Cochlear Implantation Improves Both Speech Perception and Patient-Reported Outcomes: A Prospective Follow-Up Study of Treatment Benefits among Adult Cochlear Implant Recipients
Abstract
1. Introduction
2. Materials and Methods
2.1. Cochlear Implantation
2.2. Main Outcome Measures
2.3. Audiometric Test Measures
2.3.1. Pure-Tone Audiometry (PTA) and Speech Recognition Score (SRS)
2.3.2. Dantale I
2.3.3. Hearing in Noise Test (HINT)
2.4. Patient-Reported Outcome Measures
2.4.1. Nijmegen Cochlear Implant Questionnaire (NCIQ)
2.4.2. Speech, Spatial, and Qualities of Hearing Scale (SSQ)
2.5. Statistical Analysis
3. Results
3.1. Study Subjects
3.2. Speech Perception Outcomes
3.2.1. Baseline PTA6 and SRS Results
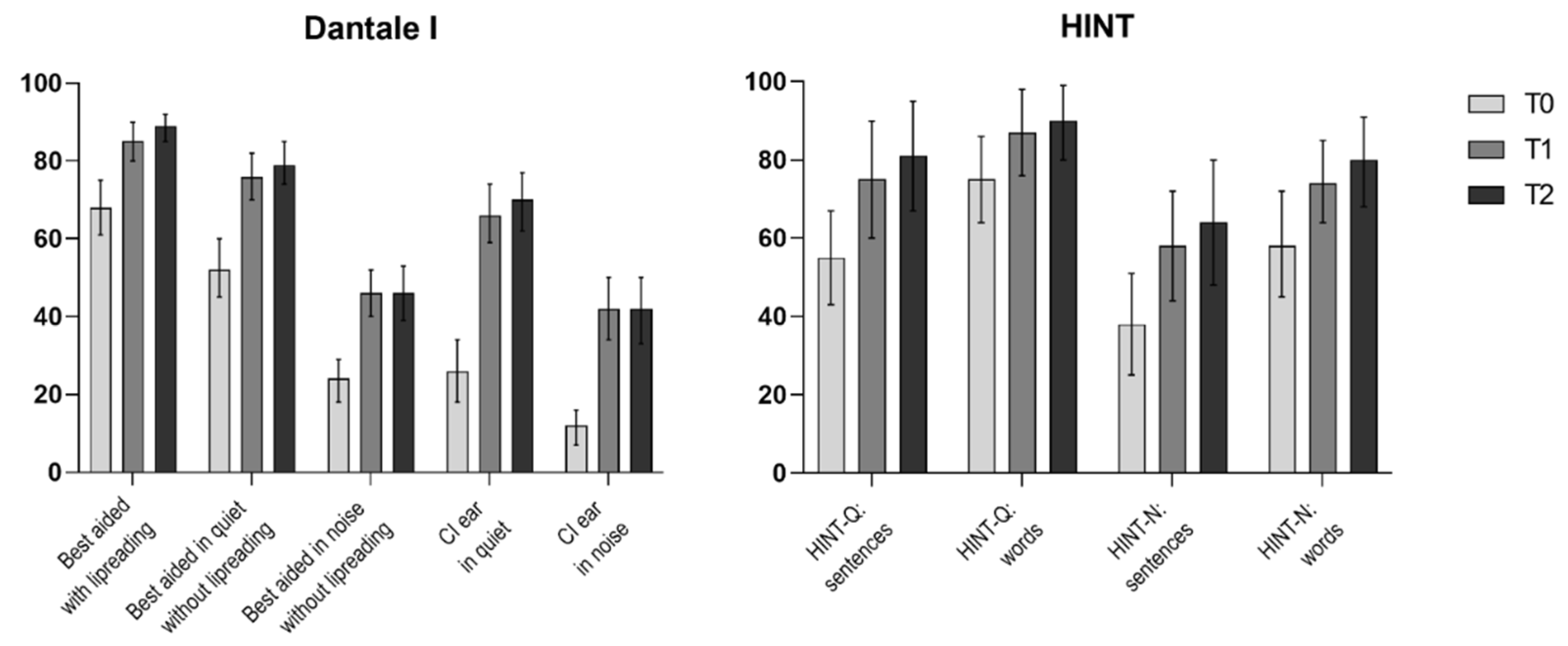
3.2.2. Dantale I Results
3.2.3. HINT Results
3.3. Patient-Reported Outcomes
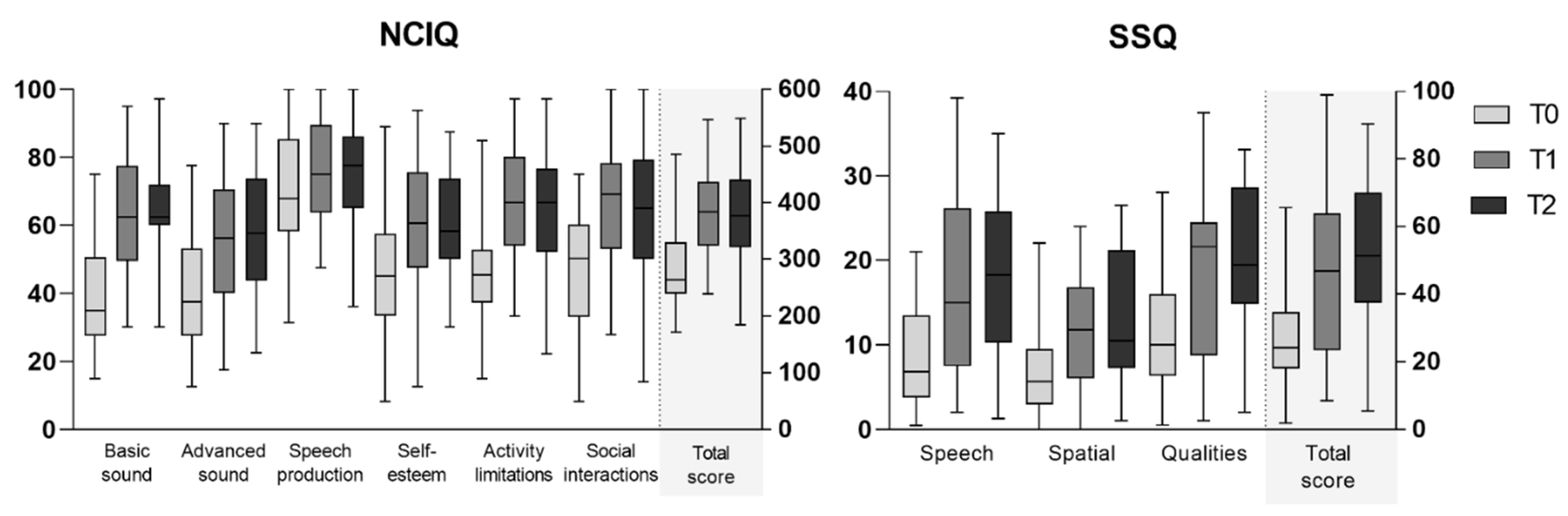
3.3.1. NCIQ Results
3.3.2. SSQ12 Results
3.4. Comparisons between Audiometric and Patient-Reported Outcome Measures
4. Discussion
Study Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | cochlear implant |
| dB | decibel |
| HA | hearing aid |
| HINT | hearing in noise test |
| NCIQ | Nijmegen Cochlear Implant Questionnaire |
| PROM | patient-reported outcome measure |
| PTA6 | pure-tone audiometry average for six frequencies |
| SNHL | sensorineural hearing loss |
| SNR | signal-to-noise ratio |
| SRS | speech recognition score |
| SSQ | Speech, Spatial, and Qualities of Hearing Scale |
References
- Choi, J.S.; Contrera, K.J.; Betz, J.F.; Blake, C.R.; Niparko, J.K.; Lin, F.R. Long-Term Use of Cochlear Implants in Older Adults. Otol. Neurotol. 2014, 35, 815–820. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Contrera, K.J.; Sung, Y.K.; Betz, J.; Li, L.; Lin, F.R. Change in loneliness after intervention with cochlear implants or hearing aids. Laryngoscope 2017, 127, 1885–1889. [Google Scholar] [CrossRef] [PubMed]
- Kelsall, D.; Lupo, J.; Biever, A. Longitudinal outcomes of cochlear implantation and bimodal hearing in a large group of adults: A multicenter clinical study. Am. J. Otolaryngol. 2021, 42, 102773. [Google Scholar] [CrossRef] [PubMed]
- Gaylor, J.M.; Raman, G.; Chung, M.; Lee, J.; Rao, M.; Lau, J.; Poe, D.S. Cochlear Implantation in Adults: A Systematic Review and Meta-analysis. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 265. [Google Scholar] [CrossRef]
