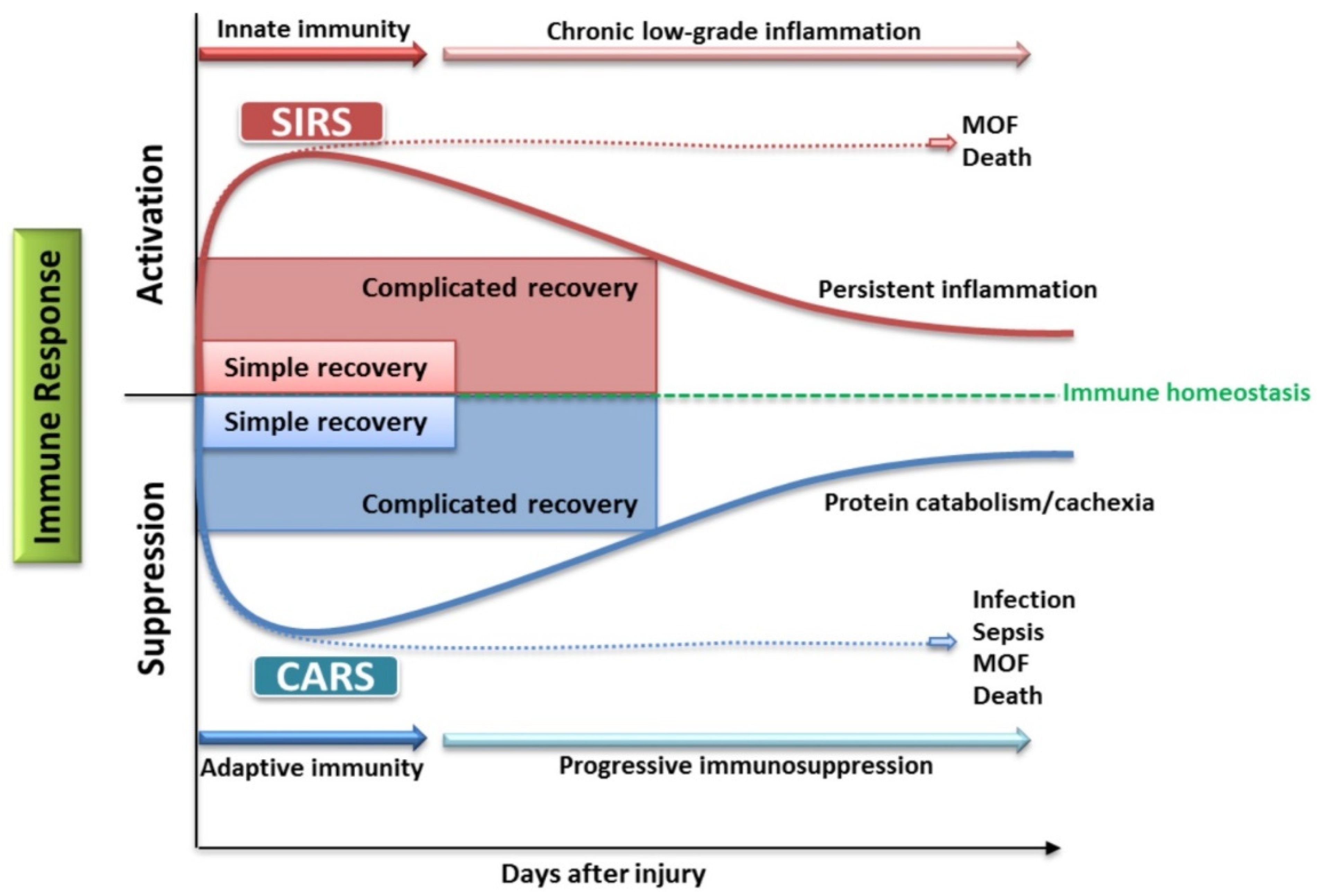
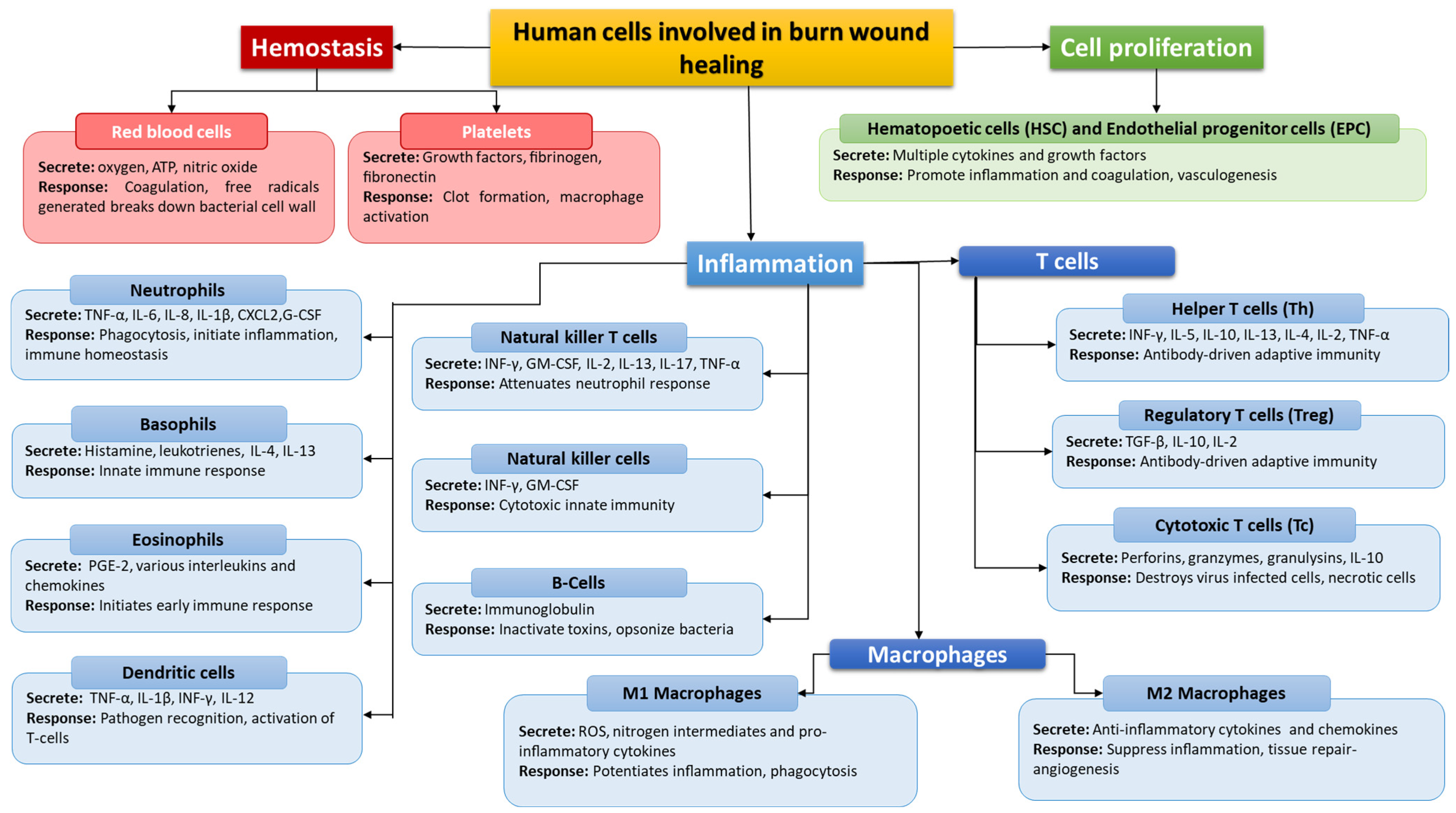
The Role of the Immune System in Pediatric Burns: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
Search Strategy, Study Selection, and Data Extraction
3. Assessment of the Effects of Burns in Pediatric Patients
3.1. Pathophysiological Effects of Burns in Pediatric Patients
3.1.1. Local Response to Burns in Pediatric Patients
3.1.2. Systemic Response to Burns in Pediatric Patients
3.1.3. Burn Shock
4. The Role of the Immune System in Burns
4.1. Importance of the Innate Response (Neutrophils, Monocytes, Macrophages, NK Cells)
4.2. Importance of the Acquired Response
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Institute of General Medical Sciences. Burns. Available online: https://www.nigms.nih.gov/education/fact-sheets/Pages/burns.aspx (accessed on 21 February 2022).
- Practical Handbook for Burns Injury Management (Draft).Pdf. Available online: https://dghs.gov.in/WriteReadData/userfiles/file/Comp-2/Practical%20handbook%20for%20burns%20injury%20management%20(draft).pdf (accessed on 13 March 2022).
