Abstract
In this study, we aimed to assess clinicopathological factors affecting early and late recurrences in patients with operable breast cancer. Patients with early (≤5 years) and late (>5 years) recurrences were assessed. Prognostic factors for disease-free survival (DFS) were also evaluated in patients with recurrence. A total of 854 patients were included. There were 432 and 205 patients in the early and late recurrence groups, respectively. In multivariate analyses, HER2+ disease, lymph node metastasis, lymphovascular invasion (LVI), and high tumor grade were associated with increased risk of early recurrence, while HER2+ disease and LVI were associated with decreased risk of late recurrence. In multivariate analyses, presence of HER2+ disease and triple-negative breast cancer (TNBC) were poor prognostic factors for DFS in patients with early recurrence. Presence of LVI and perineural invasion (PNI) were poor prognostic factors for DFS in patients with late recurrence. Molecular subtypes and LVI were effective on the early and late recurrences. However, lymph node positivity and grade were only associated with the early recurrence. After 5 years, LVI and PNI were the prognostic factors for DFS.
1. Introduction
Breast cancer is the most common cancer in women [1]. According to the Surveillance, Epidemiology, and End Results (SEER) database, the 5-year survival rate is 90.3% [2]. The most significant problem in patients with breast cancer treated with surgery is the recurrence of the disease after treatment. Recurrence is usually seen in the first 5 years, particularly within the first and second years [3]. The annual recurrence risk for the first 5 years was reported as 10.4% [3]. It has been shown that the recurrence and mortality rates decreased until 10 years and continued at a fixed rate thereafter [3].
When the patient groups are evaluated separately, there are conflicting results in the literature regarding recurrence in the first 5 years and after 5 years. In one study, estrogen receptor (ER)-negative patients were more likely to have a recurrence in the first 5 years and ER-positive patients were more likely to have a recurrence after 5 years [3]. In another study, progesterone receptor (PR)-positivity and lymph node involvement were associated with late recurrence [4]. ER-positivity was associated with late recurrence after 10 years [5]. Large and lymph node-positive tumors had a higher recurrence rate in the first 5 years while ER and PR-positive/human epidermal growth factor receptor 2 (HER2)-negative tumors were the risk factors for late recurrence after 5 years [6]. Considering the annual incidence of recurrence, the increase in follow-up interval after 5 years may lead to delayed diagnosis of recurrences in patients with a high risk of late recurrence. Closer follow up may be needed in a subgroup of patients with a high risk of late recurrence after 5 years.
In this study, we aimed to assess clinicopathological factors associated with early and late recurrences in a large group of patients with early breast cancer.
2. Materials and Methods
This retrospective study was conducted in a tertiary cancer center in Turkey. Adult patients diagnosed with early breast cancer from inception to January 2018 were included in the study and categorized according to the presence and time of recurrence; early recurrence (≤5 years), late recurrence (>5 years). Patients treated with neoadjuvant therapy and those with bilateral breast cancer were excluded. Radiological and pathological recurrences were accepted as cancer recurrence.
All clinical (age, menopausal status, obesity, date of diagnosis, surgery and recurrence, recurrence sites) and pathological (tumor size, nodal involvement, lymphovascular invasion (LVI), perineural invasion (PNI), tumor grade, type of surgery, adjuvant treatment) data were extracted from the electronic hospital records of the patients.
The median with interquartile range (IQR) and percentages were used to define the continuous and categorical variables, respectively. In univariate analysis, the chi-square test was used to determine the risk factors for early and late recurrence. Variables found to be statistically significant in univariate analysis were analyzed by using binary logistic regression models and adjusted odds ratio (aOR) was calculated. Disease-free survival (DFS) was calculated from surgery to disease recurrence. Prognostic factors for DFS in each recurrence interval (early and late) were evaluated by using Kaplan–Meier estimates and survival curves were compared by using the log-rank test. Variables found to be statistically significant in univariate analyses were analyzed by using Cox’s regression models. A p-value less than 0.05 was considered statistically significant for all statistical analyses. All analyses were done by using the SPSS 28.0 for Mac (IBM Corp., Armonk, NY, USA) and RStudio (RStudio, Boston, MA, USA).
