Short- and Long-Term Outcome of Laparoscopic- versus Robotic-Assisted Right Colectomy: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Literature Search Strategy and Eligibility Criteria
2.2. Assessment of Data Extraction and Methodological Quality
2.3. Statistical Analysis
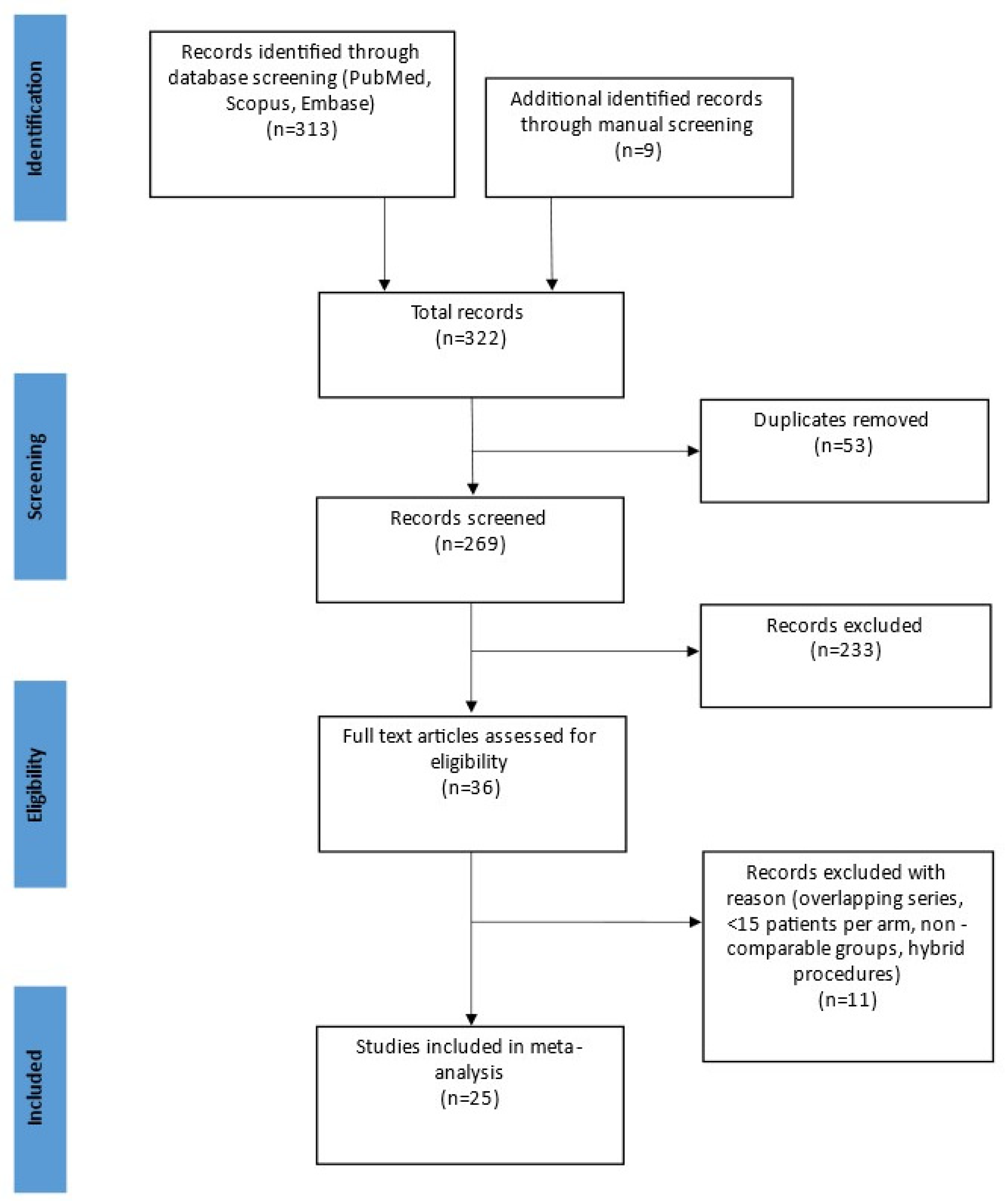
3. Results
3.1. Search Results and Study Details
3.2. Patients’ Characteristics
3.3. Perioperative Outcomes
3.4. Oncological Findings
3.5. Costs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RRC | Robotic right colectomy |
| LRC | Laparoscopic right colectomy |
| BMI | Body mass index |
| ASA | American Society of Anesthesiologists |
| OR | Odds ratio |
| CI | Confidence interval |
| CME | Complete mesocolic excision |
References
- Lim, S.W.; Kim, H.R.; Kim, Y.J. Single incision laparoscopic colectomy for colorectal cancer: Comparison with conventional laparoscopic colectomy. Ann. Surg. Treat. Res. 2014, 87, 131–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jayne, D.G.; Thorpe, H.C.; Copeland, J.; Quirke, P.; Brown, J.M.; Guillou, P.J. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br. J. Surg. 2010, 97, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, I.; Kang, S.I.; Sohn, S.-K.; Lee, K.Y. Laparoscopic right hemicolectomy with complete mesocolic excision. Surg. Endosc. 2014, 28, 2747–2751. [Google Scholar] [CrossRef] [PubMed]
- Tschann, P.; Seitinger, G.; Lechner, D.; Adler, S.; Feurstein, B.; Girotti, P.N.C.; Schmölzer, T.; Szeverinski, P.; Aigner, F.; Königsrainer, I. Reduced port versus open right hemicolectomy for colorectal cancer: A retrospective comparison study of two centers. Int. J. Colorectal Dis. 2021, 36, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.-M.; Leung, K.L.; Ng, S.S.-M.; Liu, S.Y.-W.; Lee, J.F.-Y.; Hon, S.S.-F. Laparoscopic-assisted versus open resection of right-sided colonic cancer—A prospective randomized controlled trial. Int. J. Colorectal Dis. 2012, 27, 95–102. [Google Scholar] [CrossRef]
- van der Pas, M.H.; Haglind, E.; Cuesta, M.A.; Fürst, A.; Lacy, A.M.; Hop, W.C.J.; Bonjer, H.J. Laparoscopic versus open surgery for rectal cancer (COLOR II): Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013, 14, 210–218. [Google Scholar] [CrossRef]
- Park, J.S.; Kang, H.; Park, S.Y.; Kim, H.J.; Woo, I.T.; Park, I.-K.; Choi, G.-S. Long-term oncologic after robotic versus laparoscopic right colectomy: A prospective randomized study. Surg. Endosc. 2019, 33, 2975–2981. [Google Scholar] [CrossRef]
- Spinoglio, G.; Bianchi, P.P.; Marano, A.; Priora, F.; Lenti, L.M.; Ravazzoni, F.; Petz, W.; Borin, S.; Ribero, D.; Formisano, G.; et al. Robotic Versus Laparoscopic Right Colectomy with Complete Mesocolic Excision for the Treatment of Colon Cancer: Perioperative Outcomes and 5-Year Survival in a Consecutive Series of 202 Patients. Ann. Surg. Oncol. 2018, 25, 3580–3586. [Google Scholar] [CrossRef]