- Ja, G.-E.; Vb, A.-A.; Eh, O.-V.; García-Manzano, R.; Barker Antonio, A.; Aron, J.; García-Espinoza, J. Burns: Definition, Clas-sification, Pathophysiology and Initial Approach. Int. J. Gen. Med. 2020, 5, 2327–5146. [Google Scholar] [CrossRef]
- Skin Microbes and the Immune Response. Available online: https://www.nih.gov/news-events/nih-research-matters/skin-microbes-immune-response (accessed on 21 February 2022).
- Padbury, J.F. Skin—The first line of defense. J. Pediatr. 2008, 152, A2. [Google Scholar] [CrossRef]
- Chen, C.-P.; Hwang, R.-L.; Chang, S.-Y.; Lu, Y.-T. Effects of temperature steps on human skin physiology and thermal sensation response. Build. Environ. 2011, 46, 2387–2397. [Google Scholar] [CrossRef]
- Denda, M.; Sokabe, T.; Fukumi-Tominaga, T.; Tominaga, M. Effects of Skin Surface Temperature on Epidermal Permeability Barrier Homeostasis. J. Investig. Dermatol. 2007, 127, 654–659. [Google Scholar] [CrossRef]
- Connie, J.; Mattera, M.S. RN, EMT-P NCH Paramedic Program. Burns/Thermal Trauma. Available online: http://www.nwcemss.org/assets/1/continuing_education_materials/BURNS_thermal_S19.pdf (accessed on 13 March 2022).
- Lévesque, B.; Lavoie, M.; Joly, J. Residential water heater temperature: 49 or 60 degrees Celsius? Can. J. Infect. Dis. 2004, 15, 11–12. [Google Scholar] [CrossRef]
- Hettiaratchy, S.; Dziewulski, P. ABC of burns: Pathophysiology and types of burns. BMJ 2004, 328, 1427–1429. [Google Scholar] [CrossRef]
- World Health Organization. Media Centre, Fact Sheet, Burns. January 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/burns (accessed on 21 February 2022).
- CDC Child Injury Prevention. Available online: https://www.cdc.gov/injury/features/child-injury/index.html (accessed on 21 February 2022).
- Lyons, R.; Turner, S.; Walters, A.; Kisser, R.; Rogmans, W.; Lyons, J.; Akbari, A.; Valkenberg, H.; Bejko, D.; Bauer, R.; et al. Disability Adjusted Life Year (DALY) Estimates for Injury Utilising the European Injury Data Base (IDB); LIH: Luxwembourg, 2017. [Google Scholar]
- Spronk, I.; Edgar, D.W.; Van Baar, M.E.; Wood, F.M.; Van Loey, N.E.E.; Middelkoop, E.; Renneberg, B.; Öster, C.; Orwelius, L.; Moi, A.L.; et al. Improved and standardized method for assessing years lived with disability after burns and its application to estimate the non-fatal burden of disease of burn injuries in Australia, New Zealand and the Netherlands. BMC Public Health 2020, 20, 1–15. [Google Scholar] [CrossRef]
- Alipour, J.; Mehdipour, Y.; Karimi, A. Epidemiology and outcome analysis of 3030 burn patients with an ICD-10 approach. Ann. Burn. Fire Disasters 2020, 33, 3–13. [Google Scholar]
- Fire and Burn Injuries Among Children in 2018 (2020 Update). Available online: https://www.safekids.org/fast-fact/fire-and-burn-injuries-among-children-2018-2020-update (accessed on 21 February 2022).
- World Health Organization Violence. Injuries and Disability: Biennial Report 2010–2011; World Health Organization: Geneva, Switzerland, 2012; ISBN 978-92-4-150413-3. [Google Scholar]
- Peden, M.; Oyegbite, K.; Ozanne-Smith, J.; Hyder, A.A.; Branche, C.; Rahman, A.; Rivara, F.; Bartolomeos, K. World Report on Child Injury Prevention; WHO: Geneva, Switzerland, 2008; Chapter 2. [Google Scholar]
- Trauma Service: Burns. Available online: https://www.rch.org.au/trauma-service/manual/Burns/ (accessed on 21 February 2022).