3. Results
3.1. Baseline Characteristics
A total of 854 patients with early breast cancer were included in this study. The median age was 45 (IQR: 39–53) years. The majority of patients had ductal histology (83.4%) and hormone-receptor-positive disease (70.5%). The node-negative disease was observed in 37.5% of all patients. A total of 100 (11.7%) did not receive adjuvant chemotherapy. Median follow-up was 179 months (95% Confidence Interval (CI): 171–186). There were 637 and 217 patients with and without recurrence, respectively. A total of 68% of the patients (432 out of 637) in the recurrence group had early recurrence (≤5 years), while the remaining (n = 205) had late recurrence (>5 years). About 20% of all patients had a locoregional recurrence. Baseline characteristics of patients are shown in Table 1.

Table 1.
Baseline characteristics of all patients.
3.2. Risk Factors for Early and Late Recurrences
In univariate analyses, younger patients (≤35 years old) (OR: 1.61, 95% CI: 1.11–2.33, p = 0.012), patients with ductal histology (OR: 1.48, 95% CI: 1.01–2.18, p = 0.049), HER2+ disease (OR: 2.69, 95% CI: 1.82–3.99, p < 0.001), triple-negative breast cancer (TNBC) (OR: 1.53, 95% CI: 1.01–2.32, p = 0.045), larger tumor (T3–4) (OR: 1.73, 95% CI: 1.20–2.49, p = 0.003), lymph node metastasis (OR: 2.50, 95% CI: 1.87–3.34, p < 0.001), LVI (OR: 3.77, 95% CI: 2.69–5.29, p < 0.001), PNI (OR: 2.50, 95% CI: 1.43–4.39, p = 0.001), high-grade tumor (grade 2 or 3) (OR: 3.09, 95% CI: 1.87–5.12, p < 0.001), and breast-conserving surgery (OR: 1.44, 95% CI: 1.03–2.01, p = 0.030) had a higher risk of early recurrence. On the other hand, late recurrence risk was lower in patients with HER2+ disease (OR: 0.47, 95% CI: 0.28–0.77, p = 0.003) and with LVI (OR: 0.43, 95% CI: 0.28–0.66, p < 0.001). Obesity and menopausal status were not associated with recurrence period (Table 2).

Table 2.
Univariate analysis of risk factors for early and late recurrence.
In multivariate analyses, HER2+ disease (aOR: 1.74, 95% CI: 1.10–2.75, p = 0.017), lymph node metastasis (aOR: 1.66, 95% CI: 1.12–2.47, p = 0.011), LVI (aOR: 2.40, 95% CI: 1.59–3.63, p < 0.001), and high tumor grade (aOR: 2.46, 95% CI: 1.33–4.52, p = 0.004) were associated with increased risk of early recurrence, while HER2+ disease (aOR: 0.49, 95% CI: 0.29–0.84, p = 0.010) and LVI (aOR: 0.49, 95% CI: 0.31–0.76, p = 0.001) were associated with decreased risk of late recurrence after adjusting for confounding variables (Table 3).

Table 3.
Multivariate analysis of risk factors for early and late recurrence.
3.3. Prognostic Factors for Disease-Free Survival According to Recurrence Interval
In the early recurrence group (≤5 years), the median DFS was longer in patients with hormone-receptor-positive/HER2-negative breast cancer than in patients with HER2+ disease and TNBC (31.8, 22.1, and 19.4 months in the hormone-receptor-positive/HER2-negative, HER2+, and TNBC groups, respectively; p < 0.001). In addition, presence of LVI (22.9 vs. 29.5 months, p < 0.001) was associated with shorter DFS in patients with early recurrence. In the late recurrence group (>5 years), the median DFS was shorter in patients with lymph node metastasis (102.8 vs. 103.9 months, p = 0.046), with LVI (83.7 vs. 111.7 months, p < 0.001), and with PNI (77.9 vs. 103.9 months, p = 0.046). (Table 4)

Table 4.
