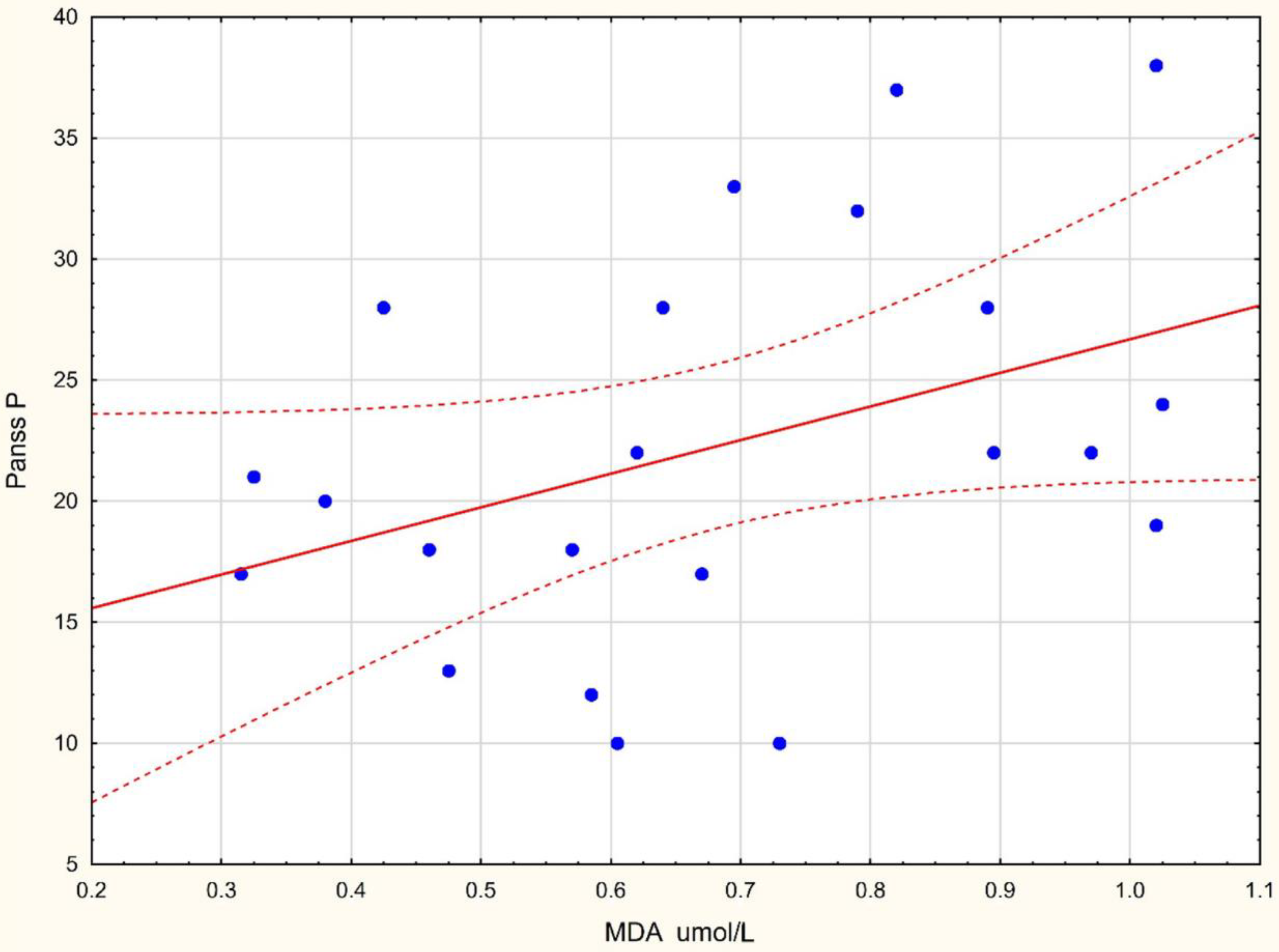
| MDA µmol/L | 0.439 * | −0.353 | 0.2125 | 0.181 |
| FRAP µmol/L | 0.368 | 0.159 | 0.359 | 0.384 |
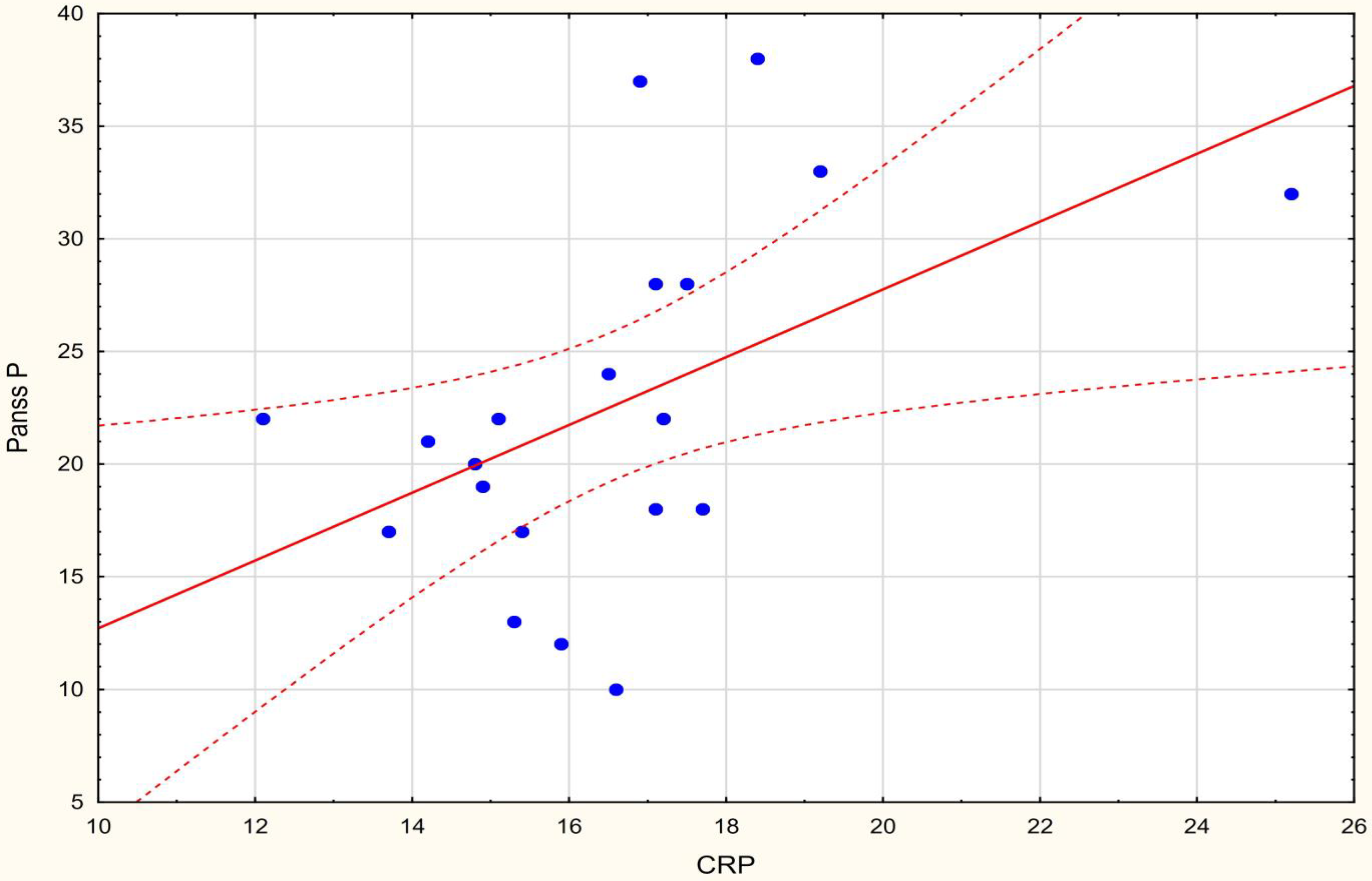
| CRP mg/L | 0.491 * | −0.173 | 0.184 | 0.319 |
| Variables | Group | |
|---|---|---|
| Ctrl | SCHI | |
| SOD (U/mL) | −0.031 | −0.172 |
| CAT U/L | 0.023 | 0.242 |
| LOOH umol/L | 0.164 | −0.036 |
| MDA umol/L | 0.065 | 0.356 |
| FRAP umol/L | −0.374 | 0.268 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudzińska, E.; Szymona, K.; Bogucki, J.; Koch, W.; Cholewińska, E.; Sitarz, R.; Ognik, K. Increased Markers of Oxidative Stress and Positive Correlation Low-Grade Inflammation with Positive Symptoms in the First Episode of Schizophrenia in Drug-Naïve Patients. J. Clin. Med. 2022, 11, 2551. https://doi.org/10.3390/jcm11092551