- Beaulieu, E.; Zheng, A.; Rajabali, F.; MacDougall, F.; Pike, I. The Economics of Burn Injuries Among Children Aged 0 to 4 Years in British Columbia. J. Burn Care Res. 2020, 42, 499–504. [Google Scholar] [CrossRef]
- Blom, L.; Klingberg, A.; Laflamme, L.; Wallis, L.; Hasselberg, M. Gender differences in burns: A study from emergency centres in the Western Cape, South Africa. Burns 2016, 42, 1600–1608. [Google Scholar] [CrossRef]
- Accidental Burns Increased for Children at Home During Pandemic. Available online: http://www.aap.org/en/news-room/news-releases/aap/2021/accidental-burns-increased-for-children-at-home-during-pandemic/ (accessed on 21 February 2022).
- Benson, A.; Dickson, W.; Boyce, D. Burns. BMJ 2006, 332, 649–652. [Google Scholar] [CrossRef]
- Mathias, E.; Murthy, M.S. Pediatric Thermal Burns and Treatment: A Review of Progress and Future Prospects. Medicines 2017, 4, 91. [Google Scholar] [CrossRef]
- Chipp, E.; Charles, L.; Thomas, C.; Whiting, K.; Moiemen, N.; Wilson, Y. A prospective study of time to healing and hypertrophic scarring in paediatric burns: Every day counts. Burn. Trauma 2017, 5, 3. [Google Scholar] [CrossRef]
- Farina, J.A.; Rosique, M.J.; Rosique, R.G. Curbing Inflammation in Burn Patients. Int. J. Inflamm. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. Sepsis in the burn patient: A different problem than sepsis in the general population. Burn. Trauma 2017, 5, 23. [Google Scholar] [CrossRef]
- Feng, J.-Y.; Chien, J.-Y.; Kao, K.-C.; Tsai, C.-L.; Hung, F.M.; Lin, F.-M.; Hu, H.-C.; Huang, K.-L.; Yu, C.-J.; Yang, K.-Y. Predictors of Early Onset Multiple Organ Dysfunction in Major Burn Patients with Ventilator Support: Experience from A Mass Casualty Explosion. Sci. Rep. 2018, 8, 10939. [Google Scholar] [CrossRef]
- Moins-Teisserenc, H.; Cordeiro, D.J.; Audigier, V.; Ressaire, Q.; Benyamina, M.; Lambert, J.; Maki, G.; Homyrda, L.; Toubert, A.; Legrand, M. Severe Altered Immune Status After Burn Injury Is Associated with Bacterial Infection and Septic Shock. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Zhang, P.; Zou, B.; Liou, Y.-C.; Huang, C. The pathogenesis and diagnosis of sepsis post burn injury. Burn. Trauma 2021, 9, tkaa047. [Google Scholar] [CrossRef]
- Hall, M.W.; Greathouse, K.C.; Thakkar, R.K.; Sribnick, E.A.; Muszynski, J.A. Immunoparalysis in Pediatric Critical Care. Pediatr. Clin. N. Am. 2017, 64, 1089–1102. [Google Scholar] [CrossRef]
- Winkler, M.S.; Rissiek, A.; Priefler, M.; Schwedhelm, E.; Robbe, L.; Bauer, A.; Zahrte, C.; Zoellner, C.; Kluge, S.; Nierhaus, A. Human leucocyte antigen (HLA-DR) gene expression is reduced in sepsis and correlates with impaired TNFα response: A diagnostic tool for immunosuppression? PLoS ONE 2017, 12, e0182427. [Google Scholar] [CrossRef]
- Nakamori, Y.; Park, E.J.; Shimaoka, M. Immune Deregulation in Sepsis and Septic Shock: Reversing Immune Paralysis by Targeting PD-1/PD-L1 Pathway. Front. Immunol. 2021, 11, 624279. [Google Scholar] [CrossRef]
- Berlot, G.; Passero, S. Immunoparalysis in Septic Shock Patients; IntechOpen: London, UK, 2019; ISBN 978-1-83880-394-0. [Google Scholar]
- Jensen, I.J.; Sjaastad, F.V.; Griffith, T.S.; Badovinac, V.P. Sepsis-Induced T Cell Immunoparalysis: The Ins and Outs of Impaired T Cell Immunity. J. Immunol. 2018, 200, 1543–1553. [Google Scholar]
- Burn Evaluation: MedlinePlus Medical Test. Available online: https://medlineplus.gov/lab-tests/burn-evaluation/ (accessed on 21 February 2022).