Univariate analysis of disease-free survival.
Survival curves for DFS in early and late recurrence groups are shown in Figure 1.
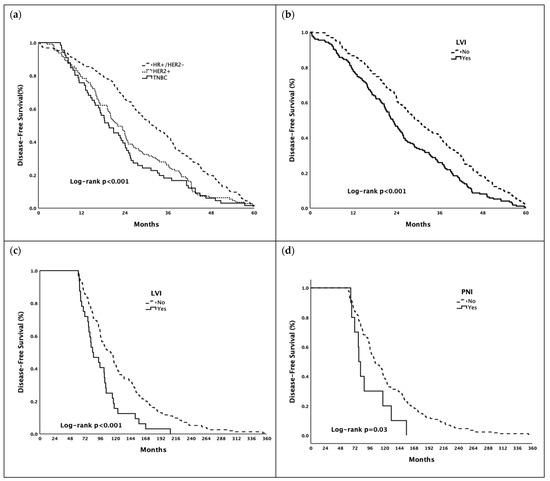
Figure 1.
Kaplan–Meier estimates of disease-free survival ((a,b) in early recurrence group, (c,d) in late recurrence group). Abbreviations: HR—Hormone Receptor; HER2—Human Epidermal Growth Factor Receptor 2; LVI—Lymphovascular Invasion; PNI—Perineural Invasion; TNBC—Triple-Negative Breast Cancer.
In multivariate analyses, the presence of HER2+ disease (HR: 1.37, 95% CI: 1.09–1.73, p = 0.007) and TNBC (HR: 1.64, 95% CI: 1.25–2.17, p < 0.001) were poor prognostic factors for DFS in patients with early recurrence (≤5 years) after adjusting for confounding variables. Presence of LVI (HR: 1.79, 95% CI: 1.19–2.69, p = 0.005) and PNI (HR: 1.99, 95% CI: 1.04–3.81, p = 0.036) were poor prognostic factors for DFS in patients with late recurrence (>5 years) after adjusting for confounding variables. (Table 5).

Table 5.
Multivariate analysis of disease-free survival.
4. Discussion
Clinical and pathological features of patients with breast cancer are easily accessible variables to define the risk of recurrence in operable patients after surgery. To the best of our knowledge, this study includes one of the largest patient numbers in the literature. In this study, we showed that HER2+ disease, lymph node involvement, presence of LVI, and higher tumor grade were the risk factors for early recurrence. On the other hand, the risk of late recurrence was lower in patients with HER2+ disease and those with LVI. When we looked at the prognostic factors, patients with HER2+ disease or TNBC and those with LVI had a poorer prognosis in the early recurrence group. Additionally, the median DFS was shorter in patients with LVI and those with PNI in the late recurrence group.
ER and/or PR positivity is one of the most important prognostic markers. They have not only prognostic but also predictive values [7]. It has been known for many years that ER positivity has a positive effect on the prognosis, especially in the early periods [8]. In ER-negative tumors, although the risk of recurrence is high in the early period, it was observed that this effect was absent in the late periods [9]. However, there are conflicting data regarding the effect of ER-positive or negative status on late prognosis. There are also publications showing that prognosis is negatively affected in patients with ER positivity after 5 years [3]. HER2 status is another prognostic and predictive marker. In a study comparing early and late recurrences, ER/PR-positive and HER2-negative patients were observed to have a higher recurrence risk after 5 years [6]. For HER2-positive tumors, the reverse situation is observed. Early recurrence has been shown to be more frequent in this patient group [10]. In our study, the risk of early recurrence was higher in patients with HER2+ disease.
The pathological grade is a prognostic factor in breast cancer [11,12]. When recurrence periods are studied separately, studies concluded that grade is effective on recurrences after 5 years [6]. Conversely, there are also studies showing that grade is effective on recurrences in the first 5 years [13,14]. In our study, it was observed that the grade was a risk factor for recurrences in the first 5 years.
