Triple Negative Breast Cancer: An Analysis of the Subtypes and the Effects of Menopausal Status on Invasive Breast Cancer
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Histopathological Examination
2.3. Breast Cancer Subtypes and Adjuvant Therapy
2.4. Statistical Analysis
3. Results
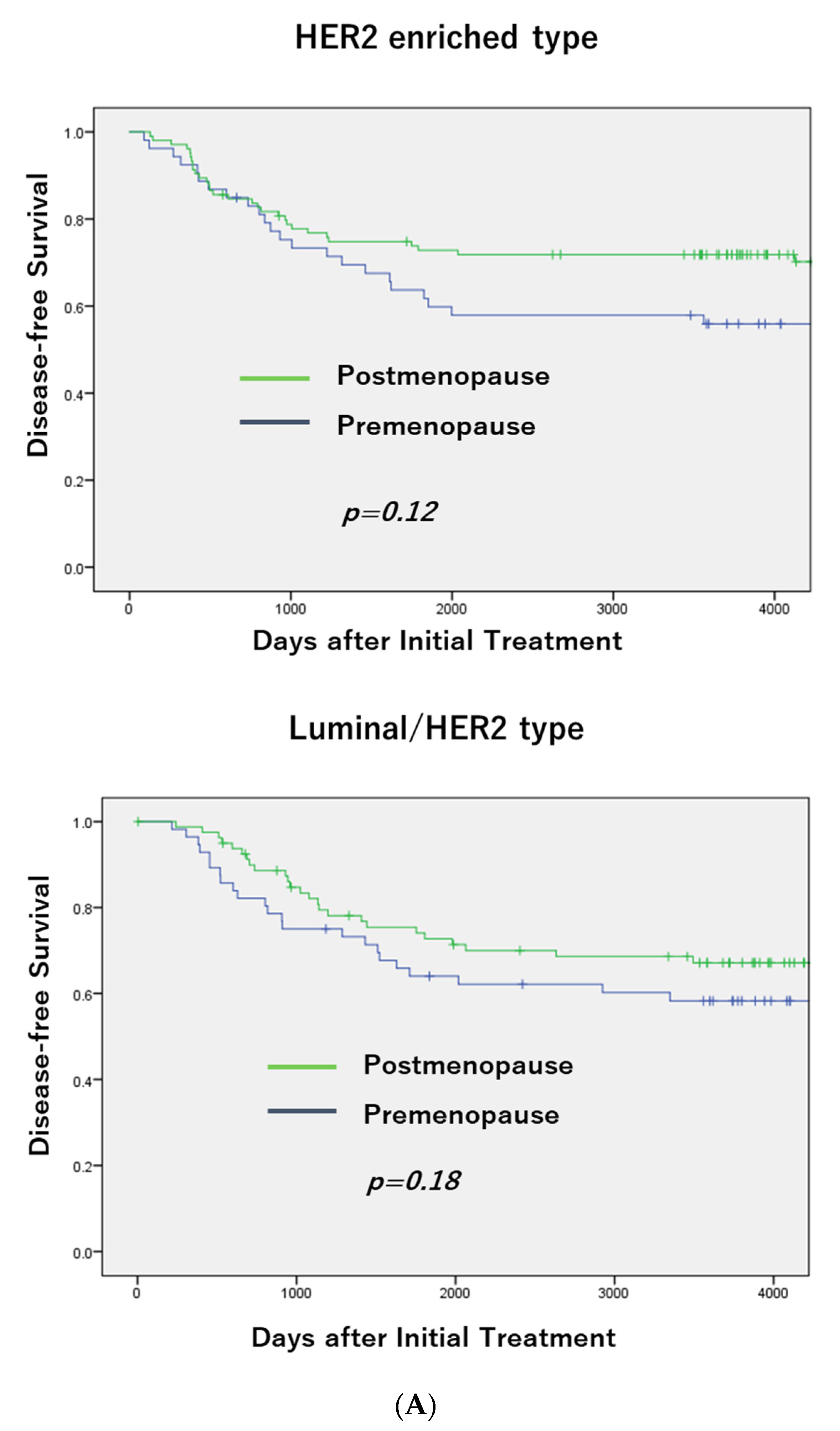
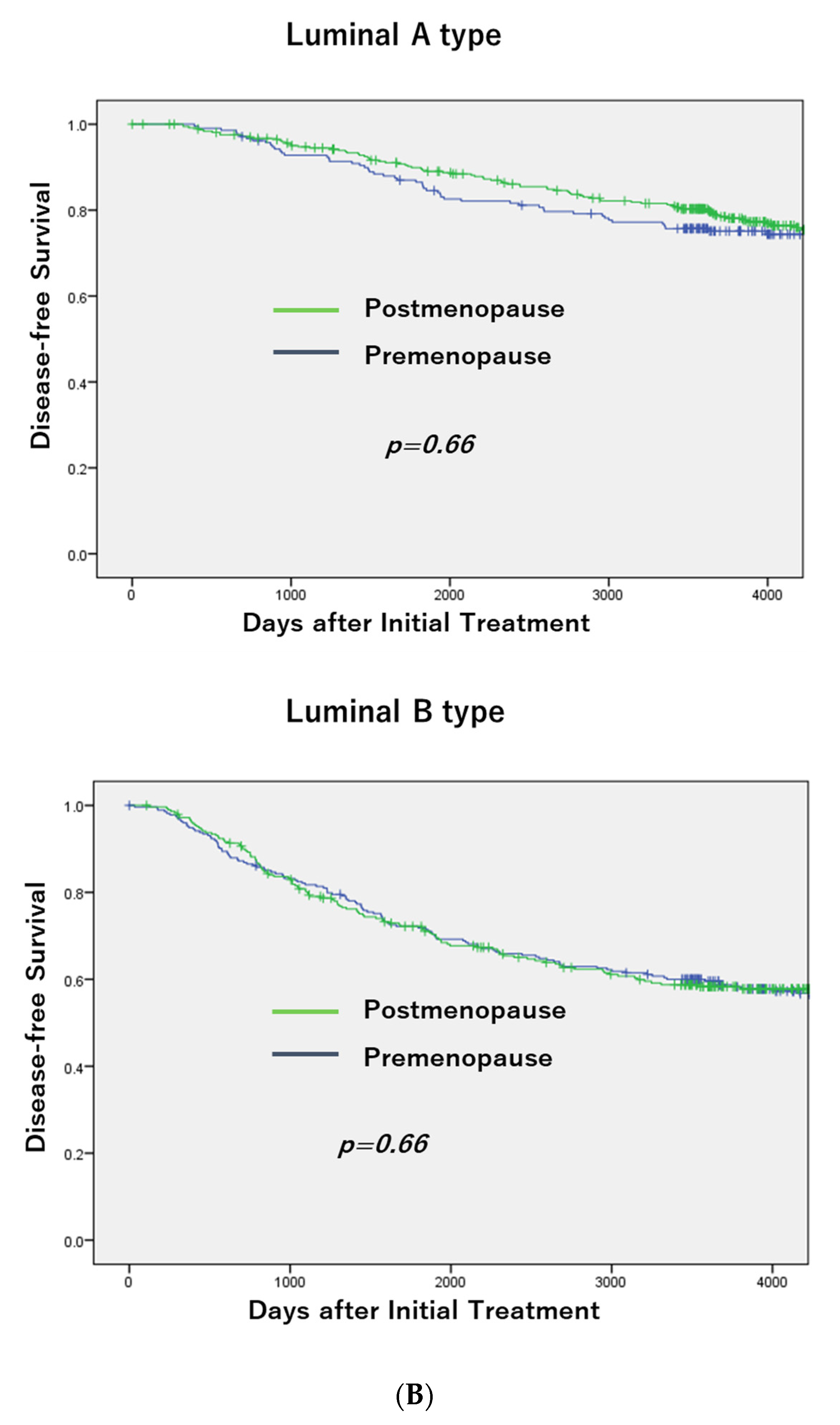
3.1. Breast Cancer Subtypes and Menopausal Status
| Subtype | Menopausal Status | Total | p Value | |
|---|---|---|---|---|
| Premenopause | Postmenopause | (vs. TN) | ||
| Luminal A | 616 (33.2) | 1238 (66.8) | 1854 | <0.0001 |
| Luminal B | 778 (41.6) | 1090 (58.4) | 1868 | <0.0001 |
| Luminal/HER2 | 159 (42.2) | 218 (57.8) | 377 | 0.046 |
| HER2 enriched | 124 (30.9) | 277 (69.1) | 401 | <0.0001 |
| Triple Negative | 165 (25.3) | 488 (74.7) | 653 | - |
| Total | 1842 (35.7) | 3313 (64.3) | 5153 | p <0.0001 |
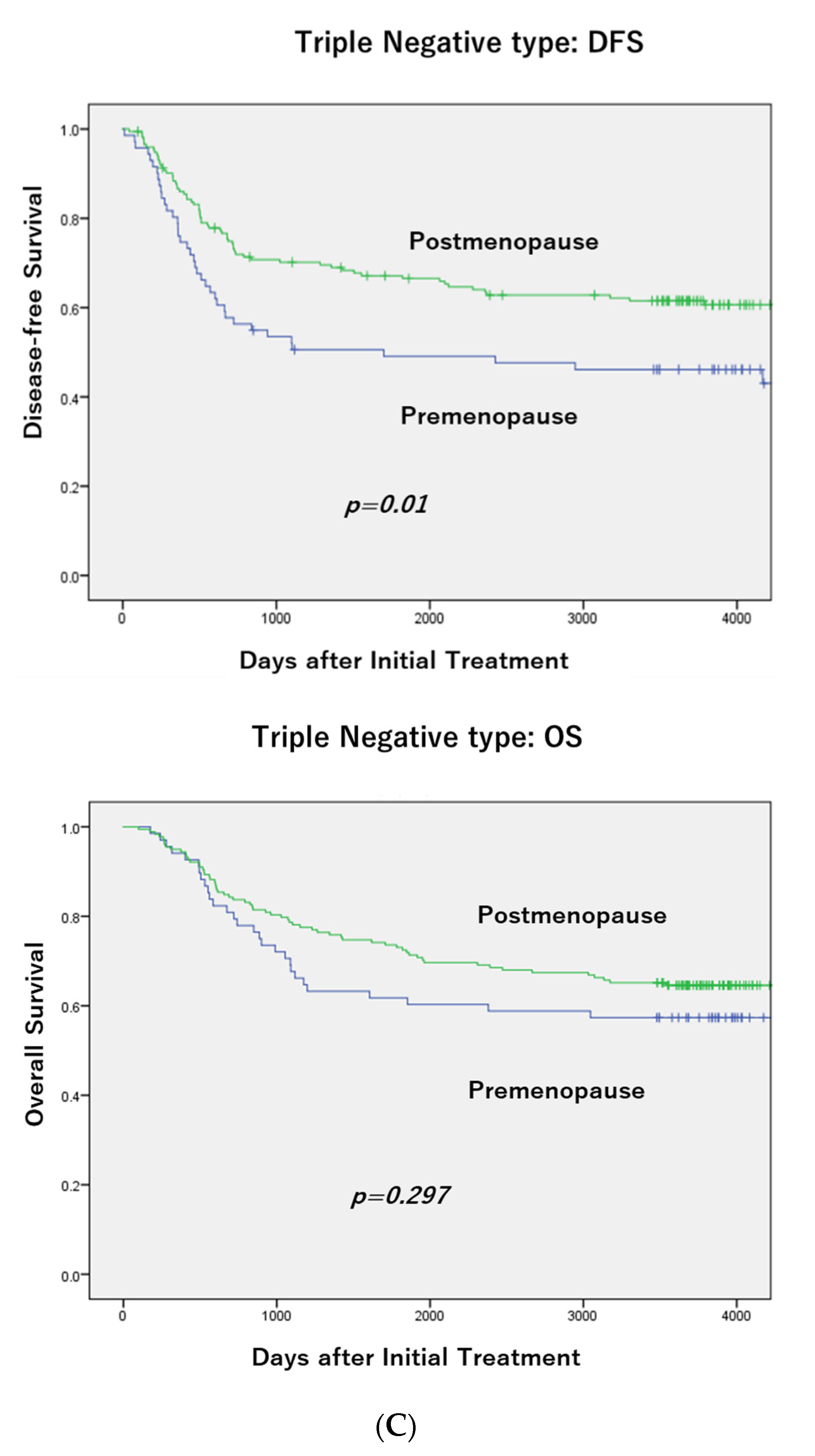
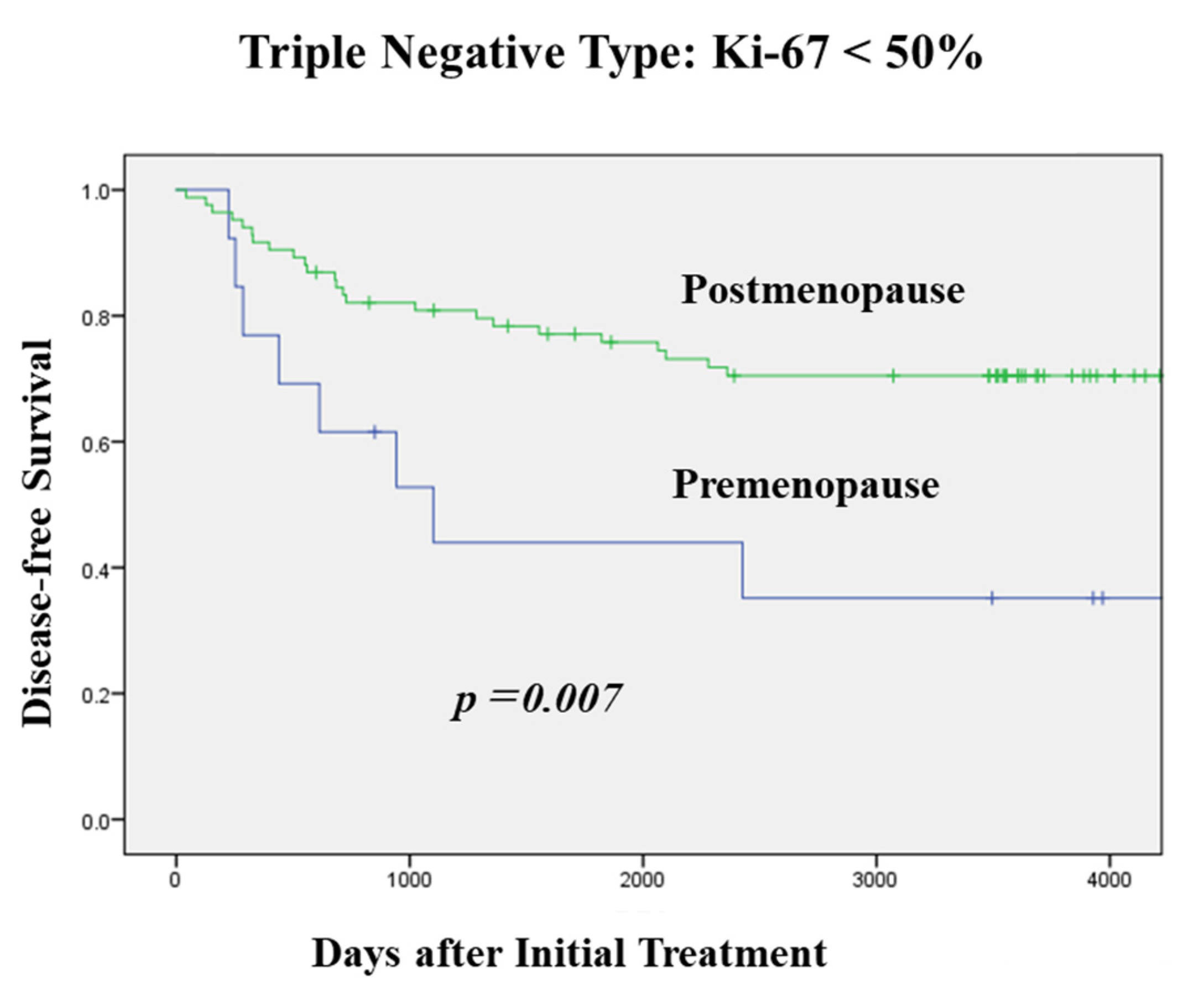
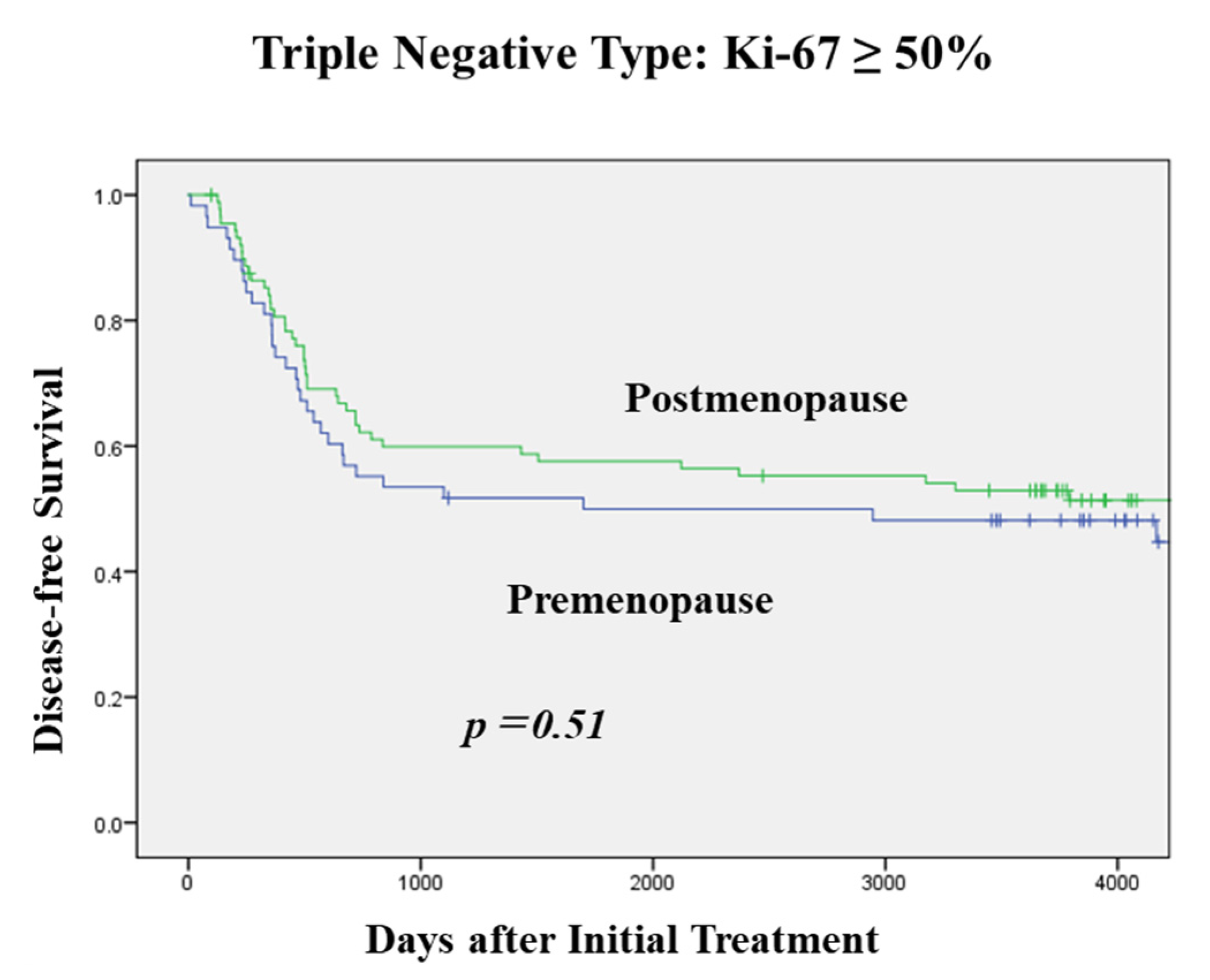
3.2. DFS According to Menopausal Status in Each Subtype and OS in TNBC
3.3. Clinicopathological Characteristics and Menopausal Status in TNBC
| Variables | Menopausal Status | Total | p Value | |
|---|---|---|---|---|
| Premenopause | Postmenopause | |||
| Tumor Size | ||||
| ≤2cm | 89 (23.2) | 294 (76.8) | 383 | |
| >2cm | 76 (28.1) | 194 (71.9) | 270 | 0.17 |