- Schaefer, T.J.; Szymanski, K.D. Burn Evaluation and Management; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Suman, A.; Owen, J. Update on the management of burns in paediatrics. BJA Educ. 2020, 20, 103–110. [Google Scholar] [CrossRef]
- Moore, R.A.; Waheed, A.; Burns, B. Rule of Nines; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn Wound Infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef]
- Hettiaratchy, S.; Papini, R. Initial management of a major burn: II—Assessment and resuscitation. BMJ 2004, 329, 101–103. [Google Scholar] [CrossRef]
- Cirillo, M.D.; Mirdell, R.; Sjöberg, F.; Pham, T.D. Improving burn depth assessment for pediatric scalds by AI based on semantic segmentation of polarized light photography images. Burns 2021, 47, 1586–1593. [Google Scholar] [CrossRef]
- King, A.; Balaji, S.; Keswani, S.G. Biology and Function of Fetal and Pediatric Skin. Facial Plast. Surg. Clin. N. Am. 2013, 21, 1–6. [Google Scholar] [CrossRef]
- Lukusa, M.; Allorto, N.; Wall, S. Hypothermia in acutely presenting burn injuries to a regional burn service: The incidence and impact on outcome. Burn. Open 2020, 5, 39–44. [Google Scholar] [CrossRef]
- Sen, S. Pediatric inhalation injury. Burn. Trauma 2017, 5, 31. [Google Scholar] [CrossRef]
- Caruso, T.J.; Janik, L.S.; Fuzaylov, G. Airway management of recovered pediatric patients with severe head and neck burns: A review. Pediatr. Anesth. 2012, 22, 462–468. [Google Scholar] [CrossRef]
- Nielson, C.B.; Duethman, N.C.; Howard, J.M.; Moncure, M.; Wood, J.G. Burns. J. Burn Care Res. 2017, 38, e469–e481. [Google Scholar] [CrossRef] [PubMed]
- Strudwick, X.L.; Cowin, A.J. The Role of the Inflammatory Response in Burn Injury; IntechOpen: London, UK, 2017; ISBN 978-1-78923-131-1. [Google Scholar]
- Noorbakhsh, S.I.; Bonar, E.M.; Polinski, R.; Amin, S. Educational Case: Burn Injury—Pathophysiology, Classification, and Treatment. Acad. Pathol. 2021, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Prakash, S. Burn: A Clinical Perspective. In Theory and Applications of Heat Transfer in Humans; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 513–527. ISBN 978-1-119-12742-0. [Google Scholar]
- Samuelsson, A. Effects of Burns and Vasoactive Drugs on Human Skin, Clinical and Experimental Studies Using Microdialysis. Ph.D. Thesis, Linköping University Electronic Press, Linköping, Sweden, 2022. [Google Scholar]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.P.G.; Vlig, M.; Boekema, B.K.H.L.; Stoop, M.M.; Pijpe, A.; van Zuijlen, P.P.M.; de Jong, E.; van Cranenbroek, B.; Joosten, I.; Koenen, H.J.P.M.; et al. Persistent Systemic Inflammation in Patients With Severe Burn Injury Is Accompanied by Influx of Immature Neutrophils and Shifts in T Cell Subsets and Cytokine Profiles. Front. Immunol. 2021, 11, 621222. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Evers, L.H.; Bhavsar, D.; Mailänder, P. The biology of burn injury. Exp. Dermatol. 2010, 19, 777–783. [Google Scholar] [CrossRef]
- Korkmaz, H.I.; Ulrich, M.M.W.; van Wieringen, W.; Vlig, M.; Emmens, R.W.; Meyer, K.W.; Sinnige, P.; Krijnen, P.; van Zuijlen, P.; Niessen, H. The Local and Systemic Inflammatory Response in a Pig Burn Wound Model With a Pivotal Role for Complement. J. Burn Care Res. 2017, 38, e796–e806. [Google Scholar] [CrossRef]
- Devine, R.; Diltz, Z.; Hall, M.W.; Thakkar, R.K. The systemic immune response to pediatric thermal injury. Int. J. Burn. Trauma 2018, 8, 6–16. [Google Scholar]
- akir, B.; Yeğen, B.Ç. Systemic Responses to Burn Injury. Turk. J. Med Sci. 2004, 34, 215–226. [Google Scholar]
- Toliver-Kinsky, T.; Kobayashi, M.; Suzuki, F.; Sherwood, E.R. 19—The Systemic Inflammatory Response Syndrome. In Total Burn Care, 5th ed.; Herndon, D.N., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 205–220.e4. ISBN 978-0-323-47661-4. [Google Scholar]
- Keller, S. Metabolic Effects of Cytokines in Burns Trauma and Sepsis—Wound Healing. Available online: https://www.alpfmedical.info/wound-healing/metabolic-effects-of-cytokines-in-burns-trauma-and-sepsis.html (accessed on 21 February 2022).