- Solaini, L.; Bazzocchi, F.; Cavaliere, D.; Avanzolini, A.; Cucchetti, A.; Ercolani, G. Robotic versus laparoscopic right colectomy: An updated systematic review and meta-analysis. Surg. Endosc. 2018, 32, 1104–1110. [Google Scholar] [CrossRef]
- Dohrn, N.; Klein, M.F.; Gögenur, I. Robotic versus laparoscopic right colectomy for colon cancer: A nationwide cohort study. Int. J. Colorectal Dis. 2021, 36, 2147–2158. [Google Scholar] [CrossRef]
- Park, J.S.; Choi, G.-S.; Park, S.Y.; Kim, H.J.; Ryuk, J.P. Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br. J. Surg. 2012, 99, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Rondelli, F.; Balzarotti, R.; Villa, F.; Guerra, A.; Avenia, N.; Mariani, E.; Bugiantella, W. Is robot-assisted laparoscopic right colectomy more effective than the conventional laparoscopic procedure? A meta-analysis of short-term outcomes. Int. J. Surg. 2015, 18, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ferri, V.; Quijano, Y.; Nuñez, J.; Caruso, R.; Duran, H.; Diaz, E.; Fabra, I.; Malave, L.; Isernia, R.; d’Ovidio, A.; et al. Robotic-assisted right colectomy versus laparoscopic approach: Case-matched study and cost-effectiveness analysis. J. Robot. Surg. 2021, 15, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Migliore, M.; Giuffrida, M.C.; Marano, A.; Pellegrino, L.; Giraudo, G.; Barili, F.; Borghi, F. Robotic versus laparoscopic right colectomy within a systematic ERAS protocol: A propensity-weighted analysis. Updates Surg. 2021, 73, 1057–1064. [Google Scholar] [CrossRef]
- Yozgatli, T.K.; Aytac, E.; Ozben, V.; Bayram, O.; Gurbuz, B.; Baca, B.; Balik, E.; Hamzaoglu, I.; Karahasanoglu, T.; Bugra, D. Robotic Complete Mesocolic Excision Versus Conventional Laparoscopic Hemicolectomy for Right-Sided Colon Cancer. J. Laparoendosc. Adv. Surg. Tech. Part A 2019, 29, 671–676. [Google Scholar] [CrossRef]
- Merola, G.; Sciuto, A.; Pirozzi, F.; Andreuccetti, J.; Pignata, G.; Corcione, F.; Milone, M.; de Palma, G.D.; Castaldo, R.; Pecchia, L.; et al. Is robotic right colectomy economically sustainable? A multicentre retrospective comparative study and cost analysis. Surg. Endosc. 2020, 34, 4041–4047. [Google Scholar] [CrossRef]
- Hannan, E.; Feeney, G.; Ullah, M.F.; Ryan, C.; McNamara, E.; Waldron, D.; Condon, E.; Coffey, J.C.; Peirce, C. Robotic versus laparoscopic right hemicolectomy: A case-matched study. J. Robot. Surg. 2021. [Google Scholar] [CrossRef]
- Tagliabue, F.; Burati, M.; Chiarelli, M.; Fumagalli, L.; Guttadauro, A.; Arborio, E.; de Simone, M.; Cioffi, U. Robotic vs laparoscopic right colectomy—The burden of age and comorbidity in perioperative outcomes: An observational study. World J. Gastrointest. Surg. 2020, 12, 287–297. [Google Scholar] [CrossRef]
- Widmar, M.; Keskin, M.; Beltran, P.; Nash, G.M.; Guillem, J.G.; Temple, L.K.; Paty, P.B.; Weiser, M.R.; Garcia-Aguilar, J. Incisional hernias after laparoscopic and robotic right colectomy. Hernia J. Hernias Abdom. Wall Surg. 2016, 20, 723–728. [Google Scholar] [CrossRef]
- Ngu, J.C.-Y.; Ng, Y.Y.-R. Robotics confers an advantage in right hemicolectomy with intracorporeal anastomosis when matched against conventional laparoscopy. J. Robot. Surg. 2018, 12, 647–653. [Google Scholar] [CrossRef]
