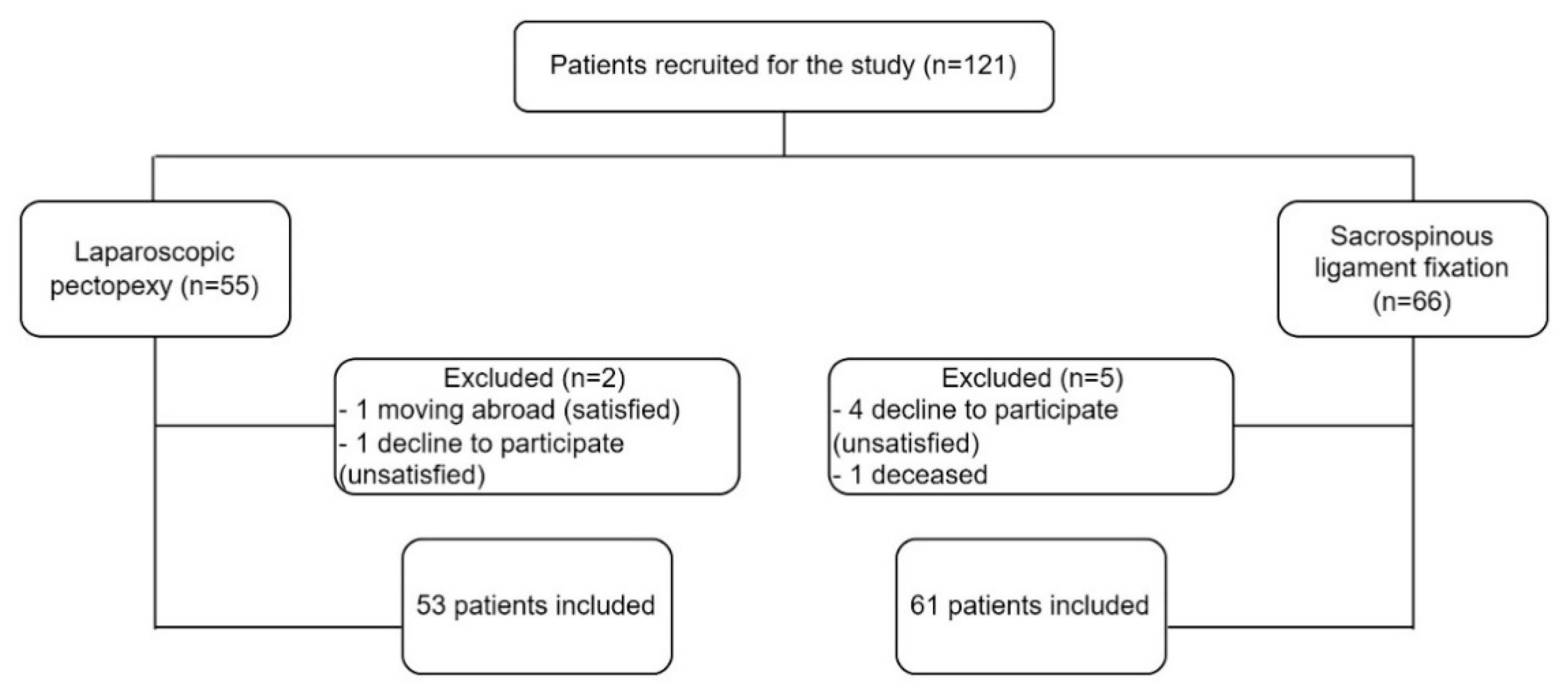
Perioperative and Long-Term Anatomical and Subjective Outcomes of Laparoscopic Pectopexy and Sacrospinous Ligament Suspension for POP-Q Stages II–IV Apical Prolapse
Abstract
:1. Introduction
2. Material and Methods
- Stage 0: No prolapse is demonstrated.
- Stage I: The most distal portion of the prolapse is more than 1 cm above the level of the hymen.
- Stage II: The most distal portion of the prolapse is situated between 1 cm above the hymen and 1 cm below the hymen.
