Multimodal Evaluation of Long-Term Salivary Gland Alterations in Sarcoidosis
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Clinical Parameter
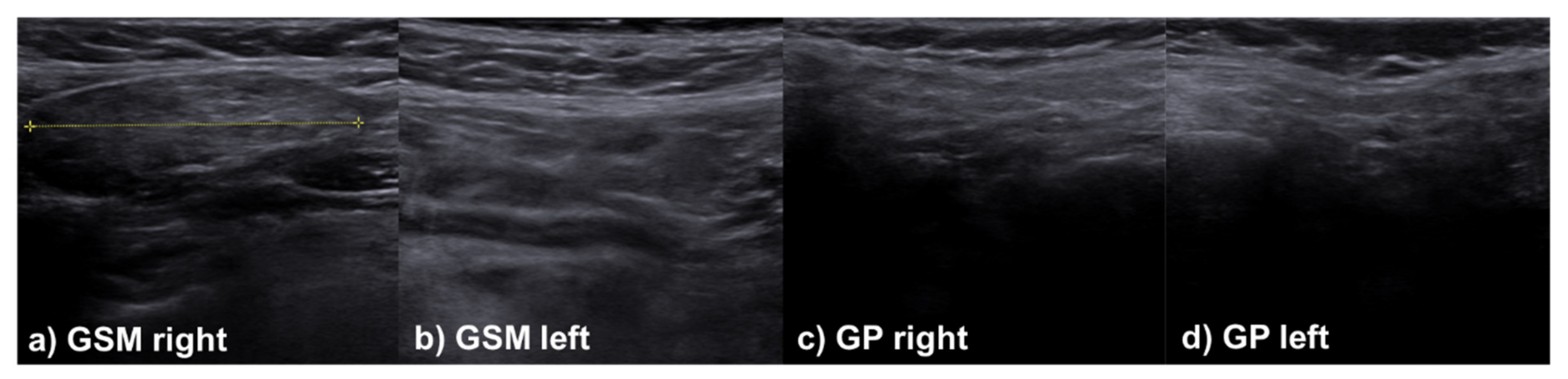
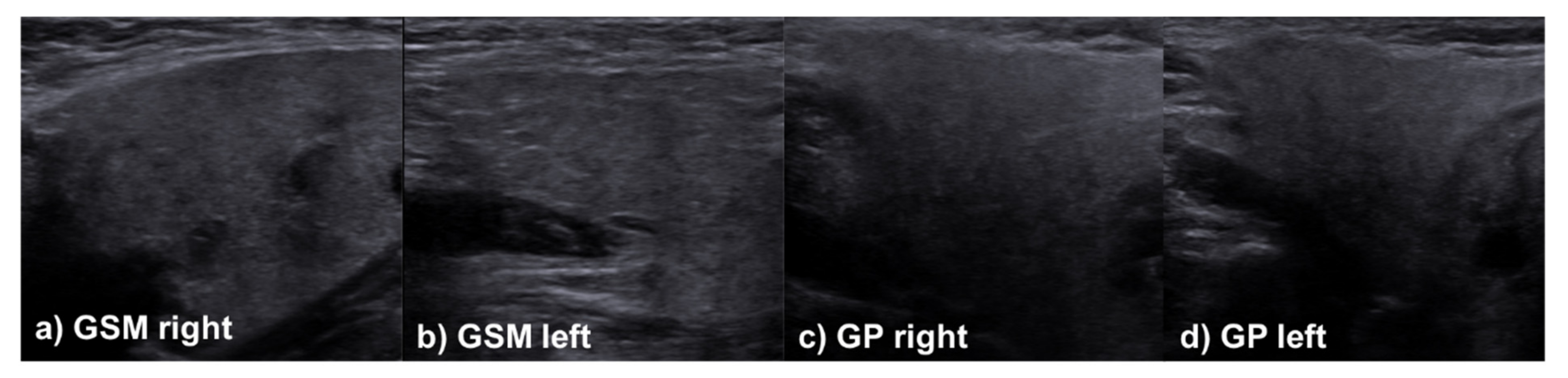
2.3. Sonographic Evaluation
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. Baseline Clinical Parameter Depending on Salivary Gland Involvement
3.3. Multimodal Evaluation at Follow-Up
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baughman, R.P.; Teirstein, A.S.; Judson, M.A.; Rossman, M.D.; Yeager, H., Jr.; Bresnitz, E.A.; De Palo, L.; Hunninghake, G.; Iannuzzi, M.C.; Johns, C.J.; et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am. J. Respir. Crit. Care Med. 2001, 164, 1885–1889. [Google Scholar] [CrossRef] [PubMed]
- Arkema, E.V.; Cozier, Y.C. Sarcoidosis epidemiology: Recent estimates of incidence, prevalence and risk factors. Curr. Opin. Pulm. Med. 2020, 26, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Knopf, A.; Bas, M.; Chaker, A.; Strassen, U.; Pickhard, A.; Stark, T.; Lahmer, T.; Thurmel, K. Rheumatic disorders affecting the head and neck: Underestimated diseases. Rheumatology 2011, 50, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Brito-Zeron, P.; Kostov, B.; Superville, D.; Baughman, R.P.; Ramos-Casals, M.; Autoimmune Big Data Study Group. Geoepidemiological big data approach to sarcoidosis: Geographical and ethnic determinants. Clin. Exp. Rheumatol. 2019, 37, 1052–1064. [Google Scholar]
- Thomas, K.W.; Hunninghake, G.W. Sarcoidosis. JAMA 2003, 289, 3300–3303. [Google Scholar] [CrossRef]
- James, W.E.; Koutroumpakis, E.; Saha, B.; Nathani, A.; Saavedra, L.; Yucel, R.M.; Judson, M.A. Clinical Features of Extrapulmonary Sarcoidosis Without Lung Involvement. Chest 2018, 154, 349–356. [Google Scholar] [CrossRef]
- Rizzato, G.; Palmieri, G.; Agrati, A.M.; Zanussi, C. The organ-specific extrapulmonary presentation of sarcoidosis: A frequent occurrence but a challenge to an early diagnosis. A 3-year-long prospective observational study. Sarcoidosis Vasc. Diffus. Lung Dis. 2004, 21, 119–126. [Google Scholar]
- Rizzato, G.; Tinelli, C. Unusual presentation of sarcoidosis. Respiration 2005, 72, 3–6. [Google Scholar] [CrossRef]
- Judson, M.A.; Thompson, B.W.; Rabin, D.L.; Steimel, J.; Knattereud, G.L.; Lackland, D.T.; Rrose, C.; Rand, C.S.; Baughman, R.P.; Teirstein, A.S.; et al. The diagnostic pathway to sarcoidosis. Chest 2003, 123, 406–412. [Google Scholar] [CrossRef]
- Hofauer, B.; Chaker, A.; Strenger, T.; Bas, M.; Mansour, N.; Knopf, A. Swelling of the submandibular and parotid glands: A description of possible differential diagnoses. HNO 2016, 64, 333–348. [Google Scholar] [CrossRef]