- Sojka, J.; Krakowski, A.C.; Stawicki, S.P. Burn Shock and Resuscitation: Many Priorities, One Goal; Intechopen: London, UK, 2020. [Google Scholar] [CrossRef]
- Schaefer, T.J.; Nunez Lopez, O. Burn Resuscitation and Management; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Rae, L.; Fidler, P.; Gibran, N. The Physiologic Basis of Burn Shock and the Need for Aggressive Fluid Resuscitation. Crit. Care Clin. 2016, 32, 491–505. [Google Scholar] [CrossRef]
- Bittner, M.E.A.; Shank, M.E.; Woodson, L.C.; Martyn, M.J.A.J. Acute and Perioperative Care of the Burn-injured Patient. Anesthesiology 2015, 122, 448–464. [Google Scholar] [CrossRef]
- Romanowski, K.S.; Palmieri, T.L. Pediatric burn resuscitation: Past, present, and future. Burn. Trauma 2017, 5, 26. [Google Scholar] [CrossRef]
- Sharma, R.K.; Parashar, A. Special considerations in paediatric burn patients. Indian J. Plast. Surg. 2010, 43, 43–50. [Google Scholar] [CrossRef]
- Wurzer, P.; Culnan, D.; Cancio, L.C.; Kramer, G.C. Pathophysiology of Burn Shock and Burn Edema. In Total Burn Care, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 66–76.e3. [Google Scholar] [CrossRef]
- Causbie, J.M.; Sattler, L.A.; Basel, A.P.; Britton, G.W.; Cancio, L.C. State of the Art: An Update on Adult Burn Resuscitation. Eur. Burn J. 2021, 2, 12. [Google Scholar] [CrossRef]
- Kara, Y.A. Burn Etiology and Pathogenesis; IntechOpen: London, UK, 2018; ISBN 978-1-78923-131-1. [Google Scholar]
- Williams, F.N.; Herndon, D.N.; Suman, O.E.; Lee, J.O.; Norbury, W.B.; Branski, L.K.; Mlcak, R.P.; Jeschke, M.G. Changes in Cardiac Physiology After Severe Burn Injury. J. Burn Care Res. 2011, 32, 269–274. [Google Scholar] [CrossRef]
- Dash, S.; Ghosh, S. Transient Diabetes Insipidus Following Thermal Burn—A Case Report and Literature Review. Bull. Emerg. Trauma 2017, 5, 311–313. [Google Scholar] [CrossRef][Green Version]
- Ciftci, A.; Esen, O.; Yazicioglu, M.B.; Haksal, M.C.; Tiryaki, C.; Gunes, A.; Civil, O.; Ozyildiz, M.; Esen, H.; Ciftci, A.; et al. Could Neutrophil-to-Lymphocyte Ratio Be a New Mortality Predictor Value in Severe Burns? J. Surg. Surg. Res. 2019, 5, 026–028. [Google Scholar]
- Andreu-Ballester, J.C.; Pons-Castillo, A.; González-Sánchez, A.; Llombart-Cussac, A.; Cano, M.J.; Cuéllar, C. Lymphopenia in hospitalized patients and its relationship with severity of illness and mortality. PLoS ONE 2021, 16, e0256205. [Google Scholar] [CrossRef]
- Singh, V.; Devgan, L.; Bhat, S.; Milner, S.M. The Pathogenesis of Burn Wound Conversion. Ann. Plast. Surg. 2007, 59, 109–115. [Google Scholar] [CrossRef]
- Mariano, F.; de Biase, C.; Hollo, Z.; Deambrosis, I.; Davit, A.; Mella, A.; Bergamo, D.; Maffei, S.; Rumbolo, F.; Papaleo, A.; et al. Long-Term Preservation of Renal Function in Septic Shock Burn Patients Requiring Renal Replacement Therapy for Acute Kidney Injury. J. Clin. Med. 2021, 10, 5760. [Google Scholar] [CrossRef]
- Lang, T.C.; Zhao, R.; Kim, A.; Wijewardena, A.; Vandervord, J.; Xue, M.; Jackson, C.J. A Critical Update of the Assessment and Acute Management of Patients with Severe Burns. Adv. Wound Care 2019, 8, 607–633. [Google Scholar] [CrossRef]