- Stage III: The most distal portion of the prolapse is more than 1 cm beyond the plane of the hymen but everted at least 2 cm less than the total vaginal length.
- Stage IV: Complete eversion or eversion at least within 2 cm of the total length of the lower genital tract is demonstrated [24].
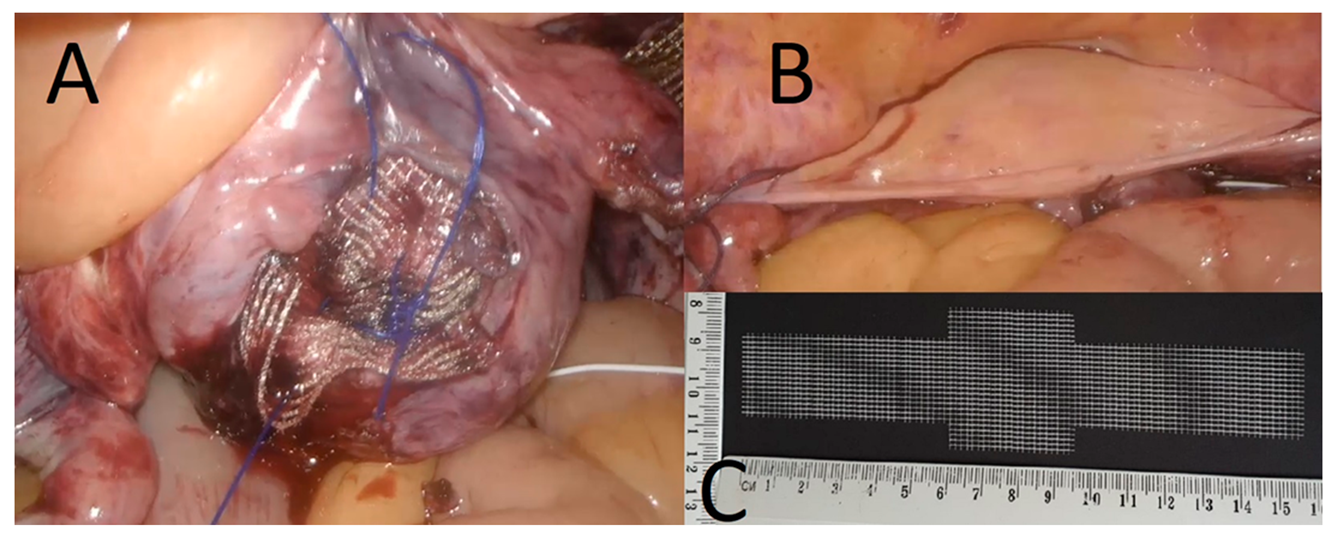
2.1. Procedure Description
2.2. Statistical Analysis
3. Results
3.1. Patient Characteristics and Complications
3.2. Urinary Incontinence
3.3. Anatomical Results
3.4. Subjective Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mattsson, N.K.; Karjalainen, P.K.; Tolppanen, A.M.; Heikkinen, A.M.; Sintonen, H.; Härkki, P.; Nieminen, K.; Jalkanen, J. Pelvic organ prolapse surgery and quality of life-a nationwide cohort study. Am. J. Obstet. Gynecol. 2020, 222, 588.e1–588.e10. [Google Scholar] [CrossRef] [PubMed]
- Favre-Inhofer, A.; Carbonnel, M.; Murtada, R.; Revaux, A.; Asmar, J.; Ayoubi, J.-M. Sacrospinous ligament fixation: Medium and long-term anatomical results, functional and quality of life results. BMC Women’s Health 2021, 21, 66. [Google Scholar] [CrossRef] [PubMed]
- Paz-Levy, D.; Yohay, D.; Neymeyer, J.; Hizkiyahu, R.; Weintraub, A.Y. Native tissue repair for central compartment prolapse: A narrative review. Int. Urogynecol. J. 2017, 28, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Dällenbach, P.; Kaelin-Gambirasio, I.; Jacob, S.; Dubuisson, J.B.; Boulvain, M. Incidence rate and risk factors for vaginal vault prolapse repair after hysterectomy. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2008, 19, 1623–1629. [Google Scholar] [CrossRef] [Green Version]
- Aboseif, C.; Liu, P. Pelvic Organ Prolapse. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Karjalainen, P.K.; Mattsson, N.K.; Nieminen, K.; Tolppanen, A.M.; Jalkanen, J.T. The relationship of defecation symptoms and posterior vaginal wall prolapse in women undergoing pelvic organ prolapse surgery. Am. J. Obstet. Gynecol. 2019, 221, 480.e1–480.e10. [Google Scholar] [CrossRef]
- Jelovsek, J.E.; Barber, M.D.; Brubaker, L.; Norton, P.; Gantz, M.; Richter, H.E.; Weidner, A.; Menefee, S.; Schaffer, J.; Pugh, N.; et al. NICHD Pelvic Floor Disorders Network. Effect of Uterosacral Ligament Suspension vs Sacrospinous Ligament Fixation with or without Perioperative Behavioral Therapy for Pelvic Organ Vaginal Prolapse on Surgical Outcomes and Prolapse Symptoms at 5 Years in the OPTIMAL Randomized Clinical Trial. JAMA 2018, 319, 1554–1565. [Google Scholar]
- Hamdy, M.A.; Ahmed, W.A.S.; Taha, O.T.; Abolill, Z.M.; Elshahat, A.M.; Aboelroose, A.A. Late suture site complications of sacrospinous ligament fixation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 242, 126–130. [Google Scholar] [CrossRef]