- James, D.G.; Sharma, O.P. Parotid gland sarcoidosis. Sarcoidosis Vasc. Diffuse. Lung Dis. 2000, 17, 27–32. [Google Scholar]
- Hofauer, B.; Mansour, N.; Heiser, C.; Gahleitner, C.; Thuermel, K.; Bas, M.; Knopf, A. Sonoelastographic Modalities in the Evaluation of Salivary Gland Characteristics in Sjogren’s Syndrome. Ultrasound Med. Biol. 2016, 42, 2130–2139. [Google Scholar] [CrossRef]
- Hofauer, B.; Chaker, A.; Thurmel, K.; Knopf, A. Manifestations of autoimmune disorders in otorhinolaryngology: Classical symptoms and diagnostic approach. HNO 2017, 65, 695–708. [Google Scholar] [CrossRef]
- Knopf, A.; Lahmer, T.; Chaker, A.; Stark, T.; Hofauer, B.; Pickhard, A.; Thurmel, K.; Bas, M. Head and neck sarcoidosis, from wait and see to tumor necrosis factor alpha therapy: A pilot study. Head Neck 2013, 35, 715–719. [Google Scholar] [CrossRef]
- Berman, J.S.; Govender, P.; Ruberg, F.L.; Mazzini, M.; Miller, E.J. Scadding revisited: A proposed staging system for cardiac sarcoidosis. Sarcoidosis Vasc. Diffus. Lung Dis. 2014, 31, 2–5. [Google Scholar]
- Makula, E.; Pokorny, G.; Rajtar, M.; Kiss, I.; Kovacs, A.; Kovacs, L. Parotid gland ultrasonography as a diagnostic tool in primary Sjogren’s syndrome. Br. J. Rheumatol. 1996, 35, 972–977. [Google Scholar] [CrossRef][Green Version]
- Li, C.W.; Tao, R.J.; Zou, D.F.; Li, M.H.; Xu, X.; Cao, W.J. Pulmonary sarcoidosis with and without extrapulmonary involvement: A cross-sectional and observational study in China. BMJ Open 2018, 8, e018865. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, J.H.; Lee, J.K. Isolated Parotid Gland Sarcoidosis Mimicking Parotid Tumor. J. Korean Med. Sci. 2016, 31, 644–645. [Google Scholar] [CrossRef]
- Teymoortash, A.; Werner, J.A. Parotid gland involvement in sarcoidosis: Sonographic features. J. Clin. Ultrasound 2009, 37, 507–510. [Google Scholar] [CrossRef]
- Shadamarshan, R.A.; Sharma, R.; Grewal, R. Sarcoidosis Mimicking Chronic Sialadenitis of Parotid Gland. J. Craniofac. Surg. 2021, 32, e424–e425. [Google Scholar] [CrossRef]
- Broggi, G.; Reggio, E.; Giuliano, L.; Palmucci, S.; Caltabiano, R.; Lanzafame, S. Parotid gland involvement in Heerfordt syndrome: A case report. Pathologica 2017, 109, 418–420. [Google Scholar]
- Law, S.T.; Jafarzadeh, S.R.; Govender, P.; Sun, X.; Sanchorawala, V.; Kissin, E.Y. Comparison of Ultrasound Features of Major Salivary Glands in Sarcoidosis, Amyloidosis, and Sjogren’s Syndrome. Arthritis Care Res. 2020, 72, 1466–1473. [Google Scholar] [CrossRef]
