Fatigue Is a Major Symptom at COVID-19 Hospitalization Follow-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Clinical Data
2.3. Pulmonary Function and Six-Minute Walk Test
2.4. Patient Reported Outcome Measures
2.5. Statistics
3. Results
3.1. Baseline Data during Hospitalization
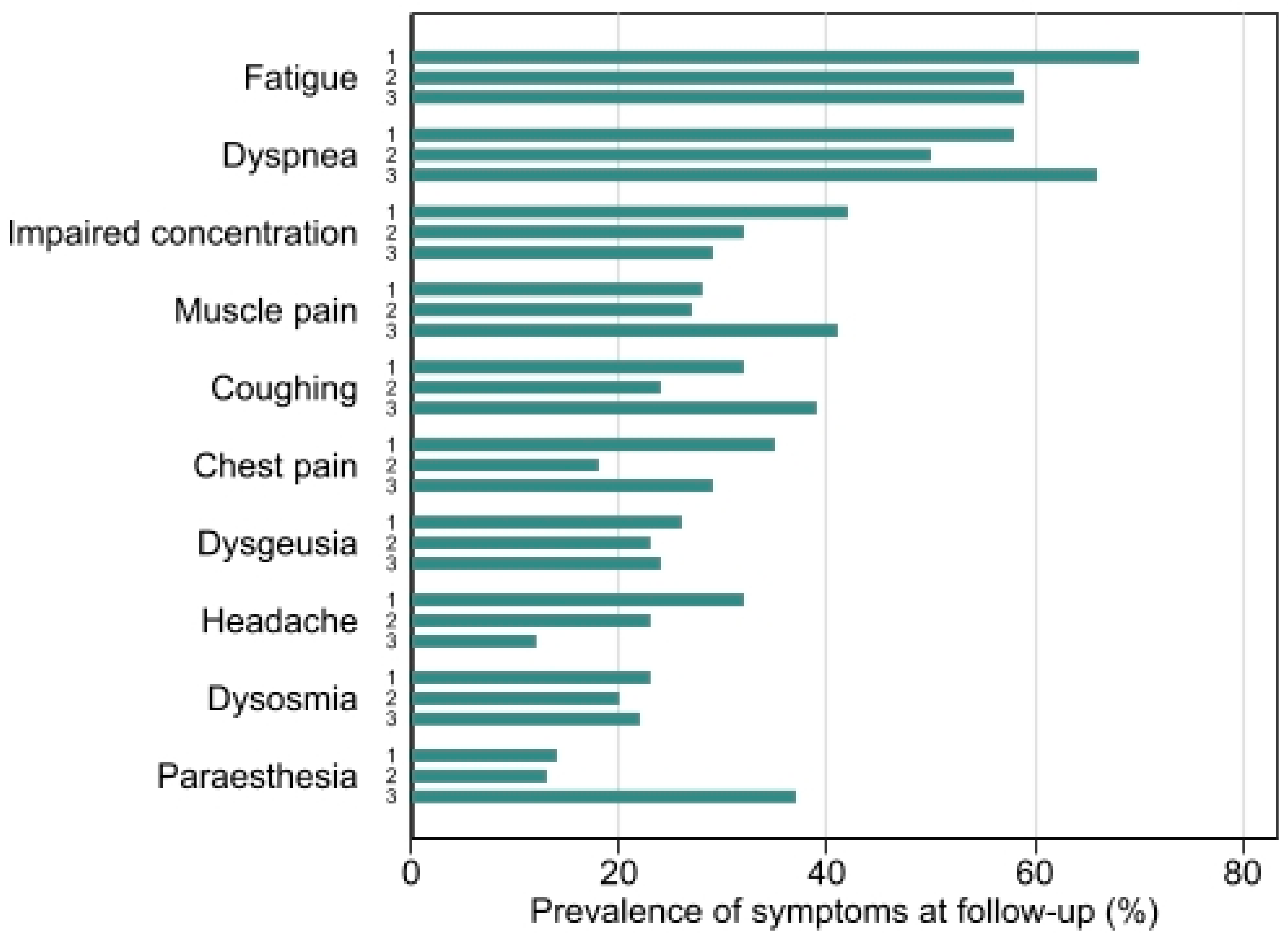
3.2. Follow-Up
3.3. Pulmonary Function Test
3.4. Six-Minute Walk Test (6MWT)
3.5. Patient Reported Outcome Measures
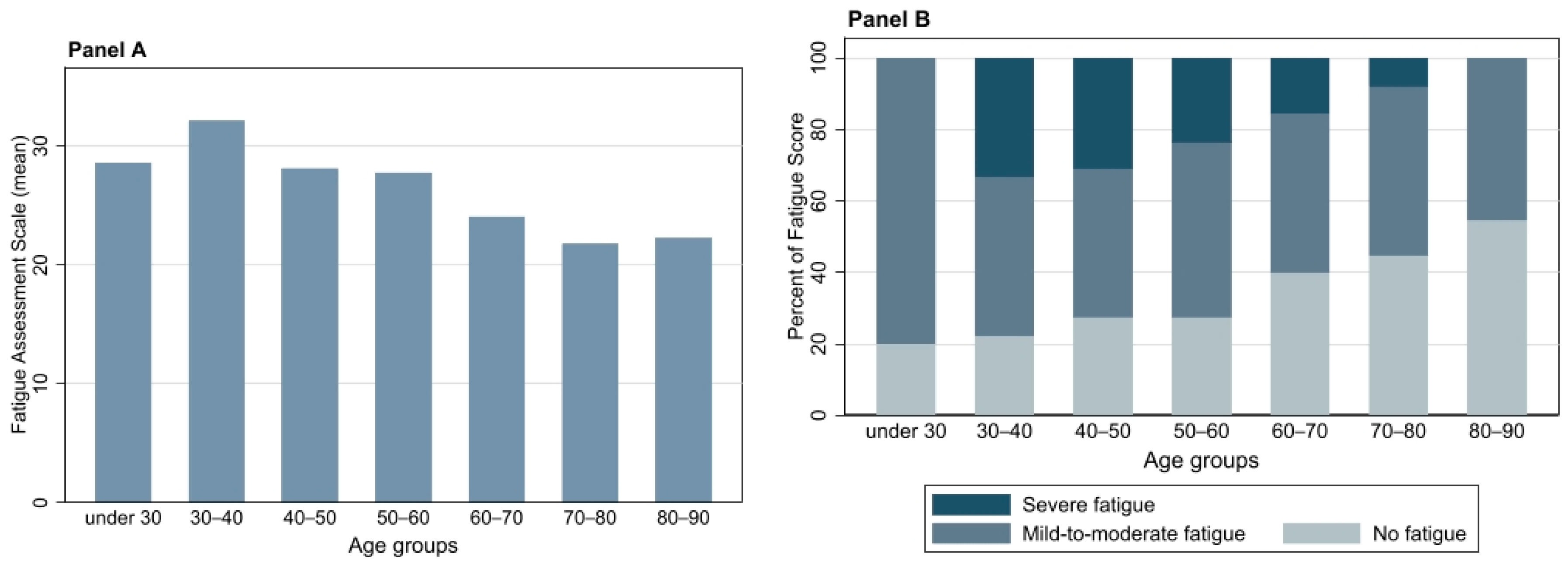
3.5.1. FAS
3.5.2. HADS
3.5.3. MRC
3.5.4. Relationship between FAS and Other Parameters
3.6. Remdesivir and Dexamethasone Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.C.; Geoghegan, L.; Arbyn, M.; Mohammed, Z.; McGuinness, L.; Clarke, E.L.; Wade, R.G. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries. PLoS ONE 2020, 15, e0234765. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Gattarello, S.; Steinberg, I.; Busana, M.; Palermo, P.; Lazzari, S.; Romitti, F.; Quintel, M.; Meissner, K.; Marini, J.J.; et al. COVID-19 pneumonia: Pathophysiology and management. Eur. Respir. Rev. 2021, 30, 210138. [Google Scholar] [CrossRef] [PubMed]
- The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. China CDC Wkly. 2020, 41, 145–151.