| Nodal Status | ||||
| n0 | 113 (24.8) | 342 (75.2) | 455 | |
| n+ | 52 (26.4) | 145 (73.6) | 197 | 0.7 |
| Ki-67 | ||||
| ≤20% | 6 (8.1) | 68 (91.9) | 74 | |
| 21–49% | 26 (15.4) | 143 (84.6) | 169 | <0.0001 |
| ≥50% | 133 (32.6) | 275 (67.4) | 408 | |
| p53 Overexpression | ||||
| without | 61 (17.8) | 282 (82.2) | 343 | |
| with | 93 (32.5) | 193 (67.5) | 286 | <0.0001 |
| Nuclear Grade | ||||
| 1 | 16 (16.3) | 82 (83.7) | 98 | |
| 2 | 31 (22.8) | 105 (77.2) | 136 | 0.045 |
| 3 | 116 (28.0) | 299 (72.0) | 415 | |
| Surgical Procedure | ||||
| Total Mastectomy | 52 (16.9) | 255 (83.1) | 307 | |
| Breast Conserving Surgery | 110 (32.8) | 227 (67.4) | 337 | <0.0001 |
3.4. Histological Type and Menopausal Status in TNBC
| Histological Type | Premenopause | Postmenopause | p Value |
|---|---|---|---|
| IBC, NST | 95 (90.5) * | 267 (81.4) * | * 0.029 |
| (with squamous differentiation) | (2) | (5) | |
| Ca w/APO | 6 (5.7) | 32 (9.8) | |
| Invasive lobular carcinoma | 1 (1) | 15 (4.6) | |
| Metaplastic carcinoma | 8 (2.4) | ||
| Mucinous carcinoma | 1 (0.3) | ||
| Adenoid cystic carcinoma | 2 (0.6) | ||
| Others | 3 (2.9) | 3 (0.9) | |
| Total | 105 | 328 |
3.5. Pre- and Post-Operative Adjuvant Chemotherapy and Menopausal Status in TNBC
| Variables | Menopausal Status | Total | p Value | |
|---|---|---|---|---|
| Premenopause | Postmenopause | |||