LVI increases the risk of recurrence in patients with early breast cancer [15,16]. Similarly, in our study, high early and late recurrence rates were observed in patients with LVI. On the other hand, there were conflicting data in the literature on the effect of LVI on DFS and recurrence periods. A study comparing early and late recurrences stated that the patients with LVI experienced early recurrence [6]. In another study, however, there was no difference in recurrence rates before and after 10 years in patients with LVI [17]. A study assessing late recurrences in patients with early-stage breast cancer stated that DFS was shorter in the patient group with LVI in recurrences after 10 years [5]. In our study, the median DFS was observed to be shorter in patients with LVI in recurrences in the first 5 years compared to the group without LVI. Furthermore, this effect was also observed in recurrences after 5 years. Since PNI is not common in cases of breast cancer, data on recurrence and survival rates are limited in the literature. However, there are studies showing that DFS rates decrease in the presence of PNI [18]. On the other hand, there are also publications showing that PNI has no significant effect on DFS [19]. In our study, presence of PNI was associated with shorter DFS in the late recurrence group.
Survival rates decrease with increasing tumor size [20]. Studies comparing the effect of the tumor size according to recurrence period have shown that large tumor size causes early recurrences [6,20,21]. There are publications showing that tumor size affects recurrence in 5–10 years [22]. Several studies stated that tumor size and T stage do not affect DFS in late recurrences, especially after 10 years [4,5]. In our study, we demonstrated no effect of the T stage on the recurrence intervals. One of the most important prognostic markers in breast cancer is the N stage, which is determined according to local and axillary lymph node involvement. In terms of the N stage, the prognosis deteriorates as the stage progresses [23]. In terms of the recurrence period, early recurrence is more frequent in patients with lymph node involvement [6,24]. However, there are studies showing that lymph node positivity is a risk factor for late recurrences after 10 years [4,24]. In our study, it was observed that node-positive tumors were higher risk of early recurrence.
Because breast cancer is a hormone-dependent tumor, menopausal states of patients play an important role in the progression of the disease. In a study analyzing the relationship between menopausal states of patients and recurrence period, lymph node-positive premenopausal patients showed to have an earlier recurrence [25]. In another study comparing early and late recurrences, there was no statistically significant difference between premenopausal and postmenopausal patient rates [6]. In our study, there was no effect of menopausal status on the risk of early and late recurrence and DFS.
Obesity is considered a risk factor, especially since postmenopausal women are likely to have increased exposure to estrogen [26]. It has been proven by many studies that patient groups with obesity have both a high recurrence rate and a shorter DFS [27,28,29]. However, in our study, there was no effect of obesity on the risk of early and late recurrence and DFS.
Our study has several limitations due to its retrospective nature. First, because treatment options for the patients were planned at the start of the treatment according to the patient and tumor characteristics and retrospective randomization was not an option for patients, it was considered to have no statistical value to analyze and compare DFS according to recurrence periods of treatment regimens. Second, in our study, molecular subtypes were studied in three groups: hormone receptor-positive/HER2-negative, HER2-positive, and TNBC. Because the follow-up time of patients was long, the Ki-67 proliferative index could not be obtained for patients whose pathological data were old. Due to the lack of data about Ki-67, patients were grouped into three subgroups. Third, we did not evaluate local and distant recurrence risks separately. In a study of assessing the late distant recurrence risk in patients with hormone receptor-positive breast cancer showed that age, nodal involvement, tumor size and grade could be used to calculate a risk score, named as “Clinical Treatment Score Post 5 Years” [30].
5. Conclusions
Our study showed that the most frequent recurrences occurred in the first 5 years in patients with operable breast cancer. Molecular subtypes and LVI were effective on the early and late recurrences. However, lymph node positivity and grade were only associated with the early recurrence. After 5 years, LVI and PNI were the prognostic factors for DFS. Because of this reason, patients with PNI and/or LVI should be followed carefully after 5 years from the surgery. Clinical and pathological factors in our study are easily accessible to determine the prognosis and recurrence risk in patients with early breast cancer. However, prospective studies including the molecular profile of those patients might present more precise information about the risk of recurrence.