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, X.; Li, Y.; Chen, H.; Chen, T.; Su, N.; Huang, F.; Zhou, J.; Zhang, B.; Yan, F.; et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am. J. Respir. Crit. Care Med. 2020, 201, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Vilches, T.N.; Moghadas, S.M.; Sah, P.; Fitzpatrick, M.C.; Shoukat, A.; Pandey, A.; Galvani, A.P. Estimating COVID-19 Infections, Hospitalizations, and Deaths Following the US Vaccination Campaigns during the Pandemic. JAMA Netw. Open 2022, 797, 2020–2023. [Google Scholar] [CrossRef] [PubMed]
- Carfi, A. Persistent Symptoms in Patietns after Acute COVID-19. JAMA-J. Am. Med. Assoc. 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Leth, S.; Gunst, J.D.; Agergaard, J. Persistent symptoms in hospitalized patients recovering from COVID-19 in Denmark. Infect. Dis. Soc. Am. 2021, 8, ofab042. [Google Scholar]
- Bulut, O.; Kilic, G.; Netea, M.G.; Netea, M.G. Disparities in case frequency and mortality of coronavirus disease 2019 (COVID-19) among various states in the United States. Ann. Med. 2020, 53, 151–159. [Google Scholar] [CrossRef]
- Vejen, M.; Hansen, E.F.; Al-Jarah, B.N.I.; Jensen, C.; Thaning, P.; Jeschke, K.N.; Ulrik, C.S. Hospital admission for COVID-19 pneumonitis–long-term impairment in quality of life and lung function. Eur. Clin. Respir. J. 2022, 9, 2024735. [Google Scholar] [CrossRef]
- Liu, C.; Ye, L.; Xia, R.; Zheng, X.; Yuan, C.; Wang, Z.; Lin, R.; Shi, D.; Gao, Y.; Yao, J.; et al. Chest CT and Clinical Follow-up of Discharged Patients with COVID-19 in Wenzhou City, Zhejiang, China. Ann. Am. Thorac. Soc. 2020, 17, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Zhang, L.; Zhang, J.; Hu, F.; Chen, L.; Dong, Y.; Yang, K.; Zhang, B.; Zhang, S. Anormal pulmonary function and residual CT abnormalities in rehabilitating COVID-19 patients after discharge. J. Infect. 2020, 81, e150–e152. [Google Scholar] [CrossRef] [PubMed]
- Huang, L. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.F.; Liu, T.; Yu, J.N.; Xu, X.R.; Zahid, K.R.; Wei, Y.C.; Wang, X.H.; Zhou, F.L. Half-year follow-up of patients recovering from severe COVID-19: Analysis of symptoms and their risk factors. J. Intern. Med. 2021, 290, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, B.; Mohn, K.G.I.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Sævik, M.; et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021, 27, 1607–1613. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. Available online: http://www.nature.com/articles/s41591-021-01292-y (accessed on 22 April 2022). [CrossRef]
- Wanger, J.; Clausen, J.L.; Coates, A.; Pedersen, O.F.; Brusasco, V.; Burgos, F.; Casaburi, R.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; Macintyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European respiratory society/American thoracic society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef] [PubMed]