- West, N.C.; Kressner, A.A.; Baungaard, L.H.; Sandvej, M.G.; Bille, M.; Cayé-Thomasen, P. Nordic results of cochlear implantation in adults: Speech perception and patient reported outcomes. Acta Oto-Laryngol. 2020, 140, 939–947. [Google Scholar] [CrossRef]
- Buchman, C.A.; Herzog, J.A.; McJunkin, J.L.; Wick, C.C.; Durakovic, N.; Firszt, J.B.; Kallogjeri, D. Assessment of Speech Understanding After Cochlear Implantation in Adult Hearing Aid Users. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 916. [Google Scholar] [CrossRef]
- Yuen, E.; Ma, C.; Nguyen, S.A.; Meyer, T.A.; Lambert, P.R. The Effect of Cochlear Implantation on Tinnitus and Quality of Life: A Systematic Review and Meta-analysis. Otol. Neurotol. 2021, 42, 1113–1122. [Google Scholar] [CrossRef]
- Vasil, K.J.; Lewis, J.; Tamati, T.; Ray, C.; Moberly, A.C. How Does Quality of Life Relate to Auditory Abilities? A Subitem Analysis of the Nijmegen Cochlear Implant Questionnaire. J. Am. Acad. Audiol. 2020, 31, 292–301. [Google Scholar] [CrossRef]
- Olze, H.; Szczepek, A.J.; Haupt, H.; Zirke, N.; Graebel, S.; Mazurek, B. The impact of cochlear implantation on tinnitus, stress and quality of life in postlingually deafened patients. Audiol. Neurootol. 2012, 17, 2–11. [Google Scholar] [CrossRef]
- Dammeyer, J.; Chapman, M. Prevalence and characteristics of self-reported physical and mental disorders among adults with hearing loss in Denmark: A national survey. Soc. Psychiatry Psychiatr. Epidemiol. 2017, 52, 807–813. [Google Scholar] [CrossRef]
- McRackan, T.R.; Bauschard, M.; Hatch, J.L.; Franko-Tobin, E.; Droghini, H.R.; Velozo, C.A.; Nguyen, S.A.; Dubno, J.R. Meta-analysis of Cochlear Implantation Outcomes Evaluated With General Health-related Patient-reported Outcome Measures. Otol. Neurotol. 2018, 39, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Sundhedsdatastyrelsen. Cochlear Implant in Denmark 2005–2018; Sundhedsdatastyrelsen: Copenhagen, Danmark, 2020. [Google Scholar]
- Dowell, R.C.; Hollow, R.; Winton, E. Outcomes for Cochlear Implant Users With Significant Residual Hearing: Implications for Selection Criteria in Children. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 575–581. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Danish Society of Otorhinolaryngology Head and Neck Surgery. Udredning af voksne til CI. 2014, Volume 1. Available online: http://dsohh.dk/wp-content/uploads/2015/04/DSOHH-KKR-CI-voksne1.pdf (accessed on 4 April 2022).