Author Contributions
Conceptualization, E.Y. and S.A.; methodology, E.Y., Ö.D., N.K. and S.A.; formal analysis, E.Y. and S.A.; investigation, E.Y., Ö.D., N.K. and S.A.; resources, E.Y., Ö.D., N.K. and S.A.; data curation, E.Y. and S.A.; writing—original draft preparation, E.Y.; writing—review and editing, Ö.D., N.K. and S.A.; visualization, Ö.D., N.K. and S.A.; supervision, Ö.D., N.K. and S.A.; project administration, E.Y. and S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Hacettepe University (GO 17/626-28).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
The data that support the findings of this study are not publicly available but are available from the corresponding author [E.Y.] upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer. J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemiology, and End Results Program Cancer Stat Facts: Female Breast Cancer. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 10 January 2022).
- Colleoni, M.; Sun, Z.; Price, K.N.; Karlsson, P.; Forbes, J.F.; Thurlimann, B.; Gianni, L.; Castiglione, M.; Gelber, R.D.; Coates, A.S.; et al. Annual Hazard Rates of Recurrence for Breast Cancer During 24 Years of Follow-Up: Results from the International Breast Cancer Study Group Trials I to V. J. Clin. Oncol. 2016, 34, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.; Osako, T.; Nishiyama, Y.; Tashima, R.; Nakano, M.; Fujisue, M.; Toyozumi, Y.; Arima, N. Evaluation of factors related to late recurrence--later than 10 years after the initial treatment--in primary breast cancer. Oncology 2013, 85, 100–110. [Google Scholar] [CrossRef]
- Grasic-Kuhar, C.; Bracko, M.; Zakotnik, B. Risk factors for late relapse and death in patients with early breast cancer. Neoplasma 2008, 55, 416–420. [Google Scholar] [PubMed]
- Wangchinda, P.; Ithimakin, S. Factors that predict recurrence later than 5 years after initial treatment in operable breast cancer. World J. Surg. Oncol. 2016, 14, 223. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; Wolff, R.A.; Koller, C.A. The MD Anderson Manual of Medical Oncology; McGraw-Hill Education: New York, NY, USA, 2011. [Google Scholar]
- Hilsenbeck, S.G.; Ravdin, P.M.; de Moor, C.A.; Chamness, G.C.; Osborne, C.K.; Clark, G.M. Time-dependence of hazard ratios for prognostic factors in primary breast cancer. Breast Cancer Res. Treat. 1998, 52, 227–237. [Google Scholar] [CrossRef]
- Hess, K.R.; Pusztai, L.; Buzdar, A.U.; Hortobagyi, G.N. Estrogen receptors and distinct patterns of breast cancer relapse. Breast Cancer Res. Treat. 2003, 78, 105–118. [Google Scholar] [CrossRef]
- Strasser-Weippl, K.; Horick, N.; Smith, I.E.; O’Shaughnessy, J.; Ejlertsen, B.; Boyle, F.; Buzdar, A.U.; Fumoleau, P.; Gradishar, W.; Martin, M.; et al. Long-term hazard of recurrence in HER2+ breast cancer patients untreated with anti-HER2 therapy. Breast Cancer Res. 2015, 17, 56. [Google Scholar] [CrossRef]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef]
- Elston, C.W. The assessment of histological differentiation in breast cancer. Aust. N. Z. J. Surg. 1984, 54, 11–15. [Google Scholar] [CrossRef]
- Yamashita, H.; Ogiya, A.; Shien, T.; Horimoto, Y.; Masuda, N.; Inao, T.; Osako, T.; Takahashi, M.; Endo, Y.; Hosoda, M.; et al. Clinicopathological factors predicting early and late distant recurrence in estrogen receptor-positive, HER2-negative breast cancer. Breast Cancer 2016, 23, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, D.J.; Lai, C.S.; Parkyn, R.F.; Walters, D.; Humeniuk, V.; Walsh, D.C. Time to breast cancer relapse predicted by primary tumour characteristics, not lymph node involvement. World J. Surg. 2014, 38, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, P.K.; Jana, D.; Mandal, P.K.; Basak, S. Effect of lymphangiogenesis and lymphovascular invasion on the survival pattern of breast cancer patients. Asian Pac. J. Cancer Prev. 2014, 15, 6287–6293. [Google Scholar] [CrossRef] [PubMed]