- Jay, S.J.; Enright, P. Reference equations for the six-minute walk in healthy adults. Am. J. Respir. Crit. Care Med. 2000, 161, 1396. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sport. Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- De Vries, J.; Michielsen, H.; Van Heck, G.L.; Drent, M. Measuring fatigue in sarcoidosis: The Fatigue Assessment Scale (FAS). Br. J. Health Psychol. 2004, 9, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, C.; Drent, M.; Elfferich, M.; De Vries, J. The Fatigue Assessment Scale: Quality and availability in sarcoidosis and other diseases. Curr. Opin. Pulm. Med. 2018, 24, 495–503. [Google Scholar] [CrossRef]
- Stenton, C. The MRC breathlessness scale. Occup. Med. 2008, 58, 226–227. [Google Scholar] [CrossRef] [Green Version]
- Snaith, R.P.; Zigmond, A.S. The hospital anxiety anddepression scale. Br. Med. J. 1986, 292, 344. [Google Scholar] [CrossRef] [Green Version]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Motiejunaite, J.; Balagny, P.; Arnoult, F.; Mangin, L.; Bancal, C.; d’Ortho, M.P.; Frija-Masson, J. Hyperventilation: A Possible Explanation for Long-Lasting Exercise Intolerance in Mild COVID-19 Survivors? Front. Physiol. 2021, 11, 614590. [Google Scholar] [CrossRef] [PubMed]
- Dennis, A.; Wamil, M.; Alberts, J.; Oben, J.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Brady, M.; Hishmeh, L.; et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open 2021, 11, 2–7. [Google Scholar]
- Goërtz, Y.M.J.; Van Herck, M.; Delbressine, J.M.; Vaes, A.W.; Meys, R.; Machado, F.V.C.; Houben-Wilke, S.; Burtin, C.; Posthuma, R.; Franssen, F.M.E.; et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: The post-COVID-19 syndrome? ERJ Open Res. 2020, 6, 00542–02020. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shang, Y.; Song, W.; Li, Q.; Xie, H.; Xu, Q.; Jia, J.; Li, L.; Mao, H.; Zhou, X.; et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020, 25, 100463. [Google Scholar] [CrossRef]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Noel, A.; Gunning, S.; Hatrick, J.; Hamilton, S.; et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax 2021, 76, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Abboud, H. COVID-19 and SARS-CoV-2 Infection: Pathophysiology and Clinical Effects on the Nervous System. World Neurusurg. 2020, 140, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Veldhuis, A.; Malhotra, T. Neuropsychiatric and Cognitive Sequelae of COVID-19. Front. Psychol. 2021, 12, 553. [Google Scholar] [CrossRef]
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020, 395, 912–920. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32112714 (accessed on 22 April 2022). [CrossRef] [Green Version]
- Morgul, E.; Bener, A.; Atak, M.; Akyel, S.; Aktaş, S.; Bhugra, D.; Ventriglio, A.; Jordan, T.R. COVID-19 pandemic and psychological fatigue in Turkey. Int. J. Soc. Psychiatry 2021, 67, 128–135. [Google Scholar] [CrossRef]