| Adjuvant Chemotherapy | ||||
| without | 10 (6.3) | 149 (93.7) | 159 | |
| with | 155 (31.4) | 339 (68.6) | 494 | <0.0001 |
| Neoadjuvant Chemotherapy | ||||
| without | 106 (20.5) | 412 (79.5) | 518 | |
| with | 59 (43.7) | 76 (56.3) | 135 | <0.0001 |
| Effect of NAC | ||||
| pCR | 27 (42.2) | 37 (57.8) | 64 | |
| non-pCR | 32 (45.1) | 39 (54.9) | 71 | 0.75 |
3.6. Uni- and Multivariate Analysis of Factors for DFS in TNBC
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauer, K.R.; Brown, M.; Cress, R.D.; Parise, C.A.; Caggiano, V. Descriptive analysis of estrogen receptor (ER)–negative, progesterone receptor (PR)–negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California Cancer Registry. Cancer 2007, 109, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin. Cancer Res. 2007, 13 Pt 1, 4429–4434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aine, M.; Boyaci, C.; Hartman, J.; Häkkinen, J.; Mitra, S.; Campos, A.B.; Nimeus, E.; Ehinger, A.; Vallon-Christersson, J.; Borg, Å.; et al. Molecular analyses of triple-negative breast cancer in the young and elderly. Breast Cancer Res. 2021, 23, 20–39. [Google Scholar] [CrossRef] [PubMed]
- Tzikas, A.-N.; Nemes, S.; Linderholm, B.K. A comparison between young and old patients with triple-negative breast cancer: Biology, survival and metastatic patterns. Breast Cancer Res. Treat. 2020, 182, 643–654. [Google Scholar] [CrossRef]