- Elberling, C.; Ludvigsen, C.; Lyregaard, P.E. Dantale: A new danish speech material. Scand. Audiol. 1989, 18, 169–175. [Google Scholar] [CrossRef] [PubMed]
- FORCE Technology, Teknisk Audiologisk Laboratorium. Instructions for Setting Up and Calibrating Equipment for Audiometry in Free Field Translated from Danish. FORCE Technology; Teknisk Audiologisk Laboratorium: Odense, Denmark, 2020; Available online: https://audiologi.dk/wp-content/uploads/2021/01/FF-Vejledning-3ed2.pdf (accessed on 4 April 2022).
- Nilsson, M.; Soli, S.D.; Sullivan, J.A. Development of the Hearing In Noise Test for the measurement of speech reception thresholds in quiet and in noise. J. Acoust. Soc. Am. 1994, 95, 1085–1099. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Dau, T. The Danish hearing in noise test. Int. J. Audiol. 2011, 50, 202–208. [Google Scholar] [CrossRef]
- Hinderink, J.B.; Krabbe, P.F.M.; Van Den Broek, P. Development and application of a health-related quality-of-life instrument for adults with cochlear implants: The Nijmegen Cochlear Implant Questionnaire. Otolaryngol. Head Neck Surg. 2000, 123, 756–765. [Google Scholar] [CrossRef]
- Gatehouse, S.; Noble, W. The Speech, Spatial and Qualities of Hearing Scale (SSQ). Int. J. Audiol. 2004, 43, 85–99. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; Version 25.0; IBM CorpReleased: Armonk, NY, USA, 2017. [Google Scholar]
- Graphpad Prism Softare. Graphpad Prism, Version 9.0.0 for Windows; Graphpad Prism Softare: San Diego, CA, USA, 2020; Available online: https://www.graphpad.com (accessed on 4 April 2022).
- Cusumano, C.; Friedmann, D.R.; Fang, Y.; Wang, B.; Roland, J.T.J.; Waltzman, S.B. Performance Plateau in Prelingually and Postlingually Deafened Adult Cochlear Implant Recipients. Otol. Neurotol. 2017, 38, 334–338. [Google Scholar] [CrossRef]
- Budenz, C.L.; Cosetti, M.K.; Coelho, D.H.; Birenbaum, B.; Babb, J.; Waltzman, S.; Roehm, P.C. The Effects of Cochlear Implantation on Speech Perception in Older Adults. J. Am. Geriatr. Soc. 2011, 59, 446–453. [Google Scholar] [CrossRef]
- Sladen, D.P.; Peterson, A.; Schmitt, M.; Olund, A.; Teece, K.; Dowling, B.; DeJong, M.; Breneman, A.; Beatty, C.W.; Carlson, M.L.; et al. Health-related quality of life outcomes following adult cochlear implantation: A prospective cohort study. Cochlear Implant. Int. 2017, 18, 130–135. [Google Scholar] [CrossRef]
- Häußler, S.M.; Knopke, S.; Wiltner, P.; Ketterer, M.; Grabel, S.; Olze, H. Long-term Benefit of Unilateral Cochlear Implantation on Quality of Life and Speech Perception in Bilaterally Deafened Patients. Otol. Neurotol. 2019, 40, e430–e440. [Google Scholar] [CrossRef]
- Wallhäusser-Franke, E.; Balkenhol, T.; Hetjens, S.; Rotter, N.; Servais, J.J. Patient Benefit Following Bimodal CI-provision: Self-reported Abilities vs. Hearing Status. Front. Neurol. 2018, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Hirschfelder, A.; Gräbel, S.; Olze, H. The impact of cochlear implantation on quality of life: The role of audiologic performance and variables. Otolaryngol. Head Neck Surg. 2008, 138, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Olze, H.; Gräbel, S.; Förster, U.; Zirke, N.; Huhnd, L.E.; Haupt, H.; Mazurek, B. Elderly patients benefit from cochlear implantation regarding auditory rehabilitation, quality of life, tinnitus, and stress. Laryngoscope 2012, 122, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Tavora-Vieira, D.; Marino, R.; Acharya, A.; Rajan, G.P. The impact of cochlear implantation on speech understanding, subjective hearing performance, and tinnitus perception in patients with unilateral severe to profound hearing loss. Otol. Neurotol. 2015, 36, 430–436. [Google Scholar] [CrossRef]