- Sorgato, N.; Mammano, E.; Contardo, T.; Vittadello, F.; Sarzo, G.; Morpurgo, E. Right colectomy with intracorporeal anastomosis for cancer: A prospective comparison between robotics and laparoscopy. J. Robot. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Gerbaud, F.; Valverde, A.; Danoussou, D.; Goasguen, N.; Oberlin, O.; Lupinacci, R.M. Experience with Transitioning From Laparoscopic to Robotic Right Colectomy. JSLS J. Soc. Laparoendosc. Surg. 2019, 23, e2019.00044. [Google Scholar] [CrossRef] [PubMed]
- Mégevand, J.L.; Amboldi, M.; Lillo, E.; Lenisa, L.; Ganio, E.; Ambrosi, A.; Rusconi, A. Right colectomy: Consecutive 100 patients treated with laparoscopic and robotic technique for malignancy. Cumulative experience in a single centre. Updates Surg. 2019, 71, 151–156. [Google Scholar] [CrossRef]
- Trastulli, S.; Coratti, A.; Guarino, S.; Piagnerelli, R.; Annecchiarico, M.; Coratti, F.; Di Marino, M.; Ricci, F.; Desiderio, J.; Cirocchi, R.; et al. Robotic right colectomy with intracorporeal anastomosis compared with laparoscopic right colectomy with extracorporeal and intracorporeal anastomosis: A retrospective multicentre study. Surg. Endosc. 2015, 29, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Costa, G.; Ferraro, V.; de Rosa, M.; Rondelli, F.; Bugiantella, W. Robotic or three-dimensional (3D) laparoscopy for right colectomy with complete mesocolic excision (CME) and intracorporeal anastomosis? A propensity score-matching study comparison. Surg. Endosc. 2021, 35, 2039–2048. [Google Scholar] [CrossRef]
- de’Angelis, N.; Lizzi, V.; Azoulay, D.; Brunetti, F. Robotic Versus Laparoscopic Right Colectomy for Colon Cancer: Analysis of the Initial Simultaneous Learning Curve of a Surgical Fellow. J. Laparoendosc. Adv. Surg. Tech. Part A 2016, 26, 882–892. [Google Scholar] [CrossRef]
- Deutsch, G.B.; Sathyanarayana, S.A.; Gunabushanam, V.; Mishra, N.; Rubach, E.; Zemon, H.; Klein, J.D.S.; Denoto, G. Robotic vs. laparoscopic colorectal surgery: An institutional experience. Surg. Endosc. 2012, 26, 956–963. [Google Scholar] [CrossRef]
- Haskins, I.N.; Ju, T.; Skancke, M.; Kuang, X.; Amdur, R.L.; Brody, F.; Obias, V.; Agarwal, S. Right Colon Resection for Colon Cancer: Does Surgical Approach Matter? J. Laparoendosc. Adv. Surg. Tech. Part A 2018, 28, 1202–1206. [Google Scholar] [CrossRef]
- Guerrieri, M.; Campagnacci, R.; Sperti, P.; Belfiori, G.; Gesuita, R.; Ghiselli, R. Totally robotic vs 3D laparoscopic colectomy: A single centers preliminary experience. World J. Gastroenterol. 2015, 21, 13152–13159. [Google Scholar] [CrossRef]
- Rawlings, A.L.; Woodland, J.H.; Vegunta, R.K.; Crawford, D.L. Robotic versus laparoscopic colectomy. Surg. Endosc. 2007, 21, 1701–1708. [Google Scholar] [CrossRef]