- Tseng, L.H.; Chen, I.; Chang, S.H.; Lee, C.-L. Modern role of sacrospinous ligament fixation for pelvic organ prolapse surgery—A systemic review. Taiwan J. Obstet. Gynecol. 2013, 52, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Richter, K. The surgical treatment of the prolapsed vaginal fundus after uterine extirpation. A contribution on Amreich’s the sacrotuberal vaginal fixation. Die operative Behandlung des prolabierten Scheidengrundes nach Uterusexstirpation. Ein Beitrag zur Vaginaefixatio sacrotuberalis nach Amreich. Geburtshilfe Frauenheilkd 1967, 27, 941–954. [Google Scholar]
- Joshi, V.M. A new technique of uterine suspension to pectineal ligaments in the management of uterovaginal prolapse. Obstet. Gynecol. 1993, 81 Pt 1, 790–793. [Google Scholar]
- Banerjee, C.; Noé, K.G. Laparoscopic pectopexy: A new technique of prolapse surgery for obese patients. Arch. Gynecol. Obstet. 2011, 284, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Noé, K.G.; Spüntrup, C.; Anapolski, M. Laparoscopic pectopexy: A randomised comparative clinical trial of standard laparoscopic sacral colpo-cervicopexy to the new laparoscopic pectopexy. Short-term postoperative results. Arch. Gynecol. Obstet. 2013, 287, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Noé, K.G.; Schiermeier, S.; Alkatout, I.; Anapolski, M. Laparoscopic pectopexy: A prospective, randomized, comparative clinical trial of standard laparoscopic sacral colpocervicopexy with the new laparoscopic pectopexy-postoperative results and intermediate-term follow-up in a pilot study. J. Endourol. 2015, 29, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Biler, A.; Ertas, I.E.; Tosun, G.; Hortu, I.; Turkay, U.; Gultekin, O.E.; Igci, G. Perioperative complications and short-term outcomes of abdominal sacrocolpopexy, laparoscopic sacrocolpopexy, and laparoscopic pectopexy for apical prolapse. Int. Braz. J. Urol. 2018, 44, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Noé, G.K.; Schiermeier, S.; Papathemelis, T.; Fuellers, U.; Khudyakovd, A.; Altmann, H.H.; Borowski, S.; Morawski, P.P.; Gantert, M.; De Vree, B.; et al. Prospective International Multicenter Pelvic Floor Study: Short-Term Follow-Up and Clinical Findings for Combined Pectopexy and Native Tissue Repair. J. Clin. Med. 2021, 10, 217. [Google Scholar] [CrossRef]
- Szymczak, P.; Grzybowska, M.E.; Sawicki, S.; Wydra, D.G. Laparoscopic Pectopexy-CUSUM Learning Curve and Perioperative Complications Analysis. J. Clin. Med. 2021, 10, 1052. [Google Scholar] [CrossRef]
- Astepe, B.S.; Karsli, A.; Köleli, I.; Aksakal, O.S.; Terzi, H.; Kale, A. Intermediate-term outcomes of laparoscopic pectopexy and vaginal sacrospinous fixation: A comparative study. Int. Braz. J. Urol. 2019, 45, 999–1007. [Google Scholar] [CrossRef] [Green Version]
- Developed by the Joint Writing Group of the American Urogynecologic Society and the International Urogynecological Association. Joint report on terminology for surgical procedures to treat pelvic organ prolapse. Int. Urogynecol. J. 2020, 31, 429–463. [Google Scholar] [CrossRef]
- Salman, S.; Kumbasar, S.; Yeniocak, A.S. Uterine preserving technique in the treatment of pelvic organ prolapse: Laparoscopic pectopexy. J. Obstet. Gynaecol. Res. 2022, 48, 850–856. [Google Scholar] [CrossRef]