- Carlson, M.L.; Driscoll, C.L.W.; Gifford, R.H.; McMenomey, S.O. Cochlear implantation: Current and future device options. Otolaryngol. Clin. N. Am. 2012, 45, 221–248. [Google Scholar] [CrossRef]
- Cohen, S.M.; Labadie, R.F.; Dietrich, M.S.; Haynes, D.S. Quality of Life in Hearing-Impaired Adults: The Role of Cochlear Implants and Hearing Aids. Otolaryngol. Head Neck Surg. 2004, 131, 413–422. [Google Scholar] [CrossRef]
- McRackan, T.R.; Bauschard, M.; Hatch, J.L.; Franko-Tobin, E.; Droghini, H.R.; Nguyen, S.A.; Dubno, J.R. Meta-analysis of quality-of-life improvement after cochlear implantation and associations with speech recognition abilities. Laryngoscope 2018, 128, 982–990. [Google Scholar] [CrossRef]
- Plath, M.; Marienfeld, T.; Sand, M.; van de Weyer, P.S.; Praetorius, M.; Plinkert, P.K.; Baumann, I.; Zaoui, K. Prospective study on health-related quality of life in patients before and after cochlear implantation. Eur. Arch. Oto-Rhino-Laryngol. 2021, 279, 115–125. [Google Scholar] [CrossRef]
- Hänsel, T.; Gauger, U.; Bernhard, N.; Behzadi, N.; Ventura, M.E.R.; Hofmann, V.; Olze, H.; Knopke, S.; Todt, I.; Coordes, A. Meta-analysis of subjective complaints of vertigo and vestibular tests after cochlear implantation. Laryngoscope 2018, 128, 2110–2123. [Google Scholar] [CrossRef]
- Damen, G.W.J.A.; Beynon, A.J.; Krabbe, P.F.M.; Mulder, J.J.S.; Mylanus, E.A.M. Cochlear implantation and quality of life in postlingually deaf adults: Long-term follow-up. Otolaryngol. Head Neck Surg. 2007, 136, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Fuller, C.; Free, R.; Maat, B.; Başkent, D. Musical background not associated with self-perceived hearing performance or speech perception in postlingual cochlear-implant users. J. Acoust. Soc. Am. 2012, 132, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Lenarz, T.; Muller, L.; Czerniejewska-Wolska, H.; Varela, H.V.; Dotú, C.O.; Durko, M.; Irujo, A.H.; Piszczatowski, B.; Zadrożniak, M.; Irwin, C.; et al. Patient-Related Benefits for Adults with Cochlear Implantation: A Multicultural Longitudinal Observational Study. Audiol. Neurootol. 2017, 22, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Dingemanse, G.; Goedegebure, A. The relation of hearing-specific patient-reported outcome measures with speech perception measures and acceptable noise levels in cochlear implant users. Int. J. Audiol. 2020, 59, 416–426. [Google Scholar] [CrossRef]
- Boisvert, I.; Reis, M.; Au, A.; Cowan, R.; Dowell, R.C. Cochlear implantation outcomes in adults: A scoping review. PLoS ONE. 2020, 15, e0232421. [Google Scholar] [CrossRef]
- Olze, H.; Szczepek, A.J.; Haupt, H.; Förster, U.; Zirke, N.; Gräbel, S.; Mazurek, B. Cochlear implantation has a positive influence on quality of life, tinnitus, and psychological comorbidity. Laryngoscope 2011, 121, 2220–2227. [Google Scholar] [CrossRef]
- Noble, W.; Tyler, R.; Dunn, C.; Bhullar, N. Unilateral and bilateral cochlear implants and the implant-plus-hearing-aid profile: Comparing self-assessed and measured abilities. Int. J. Audiol. 2008, 47, 505–514. [Google Scholar] [CrossRef]