- DeSouza, A.L.; Prasad, L.M.; Park, J.J.; Marecik, S.J.; Blumetti, J.; Abcarian, H. Robotic assistance in right hemicolectomy: Is there a role? Dis. Colon Rectum 2010, 53, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Casillas, M.A.; Leichtle, S.W.; Wahl, W.L.; Lampman, R.M.; Welch, K.B.; Wellock, T.; Madden, E.B.; Cleary, R.K. Improved perioperative and short-term outcomes of robotic versus conventional laparoscopic colorectal operations. Am. J. Surg. 2014, 208, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Park, Y.A.; Baik, S.H.; Sohn, S.-K.; Lee, K.Y. A Comparison of Open, Laparoscopic, and Robotic Surgery in the Treatment of Right-sided Colon Cancer. Surg. Laparosc. Endosc. Percutaneous Tech. 2016, 26, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Dolejs, S.C.; Waters, J.A.; Ceppa, E.P.; Zarzaur, B.L. Laparoscopic versus robotic colectomy: A national surgical quality improvement project analysis. Surg. Endosc. 2017, 31, 2387–2396. [Google Scholar] [CrossRef]
- Lujan, H.J.; Plasencia, G.; Rivera, B.X.; Molano, A.; Fagenson, A.; Jane, L.A.; Holguin, D. Advantages of Robotic Right Colectomy with Intracorporeal Anastomosis. Surg. Laparosc. Endosc. Percutaneous Tech. 2018, 28, 36–41. [Google Scholar] [CrossRef]
- Ahmadi, N.; Mor, I.; Warner, R. Comparison of outcome and costs of robotic and laparoscopic right hemicolectomies. J. Robot. Surg. 2022, 16, 429–436. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Little, J.P. Consistency of ASA grading. Anaesthesia 1995, 50, 658–659. [Google Scholar]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Jadad, A.R.; Moore, R.; Carroll, D.; Jenkinson, C.; Reynolds, D.M.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2 (Updated February 2021); Cochrane: London, UK, 2021. [Google Scholar]
- Trastulli, S.; Cirocchi, R.; Desiderio, J.; Coratti, A.; Guarino, S.; Renzi, C.; Corsi, A.; Boselli, C.; Santoro, A.; Minelli, L.; et al. Robotic versus Laparoscopic Approach in Colonic Resections for Cancer and Benign Diseases: Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0134062. [Google Scholar] [CrossRef]
- Genova, P.; Pantuso, G.; Cipolla, C.; Latteri, M.A.; Abdalla, S.; Paquet, J.-C.; Brunetti, F.; de’Angelis, N.; Di Saverio, S. Laparoscopic versus robotic right colectomy with extra-corporeal or intra-corporeal anastomosis: A systematic review and meta-analysis. Langenbeck’s Arch. Surg. 2021, 406, 1317–1339. [Google Scholar] [CrossRef] [PubMed]
- Lorenzon, L.; Bini, F.; Balducci, G.; Ferri, M.; Salvi, P.F.; Marinozzi, F. Laparoscopic versus robotic-assisted colectomy and rectal resection: A systematic review and meta-analysis. Int. J. Colorectal Dis. 2016, 31, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Petrucciani, N.; Sirimarco, D.; Nigri, G.R.; Magistri, P.; La Torre, M.; Aurello, P.; D’Angelo, F.; Ramacciato, G. Robotic right colectomy: A worthwhile procedure? Results of a meta-analysis of trials comparing robotic versus laparoscopic right colectomy. J. Minimal Access Surg. 2015, 11, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, F.; Zhang, P.; Shi, C.; Zou, Y.; Qin, H.; Ma, Y. Robot-assisted versus conventional laparoscopic surgery for colorectal disease, focusing on rectal cancer: A meta-analysis. Ann. Surg. Oncol. 2012, 19, 3727–3736. [Google Scholar] [CrossRef]