- Crozier, J.; Pezzi, T.; Hodge, C.; Janeva, S.; Lesnikoski, B.-A.; Samiian, L.; Devereaux, A.; Hammond, W.; Audisio, R.; Pezzi, C.M. Addition of chemotherapy to local therapy in women aged 70 years or older with triple-negative breast cancer: A propensity-matched analysis. Lancet Oncol. 2020, 21, 1611–1619. [Google Scholar] [CrossRef]
- Bonsang-Kitzis, H.; Chaltier, L.; Belin, L.; Savignoni, A.; Rouzier, R.; Sablin, M.-P.; Lerebours, F.; Bidard, F.-C.; Cottu, P.; Sastre-Garau, X.; et al. Beyond Axillary Lymph Node Metastasis, BMI and Menopausal Status Are Prognostic Determinants for Triple-Negative Breast Cancer Treated by Neoadjuvant Chemotherapy. PLoS ONE 2015, 10, e0144359. [Google Scholar] [CrossRef]
- Carlson, K.; Chung, A.; Mirocha, J.; Donovan, C.; Estrada, S.; Siegel, E.; Giuliano, A.; Amersi, F. Menopausal Status and Outcomes of BRCA Mutation Carriers with Breast Cancer. Am. Surg. 2018, 84, 1584–1588. [Google Scholar] [CrossRef]
- Freedman, R.A.; Keating, N.L.; Lin, N.U.; Winer, E.P.; Vaz-Luís, I.; Lii, J.; Exman, P.; Barry, W.T. Breast cancer-specific survival by age: Worse outcomes for the oldest patients. Cancer 2018, 124, 2184–2191. [Google Scholar] [CrossRef] [Green Version]
- Carr, D.N.; Vera, N.; Sun, W.; Lee, M.C.; Hoover, S.; Fulp, W.; Acs, G.; Laronga, C. Menopausal status does not predict Oncotype DX recurrence score. J. Surg. Res. 2015, 198, 27–33. [Google Scholar] [CrossRef]