- Davila-Arias, C.; Ocon, O.; Fernandez, M.F.; Arrebola, J.P.; Sanchez, M.J.; Aneiros, J.; Torne, P.; Olea, N. Disease-free survival related factors in breast cancer. Med. Clin. 2014, 143, 293–299. [Google Scholar] [CrossRef]
- Takeuchi, H.; Muto, Y.; Tashiro, H. Clinicopathological characteristics of recurrence more than 10 years after surgery in patients with breast carcinoma. Anticancer Res. 2009, 29, 3445–3448. [Google Scholar]
- McCready, D.R.; Chapman, J.A.; Hanna, W.M.; Kahn, H.J.; Murray, D.; Fish, E.B.; Trudeau, M.E.; Andrulis, I.L.; Lickley, H.L. Factors affecting distant disease-free survival for primary invasive breast cancer: Use of a log-normal survival model. Ann. Surg. Oncol. 2000, 7, 416–426. [Google Scholar] [CrossRef]
- Duraker, N.; Caynak, Z.C.; Turkoz, K. Perineural invasion has no prognostic value in patients with invasive breast carcinoma. Breast 2006, 15, 629–634. [Google Scholar] [CrossRef]
- Elkin, E.B.; Hudis, C.; Begg, C.B.; Schrag, D. The effect of changes in tumor size on breast carcinoma survival in the U.S.: 1975–1999. Cancer 2005, 104, 1149–1157. [Google Scholar] [CrossRef]
- Payandeh, M.; Sadeghi, M.; Sadeghi, E. Differences in Prognostic Factors between Early and Late Recurrence Breast Cancers. Asian Pac. J. Cancer Prev. 2015, 16, 6575–6579. [Google Scholar] [CrossRef][Green Version]
- Sestak, I.; Dowsett, M.; Zabaglo, L.; Lopez-Knowles, E.; Ferree, S.; Cowens, J.W.; Cuzick, J. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J. Natl. Cancer Inst. 2013, 105, 1504–1511. [Google Scholar] [CrossRef]
- Carter, C.L.; Allen, C.; Henson, D.E. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989, 63, 181–187. [Google Scholar] [CrossRef]
- Chen, X.L.; Fan, Y.; Xu, B.H. Clinicopathological features and prognosis of HER2-negative luminal-type breast cancer patients with early and late recurrence. Chin. J. Oncol. 2016, 38, 448–453. [Google Scholar] [CrossRef]
- Demicheli, R.; Bonadonna, G.; Hrushesky, W.J.; Retsky, M.W.; Valagussa, P. Menopausal status dependence of the timing of breast cancer recurrence after surgical removal of the primary tumour. Breast Cancer Res. 2004, 6, R689–R696. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. Available online: http://www.aicr.org/assets/docs/pdf/reports/Second_Expert_Report.pdf (accessed on 14 January 2021).
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef]
- Loi, S.; Milne, R.L.; Friedlander, M.L.; McCredie, M.R.; Giles, G.G.; Hopper, J.L.; Phillips, K.A. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer epidemiology, biomarkers & prevention: A publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology 2005, 14, 1686–1691. [Google Scholar] [CrossRef]
- Kamineni, A.; Anderson, M.L.; White, E.; Taplin, S.H.; Porter, P.; Ballard-Barbash, R.; Malone, K.; Buist, D.S. Body mass index, tumor characteristics, and prognosis following diagnosis of early-stage breast cancer in a mammographically screened population. Cancer Causes Control. 2013, 24, 305–312. [Google Scholar] [CrossRef]
- Foldi, J.; O’Meara, T.; Marczyk, M.; Sanft, T.; Silber, A.; Pusztai, L. Defining Risk of Late Recurrence in Early-Stage Estrogen Receptor-Positive Breast Cancer: Clinical Versus Molecular Tools. J. Clin. Oncol. 2019, 37, 1365–1369. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).