- Gifford, R.H.; Shallop, J.K.; Peterson, A.M. Speech recognition materials and ceiling effects: Considerations for cochlear implant programs. Audiol. Neurootol. 2008, 13, 193–205. [Google Scholar] [CrossRef]
| Age at Implantation | 28–90 Years (Mean 64) |
|---|---|
| Gender | 15 females (38%), 24 males (62%) |
| Implanted side | 23 left (59%), 16 right (41%) |
| Duration of hearing aids | |
| CI ear | 18 years (range 0–57 years) |
| Contralateral ear | 19 years (range 0–57 years) |
| Hearing loss degree | |
| CI ear | |
| Moderate–severe (56–90 dB) | 24 (62%) |
| Profound (91+ dB) | 15 (38%) |
| Contralateral ear | |
| Moderate–severe (56–90 dB) | 31 (79%) |
| Profound (91+ dB) | 8 (21%) |
| Type of implant | 1 (3%) Advanced Bionics HiRes Ultra 3D SlimJ |
| 2 (5%) Advanced Bionics HiRes90K Midscale | |
| 3 (8%) Advanced Bionics ULTRA 3D Midscale | |
| 1 (3%) MED-EL Flex 28 Synchrony | |
| 22 (56%) Nucleus Cochlear CI522 | |
| 6 (13%) Nucleus Cochlear CI622 | |
| 4 (10%) Oticon Medical Zti EVO | |
| Audiometric test—days after implantation | |
| T1 | 235 days, 110–330 (mean, range) ~ 8 months |
| T2 | 440 days, 343–636 (mean, range) ~ 15 months |
| PROMs—days after implantation | |
| T1 | 124 days, 66–241 (mean, range) ~ 4 months |
| T2 | 426 days, 301–569 (mean, range) ~ 14 months |
| ID | Etiology | Gender | Age | PTA6 Operated Ear | PTA6 Contralateral Ear | SRS CI Ear | SRS Contralateral Ear |
|---|---|---|---|---|---|---|---|
| (Years) | (dB HL) | (dB HL) | (%) | (%) | |||
| 1 | Ménière’s disease | M | 77 | 77 | 52 | 45 | 80 |
| 2 | Otitis media | M | 73 | 110 | 106 | 9 | 53 |
| 3 | Otosclerosis | F | 53 | 120 | 78 | 0 | 68 |
| 4 | Congenital (unknown etiology) | F | 46 | 108 | 115 | 0 | 0 |
| 5 | Unknown | M | 70 | 98 | 70 | 0 | 28 |
| 6 | Unknown | M | 74 | 98 | 86 | 48 | 33 |
| 7 | Late-onset progressive hereditary | F | 69 | 77 | 83 | 60 | 60 |
| 8 | Superficial Siderosis | F | 67 | 68 | 59 | 24 | 24 |
| 9 | Late-onset progressive hereditary | M | 69 | 87 | 89 | 24 | 32 |
| 10 | Hereditary congenital | M | 69 | 111 | 90 | 0 | 45 |
| 11 | Unknown | M | 81 | 78 | 69 | 14 | 56 |
| 12 | Unknown | M | 70 | 108 | 78 | 0 | 85 |
| 13 | Usher syndrome | F | 60 | 92 | 86 | 50 | 68 |
| 14 | Late-onset progressive hereditary | F | 62 | 76 | 71 | 29 | 28 |
| 15 | Late-onset progressive hereditary | M | 60 | 75 | 78 | 45 | 55 |
| 16 | Unknown | F | 75 | 103 | 82 | 0 | 9 |
| 17 | Late-onset progressive hereditary | M | 59 | 108 | 108 | 0 | 0 |
| 18 | Unknown | M | 88 | 67 | 64 | 56 | 49 |
| 19 | Ototoxicity | F | 90 | 79 | 77 | 0 | 0 |
| 20 | Unknown | M | 74 | 75 | 63 | 14 | 21 |
| 21 | Late-onset progressive hereditary | M | 80 | 63 | 55 | 20 | 35 |
| 22 | Unknown | M | 73 | 78 | 77 | 39 | 28 |
| 23 | Unknown | M | 56 | 99 | 74 | 0 | 77 |
| 24 | Neurofibromatosis | M | 56 | 68 | 72 | 15 | 35 |
| 25 | Congenital (unknown etiology) | M | 53 | 103 | 84 | 35 | 85 |
| 26 | Vestibular schwannoma | M | 75 | 83 | 118 | 50 | 0 |
| 27 | Pendred syndrome | F | 28 | 84 | 70 | 33 | 70 |
| 28 | Hereditary congenital | F | 43 | 96 | 87 | 48 | 60 |
| 29 | Unknown | F | 40 | 86 | 118 | 40 | 0 |
| 30 | Late-onset progressive hereditary | M | 72 | 78 | 74 | 35 | 65 |
| 31 | Unknown | F | 85 | 115 | 82 | 0 | 10 |
| 32 | Unknown | F | 30 | 88 | 63 | 31 | 68 |