- Xu, H.; Li, J.; Sun, Y.; Li, Z.; Zhen, Y.; Wang, B.; Xu, Z. Robotic versus laparoscopic right colectomy: A meta-analysis. World J. Surg. Oncol. 2014, 12, 274. [Google Scholar] [CrossRef][Green Version]
- Ma, S.; Chen, Y.; Chen, Y.; Guo, T.; Yang, X.; Lu, Y.; Tian, J.; Cai, H. Short-term outcomes of robotic-assisted right colectomy compared with laparoscopic surgery: A systematic review and meta-analysis. Asian J. Surg. 2019, 42, 589–598. [Google Scholar] [CrossRef]
- Shaw, D.D.; Wright, M.; Taylor, L.; Bertelson, N.L.; Shashidharan, M.; Menon, P.; Menon, V.; Wood, S.; Ternent, C.A. Robotic Colorectal Surgery Learning Curve and Case Complexity. J. Laparoendosc. Adv. Surg. Tech. Part A 2018, 28, 1163–1168. [Google Scholar] [CrossRef]
- Nasseri, Y.; Stettler, I.; Shen, W.; Zhu, R.; Alizadeh, A.; Lee, A.; Cohen, J.; Barnajian, M. Learning curve in robotic colorectal surgery. J. Robot. Surg. 2021, 15, 489–495. [Google Scholar] [CrossRef]
- Cleary, R.K.; Kassir, A.; Johnson, C.S.; Bastawrous, A.L.; Soliman, M.K.; Marx, D.S.; Giordano, L.; Reidy, T.J.; Parra-Davila, E.; Obias, V.J.; et al. Intracorporeal versus extracorporeal anastomosis for minimally invasive right colectomy: A multi-center propensity score-matched comparison of outcomes. PLoS ONE 2018, 13, e0206277. [Google Scholar] [CrossRef] [PubMed]
| First Author | Study Type | Institution City, Country | Study Period | Study Design | LRC (n) | RRC (n) | Length of Follow-Up (d) | Quality Assessment | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Yozgatli | Multicenter | Istanbul, Turkey | 2015–2017 | Retrospective | 61 | 35 | 480/450 | 15 | [15] |
| Ferri | Single | Madrid, Spain | 2013–2017 | Retrospective | 35 | 35 | 1825/1825 | 15 | [13] |
| Park | Single | Daegu, South Korea | 2009–2011 | Randomized | 35 | 35 | 1825/1825 | 4 | [7,11] |
| Spinoglio | Single | Milan, Italy | 2005–2015 | Retrospective | 100 | 100 | 1825/1825 | 19 | [8] |
| Migliore | Single | Cuneo, Italy | 2010–2018 | Retrospective | 170 | 46 | 30/30 | 16 | [14] |
| Hannan | Single | Limerick, Ireland | 2017–2020 | Retrospective | 35 | 35 | 30/30 | 13 | [17] |
| Tagliabue | Single | Lecco, Italy | 2014–2019 | Retrospective | 68 | 55 | 180/180 | 17 | [18] |
| Dohrn | Multicenter | Herlev, Denmark | 2015–2018 | Retrospective | 3621 | 381 | 90/90 | 19 | [10] |
| Merola | Multicenter | Naples, Italy | 2012–2017 | Retrospective | 94 | 94 | 180/180 | 18 | [16] |
| Ahmadi | Multicenter | Tweed Heads, Australia | 2015–2018 | Retrospective | 42 | 59 | n/a | 15 | [36] |
| Ngu | Single | Singapore, Singapore | 2015–2017 | Retrospective | 16 | 16 | 30/30 | 14 | [20] |
| Sorgato | Multicenter | Padoa, Italy | 2018–2019 | Retrospective | 40 | 48 | 30/30 | 16 | [21] |
| Widmar | Single | New York, USA | 2009–2014 | Retrospective | 207 | 69 | 500/500 | 16 | [19] |
| Gerbaud | Single | Paris, France | 2013–2019 | Retrospective | 59 | 42 | n/a | 15 | [22] |