- Kai, K.; Nishimura, R.; Arima, N.; Miyayama, H.; Iwase, H. p53 expression status is a significant molecular marker in predicting the time to endocrine therapy failure in recurrent breast cancer: A cohort study. Int. J. Clin. Oncol. 2006, 11, 426–433. [Google Scholar] [CrossRef]
- Kikuchi, S.; Osako, T.; Nishiyama, Y.; Nakano, M.; Tashima, R.; Fujisue, M.; Toyozumi, Y.; Arima, N.; Nishimura, R. P53 Overexpression in Ductal Carcinoma in situ of the Breast. J. Cytol. Histol. 2014, 5, 269–274. [Google Scholar]
- Stevens, K.N.; Vachon, C.M.; Couch, F.J. Genetic Susceptibility to Triple-Negative Breast Cancer. Cancer Res. 2013, 73, 2025–2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yam, C.; Mani, S.A.; Moulder, S.L. Targeting the Molecular Subtypes of Triple Negative Breast Cancer: Understanding the Diversity to Progress the Field. Oncology 2017, 22, 1086–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osako, T.; Nishimura, R.; Nishiyama, Y.; Okumura, Y.; Tashima, R.; Nakano, M.; Fujisue, M.; Toyozumi, Y.; Arima, N. Efficacy of intraoperative entire-circumferential frozen section analysis of lumpectomy margins during breast-conserving surgery for breast cancer. Int. J. Clin. Oncol. 2015, 20, 1093–1101. [Google Scholar] [CrossRef]