Dudzińska E, Szymona K, Bogucki J, Koch W, Cholewińska E, Sitarz R, Ognik K. Increased Markers of Oxidative Stress and Positive Correlation Low-Grade Inflammation with Positive Symptoms in the First Episode of Schizophrenia in Drug-Naïve Patients. Journal of Clinical Medicine. 2022; 11(9):2551. https://doi.org/10.3390/jcm11092551
Chicago/Turabian StyleDudzińska, Ewa, Kinga Szymona, Jacek Bogucki, Wojciech Koch, Ewelina Cholewińska, Robert Sitarz, and Katarzyna Ognik. 2022. "Increased Markers of Oxidative Stress and Positive Correlation Low-Grade Inflammation with Positive Symptoms in the First Episode of Schizophrenia in Drug-Naïve Patients" Journal of Clinical Medicine 11, no. 9: 2551. https://doi.org/10.3390/jcm11092551
APA StyleDudzińska, E., Szymona, K., Bogucki, J., Koch, W., Cholewińska, E., Sitarz, R., & Ognik, K. (2022). Increased Markers of Oxidative Stress and Positive Correlation Low-Grade Inflammation with Positive Symptoms in the First Episode of Schizophrenia in Drug-Naïve Patients. Journal of Clinical Medicine, 11(9), 2551. https://doi.org/10.3390/jcm11092551