- Bagli, I.; Tahaoglu, E.A. Pregnancy outcomes after laparoscopic pectopexy surgery: A case series. J. Obstet. Gynaecol. Res. 2020, 46, 1364–1369. [Google Scholar] [CrossRef]
- Lykke, R.; Blaakær, J.; Ottesen, B.; Gimbel, H. The indication for hysterectomy as a risk factor for subsequent pelvic organ prolapse repair. Int. Urogynecol. J. 2015, 26, 1661–1665. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.bsge.org.uk/news/nice-consultation-document-mesh-pectopexy/ (accessed on 17 February 2022).
- Haylen, B.T.; Maher, C.F.; Barber, M.D.; Camargo, S.; Dandolu, V.; Digesu, A.; Goldman, H.B.; Huser, M.; Milani, A.L.; Moran, P.A.; et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic organ prolapse (POP). Int. Urogynecol. J. 2016, 27, 165–194. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; Santibanes, E.; Pekolj, J.; Slankamenac, K.; Bassix, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzybowska, M.E.; Rechberger, T.; Wrobel, A.; Baranowski, W.; Stangel-Wojcikiewicz, K.; Rogowski, A.; Kluz, T.; Narojczyk-Swiesciak, E.; Wlazlak, E.; Burzynski, B.; et al. The Urogynecology Section of the Polish Society of Gynecologists and Obstetricians guidelines on the management of non-neurogenic overactive bladder syndrome in women. Ginekol. Pol. 2021, 92, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, S.; Robinson, D.; Cardozo, L. Validation of the patient global impression of improvement (PGI-I) for urogenital prolapse. Int. Urogynecol. J. 2010, 21, 523–528. [Google Scholar] [CrossRef]
- Sandvik, H.; Seim, A.; Vanvik, A.; Hunskaar, S. A severity index for epidemiological surveys of female urinary incontinence: Comparison with 48-hour pad-weighing tests. Neurourol. Urodyn. 2000, 19, 137–145. [Google Scholar] [CrossRef]
- Bochenska, K.; Grzybowska, M.E.; Piaskowska-Cala, J.; Mueller, M.; Lewicky-Gaupp, C.; Wydra, D.; Kenton, K. Translation and validation of the Polish version of the Pelvic Floor Impact Questionnaire short form 7. Int. Urogynecol. J. 2021, 32, 3177–3181. [Google Scholar] [CrossRef]
- Grzybowska, M.E.; Griffith, J.W.; Kenton, K.; Mueller, M.; Piaskowska-Cala, J.; Lewicky-Gaupp, C.; Wydra, D.; Bochenska, K. Validation of the Polish version of the Pelvic Floor Distress Inventory. Int. Urogynecol. J. 2019, 30, 101–105. [Google Scholar] [CrossRef]
- Grzybowska, M.E.; Piaskowska-Cala, J.; Wydra, D.G. Polish translation and validation of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire, IUGA-Revised (PISQ-IR). Int. Urogynecol. J. 2019, 30, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Noé, G.K.; Schiermeier, S.; Papathemelis, T.; Fuellers, U.; Khudyakov, A.; Altmann, H.H.; Borowski, S.; Morawski, P.P.; Gantert, M.; De Vree, B.; et al. Prospective international multicenter pectopexy trial: Interim results and findings post surgery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 244, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Paraiso, M.F.; Ballard, L.A.; Walters, M.D.; Lee, J.C.; Mitchinson, A.R. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am. J. Obstet. Gynecol. 1996, 175, 1423–1430. [Google Scholar] [CrossRef]