- Reis-Filho, J.S.; Milanezi, F.; Steele, D.; Savage, K.; Simpson, P.T.; Nesland, J.M.; Pereira, E.M.; Lakhani, S.R.; Schmitt, F.C. Metaplastic breast carcinomas are basal-like tumours. Histopathology 2006, 49, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Wu, M.; Peng, G.; Shi, D.; Zhang, J. Prognosis in triple-negative apocrine carcinomas of the breast: A population-based study. Cancer Med. 2019, 8, 7523–7531. [Google Scholar] [CrossRef]
- Gerratana, L.; Basile, D.; Buono, G.; De Placido, S.; Giuliano, M.; Minichillo, S.; Coinu, A.; Martorana, F.; De Santo, I.; Del Mastro, L.; et al. Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype. Cancer Treat. Rev. 2018, 68, 102–110. [Google Scholar] [CrossRef]
- Wang, C.; Pan, B.; Zhu, H.; Zhou, Y.; Mao, F.; Lin, Y.; Xu, Q.; Sun, Q. Prognostic value of androgen receptor in triple negative breast cancer: A meta-analysis. Oncotarget 2016, 7, 46482–46491. [Google Scholar] [CrossRef] [Green Version]
- Pujol, P.; Daures, J.; Thezenas, S.; Guilleux, F.; Rouanet, P.; Grenier, J. Changing Estrogen and Progesterone Receptor Patterns in Breast Carcinoma during the Menstrual Cycle and Menopause. Cancer 1998, 83, 698–705. [Google Scholar] [CrossRef]
- Caldarella, A.; Barchielli, A. Prognostic role of progesterone receptor expression in a population-based analysis. J. Cancer Res. Clin. Oncol. 2017, 143, 2505–2509. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Schiff, R.; Arpino, G.; Osborne, C.K.; Lee, A.V. Biology of Progesterone Receptor Loss in Breast Cancer and Its Implications for Endocrine Therapy. J. Clin. Oncol. 2005, 23, 7721–7735. [Google Scholar] [CrossRef] [PubMed]