- Taverne, J.; Salvator, H.; Leboulch, C.; Barizien, N.; Ballester, M.; Imhaus, E.; Chabi-Charvillat, M.L.; Boulin, A.; Goyard, C.; Chabrol, A.; et al. High incidence of hyperventilation syndrome after COVID-19. J. Thorac. Dis. 2021, 13, 3918–3922. [Google Scholar] [CrossRef]
- Van Den Borst, B.; Peters, J.B.; Brink, M.; Schoon, Y.; Bleeker-Rovers, C.P.; Schers, H.; Van Hees, H.W.H.; Van Helvoort, H.; Van Den Boogaard, M.; Van Der Hoeven, H.; et al. Comprehensive Health Assessment 3 Months after Recovery from Acute Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2021, 73, E1089–E1098. [Google Scholar] [CrossRef] [PubMed]
- Repurposed Antiviral Drugs for Covid-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511. [CrossRef] [PubMed]
- Boglione, L.; Meli, G.; Poletti, F.; Rostagno, R.; Moglia, R.; Cantone, M.; Esposito, M.; Scianguetta, C.; Domenicale, B.; Di Pasquale, F.; et al. Risk factors and incidence of long-COVID syndrome in hospitalized patients: Does remdesivir have a protective effect? QJM An Int. J. Med. 2022, 114, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Boon, G.J.A.M.; Barco, S.; Endres, M.; Miranda Geelhoed, J.J.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The post-COVID-19 functional status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56, 10–12. [Google Scholar] [CrossRef]

| Overall. n = 218 | Patients Not Requiring Supplemental Oxygen (Group 1) (n = 57) | Patients Requiring Supplemental Oxygen (Group 2) (n = 120) | Patients Admitted to ICU (Group 3) (n = 41) | |
|---|---|---|---|---|
| Age (years) | 59.94 (58.15, 61.73) | 54.04 (50.36, 57.71) | 62.3 (59.94, 64.66) | 61.24 (57.58, 64.90) |
| Sex | ||||
| Male | 128 (59%) | 24 (42%) | 71 (59%) | 33 (80%) |
| Female | 90 (41%) | 33 (58%) | 49 (41%) | 8 (20%) |
| Body Mass Index | 29.24 (28.51, 29.98) | 28.21 (26.71, 29.71) | 29.34 (28.31, 30.36) | 30.37 (28.90, 31.84) |
| <18.5 | 1 (0%) | 1 (2%) | 0 (0%) | 0 (0%) |
| 18.5–24.9 | 44 (21%) | 13 (24%) | 25 (21%) | 6 (15%) |
| 25.0–29.9 | 90 (42%) | 25 (45%) | 50 (43%) | 15 (37%) |
| ≥30 | 78 (37%) | 16 (29%) | 42 (36%) | 20 (49%) |
| Smoking status | ||||
| Ever | 92 (46%) | 23 (43%) | 46 (43%) | 23 (61%) |
| Never | 106 (54%) | 31(57%) | 60 (57%) | 15 (39%) |
| Comorbidities * | ||||
| Hypertension | 71 (33%) | 11 (19%) | 41 (34%) | 19 (46%) |
| Asthma | 36 (17%) | 8 (14%) | 21 (18%) | 7 (17%) |
| Diabetes | 28 (13%) | 4 (7%) | 17 (14%) | 7 (17%) |
| Malignancy | 20 (9%) | 7 (12%) | 10 (8%) | 3 (7%) |
| Chronic obstructive pulmonary disease (COPD) | 18 (8%) | 2 (4%) | 11 (9%) | 5 (12%) |
| Number of patients with more than 3 comorbidities | 41 (19) | 9 (16) | 20 (17) | 12 (29) |
| Time from symptom onset to admission (days) | 8.37 (7.36, 9.37) | 8.14 (6.20, 10.08) | 8.42 (6.88, 9.95) | 8.53 (7.24, 9.81) |
| Length of hospital stay (days) | 9.29 (7.74, 10.86) | 2.05 (1.37, 2.74) | 6.85 (5.93, 7.77) | 26.37 (21.30, 31.43) |
| Treatment during hospitalization | ||||
| Remdesivir | 92 (42%) | 6 (11%) | 69 (58%) | 17 (43%) |