- Aksakal, O.; Doğanay, M.; Onur Topçu, H.; Kokanali, K.; Erkilinç, S.; Cavkaytar, S. Long-term surgical outcomes of vaginal sacrospinous ligament fixation in women with pelvic organ prolapse. Minerva Chir. 2014, 69, 239–244. [Google Scholar] [PubMed]
- Colombo, M.; Milani, R. Sacrospinous ligament fixation and modified McCall culdoplasty during vaginal hysterectomy for advanced uterovaginal prolapse. Am. J. Obstet. Gynecol. 1998, 179, 13–20. [Google Scholar] [CrossRef]
- Tahaoglu, A.E.; Bakir, M.S.; Peker, N.; Bagli, İ.; Tayyar, A.T. Modified laparoscopic pectopexy: Short-term follow-up and its effects on sexual function and quality of life. Int. Urogynecol. J. 2018, 29, 1155–1160. [Google Scholar] [CrossRef]
- Szymczak, P.; Wydra, D.G. Evisceration of the small intestine through the vagina as a rare complication after laparoscopic pectopexy. Ginekol. Pol. 2021, 92, 673–674. [Google Scholar] [CrossRef]
- Hefni, M.; El-Toukhy, T.; Bhaumik, J.; Katsimanis, E. Sacrospinous cervicocolpopexy with uterine conservation for uterovaginal prolapse in elderly women: An evolving concept. Am. J. Obstet. Gynecol. 2003, 188, 645–650. [Google Scholar] [CrossRef]
- Pasley, W.W. Sacrospinous suspension: A local practitioner’s experience. Am. J. Obstet. Gynecol. 1995, 173, 440–448. [Google Scholar] [CrossRef]
- Sze, E.H.; Karram, M.M. Transvaginal repair of vault prolapse: A review. Obstet. Gynecol. 1997, 89, 466–475. [Google Scholar] [CrossRef]
- Greisen, S.; Axelsen, S.M.; Bek, K.M.; Guldberg, R.; Glavind-Kristensen, M. Fast track sacrospinous ligament fixation: Subjective and objective outcomes at 6 months. BMC Women’s Health 2021, 21, 154. [Google Scholar] [CrossRef]
- Zhang, W.; Cheon, W.C.; Zhang, L.; Wang, X.; Wei, Y.; Lyu, C. Comparison of the effectiveness of sacrospinous ligament fixation and sacrocolpopexy: A meta-analysis. Int. Urogynecol. J. 2022, 33, 3–13. [Google Scholar] [CrossRef]
- Karslı, A.; Karslı, O.; Kale, A. Laparoscopic Pectopexy: An Effective Procedure for Pelvic Organ Prolapse with an Evident Improvement on Quality of Life. Prague Med. Rep. 2021, 122, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, A.; Staat, M. A computational study of organ relocation after laparoscopic pectopexy to repair posthysterectomy vaginal vault prolapse. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2020, 8, 277–286. [Google Scholar] [CrossRef]
- Baki, M.; Hakan Güraslan, S.; Çakmak, Y.; Ekin, M. Bilateral sacrospinous fixation without hysterectomy: 18-month follow-up. J. Turk. Ger. Gynecol. Assoc. 2015, 16, 102–106. [Google Scholar]
- Salman, S.; Babaoglu, B.; Kumbasar, S.; Bestel, M.; Ketenci Gencer, F.; Tuna, G.; Besimoglu, B.; Yüksel, S.; Uçar, E. Comparison of Unilateral and Bilateral Sacrospinous Ligament Fixation Using Minimally Invasive Anchorage. Geburtshilfe Frauenheilkd 2019, 79, 976–982. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-46.pdf (accessed on 17 February 2022).