| 33 | Otosclerosis | M | 81 | 88 | 86 | 20 | 30 |
| 34 | Pneumococcal meningitis | M | 69 | 56 | 53 | 30 | 40 |
| 35 | Late-onset progressive hereditary | M | 87 | 79 | 59 | 55 | 40 |
| 39 | Unknown | F | 38 | 73 | 118 | 75 | 0 |
| 40 | Unknown | M | 79 | 73 | 67 | 41 | 68 |
| 42 | Late-onset progressive hereditary | F | 36 | 73 | 68 | 78 | 72 |
| 43 | Hereditary congenital | M | 33 | 104 | 91 | 8 | 40 |
| ID | Dantale I | HINT | NCIQ | SSQ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Best Aided with Lipreading | Best Aided in Quiet without Lipreading | Best Aided in Noise without Lipreading | CI Ear in Quiet | CI Ear in Noise | HINT-Q: Sentences | HINT-Q: Words | HINT-N: Sentences | HINT-N: Words | |||
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | Total Score | Total Score | |
| 1 | 85/94/89 | 85/89/84 | 51/43/60 | 46/53/51 | 29/21/38 | 50/75/100 | 80/92/100 | 35/68/80 | 62/79/88 | 257/293/265 | NA/42/42 |
| 2 | 86/98/90 | 65/81/88 | 28/49/54 | NA/81/76 | NA/45/54 | 45/85/90 | 69/96/98 | 15/45/70 | 18/67/78 | 232/268/273 | 30/37/32 |
| 3 | 59/94/91 | 34/91/96 | 21/53/59 | NA/71/76 | NA/44/59 | NA/95/100 | NA/98/100 | NA/60/80 | NA/83/90 | 239/289/377 | 27/34/NA |
| 4 | 40/63/86 | 2/44/59 | NA/NA/NA | 2/44/59 | NA/NA/NA | NA/NA/NA | NA/NA/NA | NA/NA/NA | NA/NA/NA | 329/323/348 | 25/16/47 |
| 5 | 41/94/99 | 31/88/96 | NA/49/55 | 9/71/88 | NA/49/55 | NA/95/80 | NA/98/91 | NA/65/90 | NA/83/96 | 246/448/487 | 26/77/84 |
| 6 | 86/86/84 | 49/75/78 | NA/19/33 | 8/48/58 | NA/19/33 | 10/NA/65 | 28/NA/87 | NA/NA/20 | 8/NA/44 | 263/425/410 | 23/50/56 |
| 7 | NA/98/96 | 45/93/83 | 40/71/74 | 45/91/83 | 36/65/74 | 70/95/90 | 86/99/98 | 35/75/80 | 63/91/93 | 377/423/435 | 64/57/62 |
| 8 | 88/79/84 | 78/46/65 | 30/30/21 | 48/46/65 | NA/30/21 | 35/20/20 | 46/33/33 | 15/NA/NA | 33/NA/NA | 171/248/185 | 5/20/5 |
| 9 | 70/86/94 | 30/76/78 | 10/36/14 | 16/73/78 | 4/36/14 | NA/60/60 | NA/78/80 | NA/25/40 | NA/56/65 | 253/418/403 | 39/65/59 |
| 10 | 62/93/90 | 34/68/75 | 3/58/53 | NA/68/75 | NA/58/53 | NA/20/40 | 14/42/68 | NA/10/20 | NA/34/51 | 486/533/533 | 54/90/74 |
| 11 | 84/99/99 | 75/91/95 | 64/54/54 | 29/74/90 | 26/40/53 | NA/NA/NA | NA/NA/NA | NA/NA/NA | NA/NA/NA | 266/412/328 | 27/56/42 |
| 12 | 89/98/96 | 77/88/89 | 36/58/55 | 35/88/86 | NA/56/55 | 70/100/100 | 92/100/100 | 60/75/80 | 81/91/92 | 234/328/315 | 18/31/41 |
| 13 | 86/94/95 | 63/91/88 | 24/36/39 | 18/74/79 | 18/36/30 | 55/100/100 | 80/100/100 | 55/75/85 | 73/84/96 | 373/487/NA | 46/72/74 |
| 14 | 66/88/98 | 70/93/91 | 27/60/63 | 70/86/81 | 27/60/55 | 60/90/95 | 80/96/99 | 30/60/65 | 61/76/84 | 293/434/447 | 16/57/37 |
| 15 | 92/100/99 | 69/83/89 | 21/64/69 | 36/83/89 | 13/64/69 | 75/100/85 | 88/100/95 | 45/70/60 | 65/82/78 | 240/385/425 | 20/58/90 |
| 16 | 38/53/61 | 8/40/54 | NA/5/4 | 1/28/26 | NA/5/NA | NA/NA/5 | NA/NA/22 | NA/NA/NA | NA/NA/NA | NA/226/NA | NA/NA/NA |
| 17 | 58/NA/63 | 28/NA/21 | NA/NA/1 | 10/NA/15 | NA/NA/1 | NA/NA/NA | 5/NA/NA | NA/NA/NA | NA/NA/NA | 261/341/NA | 29/9/NA |
| 18 | 83/88/NA | 66/85/85 | 14/44/51 | 40/69/73 | 6/33/41 | NA/NA/NA | NA/NA/NA | NA/NA/NA | NA/NA/NA | 373/508/549 | 32/71/71 |
| 19 | 26/76/84 | 7/73/76 | NA/NA/11 | NA/65/76 | NA/NA/NA | NA/NA/NA | NA/NA/NA | NA/NA/NA | NA/NA/NA | 256/341/364 | 17/45/NA |
| 20 | 57/88/86 | 39/66/74 | 15/30/30 | 9/46/45 | 4/19/28 | NA/NA/NA | NA/NA/NA | NA/NA/NA | NA/NA/NA | 311/355/360 | 16/25/NA |