| Systemic corticosteroids | 119 (55%) | 11 (19%) | 81 (68%) | 27 (68%) |
| Anticoagulation | 153 (71%) | 19 (33%) | 96 (80%) | 38 (97%) |
| Intravenous immunoglobin | 2 (1%) | 1 (2%) | 1 (1%) | 0 (0%) |
| All Patients (n = 218) | Patients Not Requiring Supplemental Oxygen (Group 1) (n = 57) | Patients Requiring Supplemental Oxygen ** (Group 2) (n = 120) | Patients Admitted at ICU (Group 3) (n = 41) | Group 2 vs. Group 1 Multivariable Model | Group 3 vs. Group 1 Multivariable Model | |
|---|---|---|---|---|---|---|
| Time from discharge to follow-up (days) | 127.65 (122.19, 133.11) | 133.68 (124.11, 143.26) | 123.26 (115.49, 131.03) | 132.12 (119.46, 144.78) | β (95% CI) −11.53 (−24.40, 1.35) | β (95% CI) −7.59 (−24.41, 9.23) |
| Pulmonary function | β (95% CI) | β (95% CI) | ||||
| FEV1, L/min | 2.91 (2.79, 3.02) | 3.05 (2.87, 3.23) | 2.85 (2.68, 3.02) | 2.90 (2.68, 3.12) | −0.13 (−0.34, 0.09) | −0.26 (−0.54, 0.02) |
| FEV1, % | 98.17 (95.49, 100.85) | 103.58 (99.45, 107.71) | 97.71 (93.87, 101.56) | 91.83 (85.37, 98.28) | −5.36 (−11.89, 1.18) | −10.78 (−19.23, −2.33) * |
| FVC, L/min | 3.77 (3.63, 3.91) | 3.99 (3.74, 4.25) | 3.67 (3.47, 3.86) | 3.74 (3.46, 4.03) | −0.31 (−0.59, −0.02) | −0.46 (−0.86, −0.06) * |
| FVC, % | 103.28 (100.42, 106.15) | 111.54 (106.59, 116.49) | 102.37 (98.68, 106.06) | 94.23 (86.51, 101.94) | −8.44 (−15.44, −1.43) * | −13.73 (−24.05, −3.40) * |
| DLCO, % | 80.43 (77.83, 83.04) | 88.98 (84.77, 93.19) | 79.68 (76.04, 83.32) | 70.46 (65.13, 75.79) | −9.71 (−15.49, −3.92) * | −23.08 (−30.28, −15.89) * |
| TLC, % | 94.05 (91.18, 96.91) | 99.2 (94.14, 104.26) | 94.31 (90.48, 98.14) | 84.38 (77.27, 91.50) | −5.10 (−12.55, 2.34) | −14.19 (−24.40, −3.98) * |
| RV, % | 98.37 (93.83, 102.91) | 101.73 (93.31, 110.15) | 99.99 (93.20, 106.77) | 87.96 (80.00, 95.92) | −3.96 (−15.56, 7.64) | −15.01 (−29.17, −0.85) * |
| OR (95% CI) | OR (95% CI) | |||||
| DLCO < 80% | 96 (45%) | 15 (27%) | 54 (45%) | 27 (69%) | 2.94 (1.32, 6.51) * | 13.02 (0.88, 43.65) * |
| DLCO < 60% | 35 (16%) | 2 (4%) | 22 (18%) | 11 (28%) | 4.93 (1.04, 23.31) * | 24.47 (3.05, 196.24) * |
| β (95% CI) | β (95% CI) | |||||
| MRC score | 1.95 (1.82–2.08) | 1.75 (1.54–1.95) | 1.96 (1.79–2.14) | 2.18 (1.80–2.56) | 0.21 (−0.08, 0.50) | 0.38 (−0.05, 0.81) |
| OR (95% CI) | OR (95% CI) | |||||
| MRC score (3–5) | 48 (24%) | 8 (15%) | 28 (26%) | 12 (31%) | 1.89 (0.73, 4.95) | 2.54 (0.71, 9.03) |
| 6MWT | β (95% CI) | β (95% CI) | ||||
| 6MWTD (m) | 486.90 (471.87, 501.94) | 515.33 (489.37, 541.29) | 479.65 (457.79, 501.51) | 464.65 (431.40, 497.89) | −15.78 (−45.75, 14.20) | −31.59 (−73.19, 10.01) |
| Percent predicted, male | 84.65 (81.55, 87.75) | 88.98 (83.74, 94.21) | 85.38 (81.07, 89.69) | 79.73 (72.69, 86.77) | −3.86 (−10.44, 2.72) | −9.82 (−18.30, −1.33) * |
| Percent predicted, female | 95.97 (91.35, 100.59) | 93.09 (85.42, 100.76) | 95.00 (88.71, 101.29) | 111.58 (94.94, 128.21) | −3.92 (−14.75, 6.91) | 5.83 (−8.83, 20.48) |