- Başer, E.; Seçkin, K.D.; Kadiroğullari, P.; Kiyak, H. The effect of sacrospinous ligament fixation during vaginal hysterectomy on postoperative de novo stress incontinence occurrence: A prospective study with 2-year follow-up. Turk. J. Med. Sci. 2020, 50, 978–984. [Google Scholar] [CrossRef]
| Variables | LP (n = 53) | SSLF (n = 61) | p-Value |
|---|---|---|---|
| Age (years) | 62.3 ± 8.8 | 61.8 ± 8.7 | 0.75 a |
| BMI (kg/m2) | 27.3 ± 4.0 | 27.6 ± 4.1 | 0.75 a |
| Postmenopausal | 46 (86.8%) | 55 (90.2%) | 0.57 b |
| Parity | 2.3 ± 1.1 | 2.5 ± 1.1 | 0.2 a |
| 2 [0–7] | 2 [1–7] | ||
| Pre-operative POP-Q stage | |||
| 2 | 1 (1.9%) | 0 | 0.34 b |
| 3 | 41 (77.3%) | 43 (70.5%) | |
| 4 | 11 (20.8%) | 18 (29.5%) | |
| Previous POP surgery | |||
| anterior colporrhaphy | 7 (13.2%) | 10 (16.4%) | 0.63 b |
| posterior colporrhaphy | 6 (11.3%) | 12 (19.7%) | 0.22 b |
| anterior vaginal repair with mesh | 0 | 1 (1.6%) | 0.35 b |
| posterior vaginal repair with mesh | 1 (1.9%) | 0 | 0.28 b |
| Concurrent POP surgery | <0.001 b | ||
| anterior colporrhaphy | 0 | 21 (34.2%) | |
| posterior colporrhaphy | 2 (3.8%) | 37 (60.7%) | |
| laparoscopic anterior repair | 2 (3.8%) | X | |
| laparoscopic posterior repair | 1 (1.9%) | X | |
| Operative time (min) | 151.8 ± 36.2 | 69 ± 20.4 | <0.001 a |
| Change in hemoglobin level (g/dL) | 1.5 ± 0.5 | 1.5 ± 0.6 | 0.76 a |
| Inpatient stay (days) | 2.6 ± 1.1 | 2.7 ± 1.0 | 0.42 a |
| 2 [1–7] | 2 [2–6] | ||
| Patients with perioperative complications [C-D] | 0.70 b | ||
| I | 8 (15.1%) | 12 (19.7%) | |
| II | 0 | 0 | |
| III | 1 (1.9%) | 1 (1.6%) | |
| IV | 0 | 0 | |
| Total | 9 (17%) | 13 (21.3%) |
| Variables | LP (n = 53) | SSLF (n = 61) | p-Value |
|---|---|---|---|
| Previous UI surgery | 4 (7.5%) | 3 (4.9%) | 0.56 a |
| Transobturator tape | 1 (1.9%) | 3 (4.9%) | 0.38 a |
| Kelly plication | 3 (5.7%) | 0 | 0.06 a |
| Concurrent SUI surgery | 0 | 0 | - |
| UI postoperatively | |||
| SUI de novo | 5 (9.4%) | 5 (8.2%) | 0.82 a |
| SUI persistent | 7 (13.2%) | 5 (8.2%) | 0.38 a |
| UUI de novo | 0 | 2 (3.3%) | 0.18 a |
| UUI persistent | 5 (9.4%) | 4 (6.5%) | 0.57 a |
| Mixed UI de novo | 2 (3.8%) | 2 (3.3%) | 0.88 a |