| Mégevand | Single | Milan, Italy | 2010–2015 | Retrospective | 50 | 50 | 30/30 | 17 | [23] |
| Trastulli | Multicenter | Terni, Italy | 2005–2014 | Retrospective | 134 | 102 | 30/30 | 15 | [24] |
| Ceccarelli | Single | Foligno, Italy | 2014–2019 | Retrospective | 29 | 26 | 30/30 | 15 | [25] |
| De Angelis | Single | Paris, France | 2012–2015 | Retrospective | 50 | 30 | 90/90 | 17 | [26] |
| Deutsch | Single | Roslyn, USA | 2004–2009 | Retrospective | 47 | 18 | n/a | 16 | [27] |
| Haskins | Multicenter | Washington, USA | 2012–2014 | Retrospective | 2405 | 89 | 30/30 | 15 | [28] |
| Rawlings | Single | Peoria, USA | 2002–2005 | Prospective | 15 | 17 | n/a | 15 | [30] |
| deSouza | Single | Chicago, USA | 2005–2009 | Retrospective | 135 | 40 | n/a | 16 | [31] |
| Casillas | Single | Ann Arbor, USA | 2005–2012 | Prospective | 110 | 52 | n/a | 16 | [32] |
| Kang | Single | Seoul, South Korea | 2007–2011 | Retrospective | 43 | 20 | 1200/1200 | 18 | [33] |
| Dolejs | Multicenter | Indianapolis, USA | 2012–2014 | Retrospective | 6521 | 259 | n/a | 16 | [34] |
| Lujan | Single | Jackson, USA | 2009–2015 | Retrospective | 135 | 89 | n/a | 17 | [35] |
| Author | Year | Age Mean (±SD) | Sex (n) | BMI (kg/m2) | ASA ≥2 | Neoplasm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LRC | RRC | LRC | RRC | LRC | RRC | LRC | RRC | LRC | RRC | ||||||||
| Mean | SD | Mean | SD | f | m | Total | f | m | Total | n (%) | n (%) | n (%) | n (%) | ||||
| Yozgatli [15] | 2019 | 65 | 13 | 65 | 13 | 30 | 31 | 61 | 15 | 20 | 35 | 27 | 29 | n/a | n/a | 61(100) | 35(100) |
| Ferri [13] | 2021 | 68 | n/a | 70 | n/a | 15 | 20 | 35 | 12 | 23 | 35 | 25 | 23 | 31(89) | 27(77) | 32(91) | 32(91) |
| Park [7,11] | 2019 | 66.5 | 10.5 | 62.8 | 11.4 | 19 | 16 | 35 | 21 | 14 | 35 | 23.8 | 24.4 | 14(40) | 20(57) | 35(100) | 35(100) |
| Spinoglio [8] | 2018 | 71.2 | 10.6 | 71.2 | 10.2 | 54 | 46 | 100 | 44 | 56 | 100 | 25.8 | 25.1 | 91(91) | 88(88) | 100(100) | 100(100) |
| Migliore [14] | 2021 | 71.92 | 10.1 | 68.7 | 9.2 | 74 | 96 | 170 | 24 | 22 | 46 | 25.52 | 26.05 | 153(90) | 35(76) | 163(96) | 43(93) |
| Hannan [17] | 2021 | 69.7 | n/a | 66.5 | n/a | 17 | 18 | 35 | 17 | 18 | 35 | n/a | n/a | 21(60) | 28(80) | 28(80) | 20(57) |
| Tagliabue [18] | 2020 | 72 | n/a | 72 | n/a | 28 | 40 | 68 | 23 | 32 | 55 | 24.81 | 24.31 | 56(82) | 42(76) | 55(81) | 41(75) |
| Dohrn [10] | 2021 | 73 | n/a | 73 | n/a | 2000 | 1621 | 3621 | 196 | 185 | 381 | 25.7 | 25.6 | 2956(82) | 295(77) | 3616(100) | 381(100) |
| Merola [16] | 2020 | 72.09 | 9.5 | 69.4 | 10.3 | 33 | 61 | 94 | 34 | 60 | 94 | 27.97 | 26.94 | 83(88) | 87(93) | 94(100) | 94(100) |
| Ahmadi [36] | 2021 | 75 | 12 | 75 | 13 | 22 | 20 | 42 | 29 | 30 | 59 | 27 | 27 | n/a | n/a | 35(83) | 43(73) |
| Ngu [20] | 2018 | 69.6 | 9.6 | 68.6 | 10.9 | 10 | 6 | 16 | 6 | 10 | 16 | 24.7 | 23.7 | 16(100) | 16(100) | 16(100) | 15(94) |
| Sorgato [21] | 2021 | 68 | 10 | 71 | 12.2 | 12 | 28 | 40 | 21 | 27 | 48 | 26.6 | 25.6 | 37(93) | 46(96) | 38(95) | 41(85) |