- James-Goulbourne, T.; Murugesan, V.; Kissin, E.Y. Sonographic Features of Salivary Glands in Sjogren’s Syndrome and its Mimics. Curr. Rheumatol. Rep. 2020, 22, 36. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Y.; Chen, D.; Lu, Z.; Ge, J.; Li, X.; Gao, X. Co-Existence of Sarcoidosis and Sjogren’s Syndrome with Hypercalcemia and Renal Involvement: A Case Report and Literature Review. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 768–776. [Google Scholar] [CrossRef]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjogren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef]
- Knopf, A.; Hofauer, B.; Thurmel, K.; Meier, R.; Stock, K.; Bas, M.; Manour, N. Diagnostic utility of Acoustic Radiation Force Impulse (ARFI) imaging in primary Sjoegren’s syndrome. Eur. Radiol. 2015, 25, 3027–3034. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brito-Zeron, P.; Bombardieri, S.; Bootsma, H.; De Vita, S.; Dorner, T.; Fisher, B.A.; Gottenberg, J.-E.; Hernandez-Molina, G.; Kocher, A.; et al. EULAR recommendations for the management of Sjogren’s syndrome with topical and systemic therapies. Ann. Rheum. Dis. 2020, 79, 3–18. [Google Scholar] [CrossRef]
- Hovcevar, A.; Bruyn, G.A.; Terslev, L.; De Agustin, J.J.; MacCarter, D.; Chrysidis, S.; Collado, P.; Dejaco, C.; Fana, V.; Filippou, G.; et al. Development of a new ultrasound scoring system to evaluate glandular inflammation in Sjogren’s syndrome: An OMERACT reliability exercise. Rheumatology 2021. Online ahead of print. [Google Scholar]
- Zhou, M.; Song, S.; Wu, S.; Duan, T.; Chen, L.; Ye, J.; Xiaio, J. Diagnostic accuracy of salivary gland ultrasonography with different scoring systems in Sjogren’s syndrome: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 17128. [Google Scholar] [CrossRef]

| Gender distribution [% female] | 65.8 |
| Age at diagnosis [years] | 35.1 ± 21.6 |
| Treatment department [n/%] | |
| ENT | 23/30.3 |
| Rheumatology | 27/35.5 |
| ENT + Rheumatology | 15/19.7 |
| Pneumology | 11/14.5 |
| ACE [U/L] | 85.3 ± 59.1 |
| sIL-2R [U/mL] | 1461.3 ± 959.4 |
| Chest X-ray [n/%] | |
| Normal | 6/7.9 |
| Bihilar lymphadenopathy | 26/34.2 |
| Bihilar lympha. with lung involvement | 27/35.5 |
| Lung involvement without bihilar lympha. | 4/5.3 |
| Lung fibrosis | 0/0 |
| ENT symptoms [n/%] | 60/78.9 |
| Arthralgia [n/%] | 14/18.4 |
| Uveitis [n/%] | 14/18.4 |
| Skin involvement [n/%] | 27/35.5 |
| Fever [n/%] | 17/22.4 |
| Parameter | With Salivary Gland Involvement (n = 17) | Without Salivary Gland Involvement (n = 59) | p-Value |
|---|---|---|---|
| Gender distribution [% female] | 58.8 | 67.9 | 0.495 |
| Age at diagnosis [years] | 36.6 ± 9.9 | 34.71 ± 24.0 | 0.762 |
| Interval diagnosis-follow-up [months] | 88.2 ± 73.4 | 88.3 ± 87.2 | 0.997 |