- Dunbier, A.K.; Anderson, H.; Ghazoui, Z.; Folkerd, E.J.; A'hern, R.; Crowder, R.J.; Hoog, J.; Smith, I.E.; Osin, P.; Nerurkar, A.; et al. Expression of estrogen responsive genes in breast cancers correlates with plasma estradiol levels in postmenopausal women. J. Clin. Oncol. 2010, 28, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Arima, N.; Nishimura, R.; Osako, T.; Okumura, Y.; Nakano, M.; Fujisue, M.; Nishimyama, Y.; Toyozumi, Y. Ki-67 index value and progesterone receptor status can predict prognosis and suitable treatment in node-negative breast cancer patients with estrogen receptor-positive and HER2-negative tumors. Oncol. Lett. 2019, 17, 616–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variable | Category | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| Tumor Size | ≤2 cm vs. >2 cm | 1.93 (1.30–2.86) | 0.001 | 1.58 (1.05–2.37) | 0.027 |
| Nodal Status | N− vs. n+ | 4.14 (2.77–6.18) | <0.0001 | 3.91 (2.59–5.90) | <0.0001 |
| Ki-67 | ≤20% vs. >20% | 2.78 (1.13–6.83) | 0.026 | 1.89 (0.73–4.87) | 0.19 |
| p53 overexpression | without vs. with | 1.16 (0.79–1.72) | 0.43 | - | - |
| Nuclear Grade | 1, 2 vs. 3 | 1.68 (1.12–2.51) | 0.011 | 1.42 (0.93–2.17) | 0.1 |