| Widmar [19] | 2016 | 64 | n/a | 66 | n/a | 122 | 85 | 207 | 36 | 33 | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
| Gerbaud [22] | 2019 | 72 | 8.6 | 67 | 8.6 | 28 | 31 | 59 | 21 | 21 | 42 | 24 | 26 | n/a | n/a | 37(63) | 30(71) |
| Mégevand [23] | 2019 | 69.6 | n/a | 70.3 | n/a | 26 | 24 | 50 | 22 | 28 | 50 | 25.25 | 26.2 | 43(86) | 44(88) | 35(70) | 41(82) |
| Trastulli [24] | 2015 | 71.01 | n/a | 71.2 | 11.6 | 57 | 77 | 134 | 46 | 56 | 102 | 25.76 | 25.6 | 122(91) | 94(92) | 113(84) | 81(79) |
| Ceccarelli [25] | 2021 | 75 | 11.7 | 69.1 | 9.4 | 14 | 15 | 29 | 6 | 20 | 26 | 24.2 | 24.4 | 20(69) | 24(92) | 24(83) | 24(92) |
| De Angelis [26] | 2016 | 71.1 | 12.9 | 71 | 8.5 | 31 | 19 | 50 | 15 | 15 | 30 | 25.26 | 26.43 | 46(92) | 30(100) | 50(100) | 30(100) |
| Deutsch [27] | 2012 | 70.8 | 14.6 | 65.2 | 12 | 22 | 25 | 47 | 6 | 12 | 18 | 28 | 25 | 24(96) | 4(22) | 28(60) | 18(100) |
| Haskins [28] | 2018 | 68.3 | 12.6 | 68.9 | 11.8 | 1279 | 1126 | 2405 | 40 | 49 | 89 | 28.5 | 29.3 | 2363(98) | 89(100) | 2405(100) | 89(100) |
| Rawlings [30] | 2007 | 63.1 | 17.5 | 64.6 | 11.7 | 9 | 6 | 15 | 9 | 8 | 17 | 28.3 | 25.7 | n/a | n/a | 6(40) | 2(12) |
| deSouza [31] | 2010 | 65.32 | 18.7 | 71.4 | 14.1 | 73 | 62 | 135 | 18 | 22 | 40 | 26.57 | 27.33 | 118(87) | 35(88) | 66(49) | 18(54) |
| Casillas [32] | 2014 | 71 | 12 | 65 | 12 | 41 | 69 | 110 | 27 | 25 | 52 | 27 | 26.9 | 108(98) | 51(98) | 110(100) | 52(100) |
| Kang [33] | 2016 | 65.7 | 13.2 | 66 | 9.6 | 21 | 22 | 43 | 11 | 9 | 20 | 23 | 23.5 | 22(51) | 9(45) | 43(100) | 20(100) |
| Dolejs [34] | 2017 | n/a | n/a | n/a | n/a | 2913 | 3608 | 6521 | 133 | 126 | 259 | n/a | n/a | n/a | n/a | 3247(50) | 116(45) |
| Lujan [35] | 2018 | 72.6 | 11.4 | 70.9 | 9.6 | 74 | 61 | 135 | 41 | 48 | 89 | 27.1 | 27.8 | 130(96) | 88(99) | n/a | n/a |
| Variable | LRC | RRC | OR/MD | p-Value | I2 | References |
|---|---|---|---|---|---|---|
| Age, years | 70.27 ± 3.00 | 68.79 ± 2.90 | 1.48 (0.11–2.84) | 0.03 | 47% | [3,7,8,11,15,16,20,21,22,25,26,27,28,30,31,32,35,36] |
| Neoplasm, n | 7539/11,017 | 946/1229 | 1.22 (0.91–1.64) | 0.17 | 27% | [10,13,14,17,18,20,21,22,23,24,25,27,30,31,34,36] |
| Operative time (min) | 165.31 ± 43.08 | 207.38 ± 189.13 | −42.01 (−51.06–32.96) | <0.001 | 89% | [7,8,11,13,14,16,18,19,20,21,22,23,24,25,26,27,28,30,31,33,34,36] |
| Blood loss (mL) | 63.57 ± 35.21 | 53.62 ± 34.02 | 10.03 (1.61–18.45) | 0.02 | 65% | [7,10,11,15,22,24,26,27,30,31,33,35] |
| Conversion, n | 1155/11629 | 94/1534 | 1.53 (1.08–2.17) | 0.02 | 14% | [8,10,13,14,16,17,18,19,22,23,24,26,27,30,31,32,33,34,35] |
| Intracorporeal Anastomosis, n | 329/4308 | 468/860 | 0.03 (0.00–0.20) | <0.001 | 90% | [7,10,11,13,19,21,22,35,36,43] |
| Time to first flatus (d) | 2.46 ± 2.14 | 2.30 ± 2.08 | 0.15 (−0.18–0.48) | 0.38 | 93% | [7,8,11,13,14,15,16,18,20,23,24,26,27,33] |
| Mortality, n | 126/13,388 | 18/1198 | 0.66 (0.41–1.06) | 0.08 | 0% | [8,10,14,16,26,27,28,31,32,34,35] |
| Overall Morbidity, n | 3093/14,242 | 464/1825 | 1.01 (0.86–1.19) | 0.88 | 22% | [7,8,10,11,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,31,32,33,34,35,36] |