| 21 | 94/96/91 | 70/84/80 | 21/43/45 | 31/84/68 | 3/43/33 | 95/90/85 | 99/98/93 | 80/85/75 | 91/93/88 | 325/396/368 | 52/56/72 |
| 22 | 79/96/95 | 65/98/94 | 13/73/69 | 23/98/88 | 13/73/61 | 40/85/NA | 74/96/NA | 40/NA/NA | 64/NA/NA | 444/547/532 | 66/64/NA |
| 23 | 89/98/98 | 61/96/93 | 31/49/68 | NA/78/90 | NA/49/59 | 85/100/NA | 95/100/NA | 35/90/NA | 61/98/NA | 238/308/NA | 19/16/NA |
| 24 | 65/88/85 | 59/81/78 | 14/38/36 | NA/81/78 | NA/38/36 | NA/84/75 | NA/94/93 | NA/49/65 | NA/65/87 | 259/371/404 | 9/19/NA |
| 25 | 87/100/96 | 70/81/91 | 44/60/54 | NA/61/73 | NA/31/26 | 35/70/85 | 59/86/93 | 40/40/15 | 56/66/44 | 310/414/433 | 38/49/69 |
| 26 | 40/75/86 | 29/84/84 | 8/46/55 | 29/84/84 | 8/46/55 | NA/70/75 | NA/85/90 | NA/20/50 | NA/42/72 | 340/339/374 | 32/16/40 |
| 27 | 88/93/91 | 74/89/95 | 40/75/45 | 41/68/69 | 11/33/44 | 50/65/80 | 72/87/89 | 15/45/60 | 42/78/78 | 384/461/478 | 47/99/NA |
| 28 | 88/96/99 | 79/98/91 | 20/66/73 | 33/91/88 | 16/66/73 | 75/80/100 | 85/95/100 | 20/93/90 | 47/80/92 | 332/526/529 | 18/74/60 |
| 29 | 39/83/93 | 34/54/69 | 16/30/51 | NA/54/69 | NA/30/51 | NA/5/15 | NA/35/54 | NA/NA/NA | NA/NA/21 | 177/239/291 | 9/NA/19 |
| 30 | 73/NA/96 | 64/91/93 | 24/85/63 | 41/85/89 | 4/85/63 | 80/85/100 | 90/99/100 | 75/80/90 | 80/94/98 | 340/487/432 | 24/73/51 |
| 31 | 20/48/69 | 6/23/33 | NA/NA/NA | NA/23/33 | NA/NA/NA | NA/NA/NA | NA/NA/NA | NA/NA/NA | NA/NA/NA | 315/384/332 | 24/19/22 |
| 32 | 70/93/98 | 49/75/90 | 46/28/49 | NA/75/85 | NA/28/49 | 30/95/95 | 74/95/99 | 15/45/90 | 63/66/98 | 206/385/474 | 18/35/66 |
| 33 | 85/96/90 | 58/74/73 | 29/43/41 | NA/65/73 | NA/43/30 | NA/78/85 | NA/87/90 | NA/60/35 | NA/88/61 | 194/495/421 | 7/86/52 |
| 34 | 52/53/64 | 50/40/30 | 45/45/24 | 25/40/30 | 16/45/24 | NA/NA/NA | 9/15/NA | NA/NA/NA | NA/NA/NA | 288/287/304 | 29/34/26 |
| 35 | 43/65/69 | 23/48/41 | 10/18/1 | 10/44/31 | 8/18/NA | NA/NA/NA | NA/NA/NA | NA/NA/NA | NA/NA/NA | 235/308/290 | 19/31/18 |
| 39 | 74/79/90 | 74/79/81 | 44/45/39 | 74/79/81 | 44/45/39 | 55/25/30 | 80/71/67 | 55/5/NA | 73/35/35 | 210/355/351 | 21/51/76 |
| 40 | 70/58/80 | 56/53/69 | 17/30/16 | 19/43/64 | 15/30/14 | 40/75/NA | 65/86/NA | 25/35/NA | 50/65/NA | 242/329/308 | 20/15/40 |
| 42 | 85/98/100 | 72/94/95 | 42/70/74 | 57/86/86 | 36/70/68 | 35/NA/NA | 65/NA/NA | 20/NA/NA | 54/NA/NA | 322/NA/NA | 40/50/NA |
| 43 | 58/86/85 | 59/88/91 | 10/53/69 | 19/63/73 | NA/46/55 | 10/NA/NA | 41/NA/NA | NA/NA/NA | 16/NA/NA | 328/418/NA | 2/41/NA |
| n | T0 | T1 | T2 | Mean Difference (T0–T2) a | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Dantale | ||||||||
| Best aided with lipreading | (%) | 35 | 68 (61–75) | 85 (80–90) | 89 (85–92) | 21 (13–28) | <0.001 | 0.588 |
| Best aided in quiet without lipreading | (%) | 38 | 52 (45–60) | 76 (70–82) | 79 (74–85) | 27 (19–35) | <0.001 | 0.667 |
| Best aided in noise without lipreading | (%) | 36 | 24 (18–29) | 46 (40–52) | 46 (39–53) | 22 (13–31) | <0.001 | 0.516 |
| CI ear in quiet | (%) | 31 | 26 (18–34) | 66 (59–74) | 70 (62–77) | 43 (33–53) | <0.001 | 0.794 |
| CI ear in noise | (%) | 31 | 12 (7–16) | 42 (34–50) | 42 (33–50) | 30 (20–40) | <0.001 | 0.663 |
| HINT | ||||||||
| HINT-Q: sentences | (%) | 16 | 55 (43–67) | 75 (60–90) | 81 (67–95) | 26 (9–43) | <0.001 | 0.528 |
| HINT-Q: words | (%) | 16 | 75 (64–86) | 87 (76–98) | 90 (80–99) | 15 (3–26) | 0.003 | 0.446 |