| Desaturation (%-point) | 2.89 (2.39, 3.39) | 2.11 (1.45, 2.77) | 3.05 (2.25, 3.85) | 3.65 (2.56, 4.73) | 0.59 (−0.41, 1.59) | 1.39 (−0.00, 2.79) |
| Borg scale before test | 0.79 (0.58, 1.01) | 0.57 (0.20, 0.94) | 0.85 (0.56, 1.13) | 1.02 (0.35, 1.69) | 0.17 (−0.36, 0.69) | 0.34 (−0.44, 1.12) |
| Borg scale after test | 3.64 (3.24, 4.04) | 3.41 (2.67, 4.15) | 3.73 (3.19, 4.28) | 3.73 (2.67, 4.78) | 0.34 (−0.60, 1.29) | 0.27 (−1.08, 1.63) |
| Change in Borg scale | 2.82 (2.46, 3.18) | 2.83 (2.15, 3.49) | 2.85 (2.34, 3.35) | 2.69 (1.86, 3.53) | 0.13 (−0.68, 0.95) | −0.06 (−1.23, 1.11) |
| OR (95% CI) | OR (95% CI) | |||||
| Desaturation below 92 | 37 (19%) | 6 (11%) | 19 (19%) | 12 (32%) | 1.11 (0.39, 3.19) | 3.94 (1.07, 14.48) * |
| Desaturation ≥4%-point | 53 (27%) | 12 (22%) | 23 (23%) | 18 (49%) | 0.82 (0.35, 1.91) | 3.44 (1.19, 9.91) * |
| β (95% CI) | β (95% CI) | |||||
| Fatigue assessment scale | 25.61 (24.29, 26.93) | 28.08 (25.45, 30.71) | 24.66 (22.81, 26.52) | 25.08 (22.42, 27.74) | −1.32 (−4.80, 2.16) | −2.05 (−6.29, 2.19) |
| OR (95% CI) | OR (95% CI) | |||||
| No fatigue | 67 (35%) | 11 (23%) | 42 (40%) | 14 (38%) | 1.96 (0.86, 4.45) | 2.57 (0.86, 7.68) |
| Mild-to-moderate fatigue | 88 (47%) | 25 (52%) | 45 (43%) | 18 (49%) | 0.82 (0.41, 1.65) | 1.31 (0.52, 3.32) |
| Severe fatigue | 34 (18%) | 12 (25%) | 17 (16%) | 5 (14%) | 0.87 (0.34, 2.22) | 0.57 (0.16, 2.03) |
| β (95% CI) | β (95% CI) | |||||
| HADS score total | 7.94 (6.95, 8.93) | 9.60 (7.54, 11.67) | 7.59 (6.27, 8.91) | 6.76 (4.52, 8.99) | −1.17(−3.79, 1.45) | −2.90 (−6.15, 0.34) |
| HADS-D score | 3.22 (2.71, 3.67) | 3.56 (2.53, 4.59) | 3.26 (2.59, 3.94) | 2.49 (1.59, 3.38) | −0.05 (−1.41, 1.31) | −1.29 (−2.84, 0.26) |
| HADS-A score | 4.75 (4.17, 5.34) | 6.04 (4.87, 7.21) | 4.32 (3.56, 5.08) | 4.27 (2.80, 5.74) | −1.12 (−2.59, 0.35) | −1.61 (−3.53, 0.31) |
| OR (95% CI) | OR (95% CI) | |||||
| HADS-D abnormal score (≥8) | 29 (16%) | 9 (19%) | 16 (16%) | 4 (11%) | 0.94 (0.35, 2.49) | 0.54 (0.14, 2.14) |
| HADS-A abnormal score (≥8) | 43 (23%) | 15 (31%) | 21 (21%) | 7 (19%) | 0.73 (0.32) | 0.69 (0.22, 2.20) |
| Oxygen-Dependent Patients Not Receiving Systemic Corticosteroids and Remdesivir (RaD÷) n = 52 | Oxygen-Dependent Patients Receiving Systemic Corticosteroids and Remdesivir (RaD+) n = 86 | RaD+ vs. RaD÷ Multivariable Model | |
|---|---|---|---|
| β (95% CI) | |||
| Age | 63.54 (59.62, 67.45) | 61.88 (59.24, 64.53) | −5.27 (−9.33, −1.22) * |
| Sex | OR (95% CI) | ||
| Male | 31 (60%) | 56 (65%) | 1.18 (0.55, 2.51) |
| Female | 21 (40%) | 30 (35%) | |
| Smoking status | OR (95% CI) | ||
| Ever | 23 (48%) | 37 (47%) | 1.18 (0.55, 2.51) |
| Never | 25 (52%) | 41 (53%) | |
| β (95% CI) | |||
| Number of comorbidities | 1.13 (0.84, 1.43) | 1.79 (1.55, 2.03) | 0.72 (0.40, 1.04) * |
| β (95% CI) | |||
| Length of hospital stay | 12.27 (8.84, 15.69) | 9.22 (7.08, 11.36) | −4.04 (−8.38, 0.29) |
| OR (95% CI) | |||