| ACE [U/L] # | 103.3 ± 68.3 | 79.0 ± 55.6 | 0.294 |
| sIL-2R [U/mL] § | 1059.0 * | 1494.83 ± 994.1 | 0.682 |
| Xerostomia [VAS 0–10] | 4.5 ± 4.6 | 2.1 ± 2.9 | 0.009 |
| Keratoconjunctivitis sicca [VAS 0–10] | 4.5 ± 3.8 | 2.2 ± 3.1 | 0.014 |
| Uveitis [n/%] | 5/29.4 | 8/13.6 | 0.129 |
| Facial nerve palsy [n/%] | 5/29.4 | 8/13.6 | 0.129 |
| Fever [n/%] | 5/29.4 | 11/18.6 | 0.358 |
| Parameter | With Salivary Gland Involvement (n = 17) | Without Salivary Gland Involvement (n = 59) | p-Value |
|---|---|---|---|
| Schirmer test [mm] | 20.0 ± 11.2 | 15.9 ± 9.8 | 0.144 |
| UWSF [mL/min] | 1.3 ± 0.9 | 1.2 ± 1.1 | 0.706 |
| Xerostomia [VAS 0–10] | 2.7 ± 3.8 | 2.4 ± 3.3 | 0.760 |
| Keratoconjunctivitis sicca [VAS 0–10] | 3.5 ± 3.8 | 2.4 ± 3.2 | 0.241 |
| Uveitis [n/%] | 2/11.8 | 3/5.1 | 0.310 |
| Facial nerve palsy [n/%] | 1/5.9 | 0/0 | 0.224 |
| Fever [n/%] | 0/0 | 1/1.7 | 0.776 |
| Parameter | With Salivary Gland Involvement (n = 17) | Without Salivary Gland Involvement (n = 59) | p-Value |
|---|---|---|---|
| B-Mode all glands [Δ score 0–4] | 0.5 ± 0.6 | 0.4 ± 0.5 | 0.523 |
| B-Mode parotid glands [Δ score 0–4] | 0.5 ± 0.8 | 0.3 ± 0.7 | 0.106 |
| RTE all glands [Δ score 1–3] | 3.0 ± 0.4 | 3.0 ± 0.1 | 0.502 |
| RTE parotid glands [Δ score 1–3] | 2.9 ± 0.2 | 3.0 ± 0.1 | 0.697 |
| VTI all glands [Δ score 1–3] | 2.1 ± 0.4 | 1.9 ± 0.4 | 0.041 |
| VTI parotid glands [Δ score 1–3] | 2.4 ± 0.5 | 2.2 ± 0.5 | 0.082 |
| VTQ all glands [m/s] | 1.9 ± 0.4 | 1.7 ± 0.3 | 0.235 |
| VTQ parotid glands [m/s] | 2.4 ± 0.5 | 2.2 ± 0.5 | 0.082 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofauer, B.; Wiesner, M.; Stock, K.; Peltz, F.; Johnson, F.; Zhu, Z.; Chaker, A.; Knopf, A. Multimodal Evaluation of Long-Term Salivary Gland Alterations in Sarcoidosis. J. Clin. Med. 2022, 11, 2292. https://doi.org/10.3390/jcm11092292
Hofauer B, Wiesner M, Stock K, Peltz F, Johnson F, Zhu Z, Chaker A, Knopf A. Multimodal Evaluation of Long-Term Salivary Gland Alterations in Sarcoidosis. Journal of Clinical Medicine. 2022; 11(9):2292. https://doi.org/10.3390/jcm11092292
Chicago/Turabian StyleHofauer, Benedikt, Miriam Wiesner, Konrad Stock, Friedhelm Peltz, Felix Johnson, Zhaojun Zhu, Adam Chaker, and Andreas Knopf. 2022. "Multimodal Evaluation of Long-Term Salivary Gland Alterations in Sarcoidosis" Journal of Clinical Medicine 11, no. 9: 2292. https://doi.org/10.3390/jcm11092292
APA StyleHofauer, B., Wiesner, M., Stock, K., Peltz, F., Johnson, F., Zhu, Z., Chaker, A., & Knopf, A. (2022). Multimodal Evaluation of Long-Term Salivary Gland Alterations in Sarcoidosis. Journal of Clinical Medicine, 11(9), 2292. https://doi.org/10.3390/jcm11092292