| HINT-N: sentences | (%) | 15 | 38 (25–51) | 58 (44–72) | 64 (48–80) | 26 (1–50) | 0.012 | 0.370 |
| HINT-N: words | (%) | 15 | 58 (45–72) | 74 (64–85) | 80 (68–91) | 21 (4–39) | 0.006 | 0.432 |
| T0 | T1 | T2 | Wilcoxon Signed Rank Test | ||||
|---|---|---|---|---|---|---|---|
| T0–T1 | T0–T2 | ||||||
| Z | p-Value | Z | p-Value | ||||
| NCIQ total score | 264 (239–330) | 384 (324–437) | 377 (321–441) | −5.2 | <0.001 | −5.0 | <0.001 |
| Physical subdomains | |||||||
| Basic sound perception | 35 (28–51) | 63 (49–78) | 63 (60–72) | −5.2 | <0.001 | −4.9 | <0.001 |
| Advanced sound perception | 38 (28–53) | 56 (40–71) | 58 (44–74) | −4.8 | <0.001 | −4.4 | <0.001 |
| Speech production | 68 (58–85) | 75 (64–90) | 78 (65–86) | −2.6 | 0.009 | −2.1 | 0.024 |
| Psychological subdomain | |||||||
| Self-esteem | 45 (33–58) | 61 (48–76) | 58 (50–74) | −4.5 | <0.001 | −4.1 | <0.001 |
| Social subdomains | |||||||
| Activity limitations | 45 (37–53) | 67 (54–80) | 67 (52–77) | −5.2 | <0.001 | −4.8 | <0.001 |
| Social interactions | 50 (33–60) | 69 (53–78) | 65 (50–79) | −4.8 | <0.001 | −4.7 | <0.001 |
| SSQ total score | 24 (18–35) | 47 (23–64) | 51 (37–70) | −3.7 | <0.001 | −4.1 | <0.001 |
| Speech | 7 (4–14) | 15 (8–26) | 18 (10–26) | −4.4 | <0.001 | −4.4 | <0.001 |
| Spatial | 6 (3–10) | 12 (6–17) | 11 (7–21) | −3.6 | <0.001 | −3.2 | 0.002 |
| Qualities | 10 (6–16) | 22 (9–25) | 20 (15–29) | −2.9 | 0.004 | −4.0 | <0.001 |
| Dantale | HINT | |||||||
|---|---|---|---|---|---|---|---|---|
| Test Condition | Best Aided in Quiet without Lipreading | Best Aided in Noise without Lipreading | CI Ear in Quiet | CI Ear in Noise | HINT-Q: Sentences | HINT-Q: Words | HINT-N: Sentences | HINT-N: Words |
| NCIQ total score | 0.53 *** | 0.44 ** | 0.48 ** | 0.47 ** | 0.15 | 0.13 | 0.21 | 0.26 |
| SSQ total score | 0.45 * | 0.30 | 0.45 * | 0.32 | −0.02 | 0.02 | 0.07 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasmussen, K.M.B.; West, N.C.; Bille, M.; Sandvej, M.G.; Cayé-Thomasen, P. Cochlear Implantation Improves Both Speech Perception and Patient-Reported Outcomes: A Prospective Follow-Up Study of Treatment Benefits among Adult Cochlear Implant Recipients. J. Clin. Med. 2022, 11, 2257. https://doi.org/10.3390/jcm11082257
Rasmussen KMB, West NC, Bille M, Sandvej MG, Cayé-Thomasen P. Cochlear Implantation Improves Both Speech Perception and Patient-Reported Outcomes: A Prospective Follow-Up Study of Treatment Benefits among Adult Cochlear Implant Recipients. Journal of Clinical Medicine. 2022; 11(8):2257. https://doi.org/10.3390/jcm11082257
Chicago/Turabian StyleRasmussen, Kasper Møller Boje, Niels Cramer West, Michael Bille, Matilde Grønborg Sandvej, and Per Cayé-Thomasen. 2022. "Cochlear Implantation Improves Both Speech Perception and Patient-Reported Outcomes: A Prospective Follow-Up Study of Treatment Benefits among Adult Cochlear Implant Recipients" Journal of Clinical Medicine 11, no. 8: 2257. https://doi.org/10.3390/jcm11082257
APA StyleRasmussen, K. M. B., West, N. C., Bille, M., Sandvej, M. G., & Cayé-Thomasen, P. (2022). Cochlear Implantation Improves Both Speech Perception and Patient-Reported Outcomes: A Prospective Follow-Up Study of Treatment Benefits among Adult Cochlear Implant Recipients. Journal of Clinical Medicine, 11(8), 2257. https://doi.org/10.3390/jcm11082257