| ICU admission | 13 (25%) | 17 (20%) | 0.19 (0.05, 0.74) * |
| Number of symptoms at follow-up | β (95% CI) | ||
| 3.27 (2.49, 4.04) | 3.86 (3.15, 4.57) | 0.13 (−0.97, 1.24) | |
| β (95% CI) | |||
| MRC score | 1.89 (1.64, 2.15) | 2.08 (1.85, 2.30) | −0.03 (−0.36, 0.29) |
| OR (95% CI) | |||
| MRC score 3–5 | 12 (25%) | 22 (28%) | 0.81 (0.32, 2.04) |
| Pulmonary function | β (95% CI) | ||
| FEV1, % | 101.69 (96.30, 107.07) | 95.0 (90.84, 99.16) | −4.17 (−11.11, 2.78) |
| FVC, % | 107.37 (101.08, 113.66) | 97.92 (93.89, 101.94) | −6.64 (−13.86, 0.58) |
| DLCO, % | 80.41 (74.79, 86.04) | 76.39 (72.21, 80.57) | −2.15 (−8.89, 4.59) |
| 6MWT | β (95% CI) | ||
| 6MWTD (m) | 471.73 (438.67, 504.79) | 471.55 (445.56, 497.54) | 20.02 (−17.25, 57.31) |
| Desaturation during 6MWT (%-point) | 2.09 (1.25, 2.93) | 3.79 (2.76, 4.81) | 1.77 (0.27, 3.27) * |
| Change in Borg scale score | |||
| 2.28 (1.60, 2.95) | 3.37 (2.67, 4.07) | 1.16 (0.05, 2.27) * | |
| β (95% CI) | |||
| FAS score | 23.73 (21.28, 26.19) | 25.37 (23.17, 27.57) | 0.61 (−2.67, 3.89) |
| OR (95% CI) | |||
| No fatigue | 22 (45%) | 28 (38%) | 0.78 (0.36, 1.67) |
| Mild-to-moderate fatigue | 23 (47%) | 31 (42%) | 0.73 (0.34, 1.54) |
| Severe fatigue | 4 (8%) | 14 (19%) | 1.65 (0.43, 6.19) |
| β (95% CI) | |||
| HADS score total | 5.69 (4.17, 7.21) | 8.82 (7.04, 10.60) | 2.52 (0.21, 4.83) * |
| HADS-D score | 2.22 (1.52, 2.91) | 3.89 (3.00, 4.79) | 1.29 (0.20, 2.38) * |
| HADS-A score | 3.47 (2.20, 4.44) | 4.93 (3.88, 5.97) | 1.23 (−0.22, 2.67) |
| OR (95% CI) | |||
| HADS-D abnormal score (≥8) | 3 (6%) | 15 (22%) | 3.17 (0.78, 12.94) |
| HADS-A abnormal score (≥8) | 5 (10%) | 18 (26%) | 2.54 (0.82, 7.84) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sperling, S.; Fløe, A.; Leth, S.; Hyldgaard, C.; Gissel, T.; Topcu, A.; Kristensen, L.; Sønderskov, L.D.; Schmid, J.M.; Jensen-Fangel, S.; et al. Fatigue Is a Major Symptom at COVID-19 Hospitalization Follow-Up. J. Clin. Med. 2022, 11, 2411. https://doi.org/10.3390/jcm11092411
Sperling S, Fløe A, Leth S, Hyldgaard C, Gissel T, Topcu A, Kristensen L, Sønderskov LD, Schmid JM, Jensen-Fangel S, et al. Fatigue Is a Major Symptom at COVID-19 Hospitalization Follow-Up. Journal of Clinical Medicine. 2022; 11(9):2411. https://doi.org/10.3390/jcm11092411
Chicago/Turabian StyleSperling, Søren, Andreas Fløe, Steffen Leth, Charlotte Hyldgaard, Tina Gissel, Ayfer Topcu, Lars Kristensen, Lene Dahl Sønderskov, Johannes Martin Schmid, Søren Jensen-Fangel, and et al. 2022. "Fatigue Is a Major Symptom at COVID-19 Hospitalization Follow-Up" Journal of Clinical Medicine 11, no. 9: 2411. https://doi.org/10.3390/jcm11092411
APA StyleSperling, S., Fløe, A., Leth, S., Hyldgaard, C., Gissel, T., Topcu, A., Kristensen, L., Sønderskov, L. D., Schmid, J. M., Jensen-Fangel, S., & Bendstrup, E. (2022). Fatigue Is a Major Symptom at COVID-19 Hospitalization Follow-Up. Journal of Clinical Medicine, 11(9), 2411. https://doi.org/10.3390/jcm11092411