| Menopausal Status | Pre- vs Post- | 0.61 (0.41–0.91) | 0.014 | 0.54 (0.36–0.82) | 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, R.; Osako, T.; Okumura, Y.; Nakano, M.; Otsuka, H.; Fujisue, M.; Arima, N. Triple Negative Breast Cancer: An Analysis of the Subtypes and the Effects of Menopausal Status on Invasive Breast Cancer. J. Clin. Med. 2022, 11, 2331. https://doi.org/10.3390/jcm11092331
Nishimura R, Osako T, Okumura Y, Nakano M, Otsuka H, Fujisue M, Arima N. Triple Negative Breast Cancer: An Analysis of the Subtypes and the Effects of Menopausal Status on Invasive Breast Cancer. Journal of Clinical Medicine. 2022; 11(9):2331. https://doi.org/10.3390/jcm11092331
Chicago/Turabian StyleNishimura, Reiki, Tomofumi Osako, Yasuhiro Okumura, Masahiro Nakano, Hiroko Otsuka, Mamiko Fujisue, and Nobuyuki Arima. 2022. "Triple Negative Breast Cancer: An Analysis of the Subtypes and the Effects of Menopausal Status on Invasive Breast Cancer" Journal of Clinical Medicine 11, no. 9: 2331. https://doi.org/10.3390/jcm11092331
APA StyleNishimura, R., Osako, T., Okumura, Y., Nakano, M., Otsuka, H., Fujisue, M., & Arima, N. (2022). Triple Negative Breast Cancer: An Analysis of the Subtypes and the Effects of Menopausal Status on Invasive Breast Cancer. Journal of Clinical Medicine, 11(9), 2331. https://doi.org/10.3390/jcm11092331