| Non-surgical complications, n | 693/13,515 | 119/1406 | 0.93 (0.70–1.23) | 0.6 | 9% | [8,10,15,18,21,22,23,24,25,26,27,28,31,32,33,34,36] |
| Incisional hernia, n | 53/389 | 12/176 | 1.51 (0.78–2.95) | 0.22 | 0% | [19,27,35] |
| Postoperative hemorrhage, n | 573/10,013 | 55/1178 | 0.88 (0.64–1.21) | 0.43 | 0% | [7,8,11,15,16,18,21,22,23,24,25,27,28,30,31,33,34,35,36] |
| Postoperative ileus, n | 962/10,257 | 70/1209 | 1.30 (0.91–1.87) | 0.14 | 18% | [7,8,11,15,18,19,21,22,23,24,26,27,28,30,31,32,33,34,35,36] |
| Wound infection, n | 618/10,074 | 60/1076 | 1.15 (0.84–1.57) | 0.39 | 0% | [7,8,11,15,17,18,19,21,22,24,25,28,31,32,34,35] |
| Anastomotic leakage, n | 273/11,552 | 34/1557 | 1.02 (0.69–1.50) | 0.94 | 0% | [7,8,10,11,14,15,16,17,18,19,21,22,23,24,26,27,30,32,34,35] |
| Abdominal abscess, n | 13/966 | 10/526 | 0.75 (0.34–1.64) | 0.47 | 0% | [7,11,14,15,18,19,20,21,23,24,26,31] |
| Hospital stay (d) | 6.15 ± 31.77 | 5.31 ± 1.65 | 0.84 (0.29–1.38) | 0.003 | 87% | [7,8,11,14,16,18,20,21,22,23,24,25,26,27,28,30,31,33,34,35] |
| Variable | LRC | RRC | OR/MD | p-Value | I2 | References |
|---|---|---|---|---|---|---|
| Lymph nodes harvested | 22.97 ± 5.94 | 23.82 ± 6.76 | −0.85 (−2.19–0.48) | 0.21 | 75% | [7,8,10,11,14,15,16,18,20,21,22,23,25,28,33,35,36,43] |
| Disease free survival (5 years) | 178/213 | 162/190 | 0.87 (0.50–1.51) | 0.62 | 0% | [7,8,11,13,33] |
| Overall survival (5 years) | 172/213 | 157/190 | 0.90 (0.54–1.52) | 0.7 | 0% | [7,8,11,13,33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tschann, P.; Szeverinski, P.; Weigl, M.P.; Rauch, S.; Lechner, D.; Adler, S.; Girotti, P.N.C.; Clemens, P.; Tschann, V.; Presl, J.; et al. Short- and Long-Term Outcome of Laparoscopic- versus Robotic-Assisted Right Colectomy: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 2387. https://doi.org/10.3390/jcm11092387
Tschann P, Szeverinski P, Weigl MP, Rauch S, Lechner D, Adler S, Girotti PNC, Clemens P, Tschann V, Presl J, et al. Short- and Long-Term Outcome of Laparoscopic- versus Robotic-Assisted Right Colectomy: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(9):2387. https://doi.org/10.3390/jcm11092387
Chicago/Turabian StyleTschann, Peter, Philipp Szeverinski, Markus P. Weigl, Stephanie Rauch, Daniel Lechner, Stephanie Adler, Paolo N. C. Girotti, Patrick Clemens, Veronika Tschann, Jaroslav Presl, and et al. 2022. "Short- and Long-Term Outcome of Laparoscopic- versus Robotic-Assisted Right Colectomy: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 9: 2387. https://doi.org/10.3390/jcm11092387
APA StyleTschann, P., Szeverinski, P., Weigl, M. P., Rauch, S., Lechner, D., Adler, S., Girotti, P. N. C., Clemens, P., Tschann, V., Presl, J., Schredl, P., Mittermair, C., Jäger, T., Emmanuel, K., & Königsrainer, I. (2022). Short- and Long-Term Outcome of Laparoscopic- versus Robotic-Assisted Right Colectomy: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(9), 2387. https://doi.org/10.3390/jcm11092387