- Sobouti, B.; Fallah, S.; Ghavami, Y.; Moradi, M. Serum immunoglobulin levels in pediatric burn patients. Burns 2013, 39, 473–476. [Google Scholar] [CrossRef]
- Megha, K.B.; Mohanan, P.V. Role of immunoglobulin and antibodies in disease management. Int. J. Biol. Macromol. 2020, 169, 28–38. [Google Scholar] [CrossRef]
- Boldeanu, L.; Boldeanu, M.V.; Bogdan, M.; Meca, A.D.; Coman, C.G.; Buca, B.R.; Tartau, C.G.; Tartau, L.M. Immunological approaches and therapy in burns (Review). Exp. Ther. Med. 2020, 20, 2361–2367. [Google Scholar] [CrossRef]
- Yussof, S.J.M.; Omar, E.; Pai, D.; Sood, S. Cellular events and biomarkers of wound healing. Indian J. Plast. Surg. 2012, 45, 220–228. [Google Scholar] [CrossRef]
- Strbo, N.; Yin, N.; Stojadinovic, O. Innate and Adaptive Immune Responses in Wound Epithelialization. Adv. Wound Care 2014, 3, 492–501. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef]
- Suda, S.; Williams, H.; Medbury, H.J.; Holland, A.J.A. A Review of Monocytes and Monocyte-Derived Cells in Hypertrophic Scarring Post Burn. J. Burn Care Res. 2016, 37, 265–272. [Google Scholar] [CrossRef]
- Beckmann, N.; Schumacher, F.; Kleuser, B.; Gulbins, E.; Nomellini, V.; Caldwell, C.C. Burn Injury Impairs Neutrophil Chemotaxis through Increased Ceramide. Shock 2020, 56, 125–132. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum. Vaccines Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef]
- Grywalska, E.; Smarz-Widelska, I.; Korona-Głowniak, I.; Mertowski, S.; Gosik, K.; Hymos, A.; Ludian, J.; Niedźwiedzka-Rystwej, P.; Roliński, J.; Załuska, W. PD-1 and PD-L1 Expression on Circulating Lymphocytes as a Marker of Epstein-Barr Virus Reactivation-Associated Proliferative Glomerulonephritis. Int. J. Mol. Sci. 2020, 21, 8001. [Google Scholar] [CrossRef] [PubMed]
- Van Coillie, S.; Wiernicki, B.; Xu, J. Molecular and Cellular Functions of CTLA-4. In Regulation of Cancer Immune Checkpoints: Molecular and Cellular Mechanisms and Therapy; Xu, J., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2020; pp. 7–32. ISBN 9789811532665. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korzeniowski, T.; Mertowska, P.; Mertowski, S.; Podgajna, M.; Grywalska, E.; Strużyna, J.; Torres, K. The Role of the Immune System in Pediatric Burns: A Systematic Review. J. Clin. Med. 2022, 11, 2262. https://doi.org/10.3390/jcm11082262
Korzeniowski T, Mertowska P, Mertowski S, Podgajna M, Grywalska E, Strużyna J, Torres K. The Role of the Immune System in Pediatric Burns: A Systematic Review. Journal of Clinical Medicine. 2022; 11(8):2262. https://doi.org/10.3390/jcm11082262
Chicago/Turabian StyleKorzeniowski, Tomasz, Paulina Mertowska, Sebastian Mertowski, Martyna Podgajna, Ewelina Grywalska, Jerzy Strużyna, and Kamil Torres. 2022. "The Role of the Immune System in Pediatric Burns: A Systematic Review" Journal of Clinical Medicine 11, no. 8: 2262. https://doi.org/10.3390/jcm11082262
APA StyleKorzeniowski, T., Mertowska, P., Mertowski, S., Podgajna, M., Grywalska, E., Strużyna, J., & Torres, K. (2022). The Role of the Immune System in Pediatric Burns: A Systematic Review. Journal of Clinical Medicine, 11(8), 2262. https://doi.org/10.3390/jcm11082262