| Mixed UI persistent | 9 (17%) | 12 (19.7%) | 0.71 a |
| Positive cough stress test | |||
| de novo | 2 (3.8%) | 5 (8.2%) | 0.33 a |
| persistent | 10 (18.9%) | 15 (24.6%) | 0.46 a |
| Rehospitalizations with MUS insertion | |||
| total | 5 (9.4%) | 1 (1.6%) | 0.06 a |
| transobturator tape | 5 (9.4%) | 0 | 0.01 a |
| retropubic tape | 0 | 1 (1.6%) | 0.35 a |
| LP (n = 53) | SSLF (n = 61) | Comparison of Change between Groups | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Change | p-Value | Pre | Post | Change | p-Value | p-Value | |
| Aa | 1.7 ± 2.1 | −0.4 ± 2.6 | −2.1 ± 2.4 | <0.001 a | 1.0 ± 2.0 | −0.2 ± 2.3 | −0.8 ± 2.3 | 0.01 a | 0.003 b |
| Ba | 3.6 ± 4.0 | 0.7 ± 4.0 | −3.0 ± 4.0 | <0.001 a | 3.7 ± 3.4 | 1.4 ± 3.5 | −2.3 ± 3.6 | <0.001 a | 0.37 b |
| Ap | 0.1 ± 2.2 | −1.2 ± 1.9 | −0.8 ± 2.6 | 0.03 a | 0.02 ± 2.3 | −2.2 ± 1.2 | −1.5 ± 2.8 | <0.001 a | 0.17 b |
| Bp | 2 ± 4.5 | −0.6 ± 3.1 | −2.1 ± 4.4 | <0.001 a | 2.1 ± 4.1 | −1.7 ± 2.3 | −3.0 ± 4.6 | <0.001 a | 0.25 b |
| C | 5.4 ± 3.0 | −4.2 ± 4.6 | −8.6 ± 5.1 | <0.001 a | 5.6 ± 2.6 | −3.4 ± 4.8 | −3.7 ± 3.4 | <0.001 a | 0.09 b |
| TVL | 9.5 ± 1.4 | 9.0 ± 1.5 | −0.5 ± 0.8 | <0.001 a | 9.8 ± 1.6 | 8.8 ± 1.6 | −1.3 ± 1.3 | <0.001 a | 0.001 b |
| Variables | LP (n = 53) | SSLF (n = 61) | p-Value |
|---|---|---|---|
| Follow-up (months) | 26.9 ± 12 | 37.3 ± 17.5 | <0.001 a |
| 25 [5–50] | 39 [6–99] | ||
| Surgical failure POP-Q C ≥ −1 | 13 (24.5%) | 25 (41%) | 0.06 b |
| Reoperation for apical prolapse | |||
| Bilateral SSHP with graft | 2 (3.8%) | 1 (1.6%) | |
| LP | 3 (5.7%) | 11 (18%) | |
| SSLF | 0 | 3 (5%) | |
| LeFort colpocleisis | 0 | 1 (1.6%) | |
| Total | 5 (9.4%) | 16 (26.2%) | 0.02 b |
| Mesh complications | X | - | |
| mesh detachment | 7 (13.2%) | ||
| mesh exposure | 2 (3.8%) |
| Variables | LP (n = 53) | SSLF (n = 61) | p-Value |
|---|---|---|---|
| Change in pain intensity (VAS, score 0–10) | 0.03 ± 1.8 | −0.67 ± 1.5 | 0.03 a |
| Constipation | |||
| de novo | 1 (1.9%) | 2 (3.3%) | 0.64 b |
| persistent | 16 (30.2%) | 17 (27.9%) | 0.78 b |
| Sexually active | |||
| preoperative | 24 (45.3%) | 27 (44.3%) | 0.91 b |
| postoperative | 23 (43.4%) | 26 (42.6%) | 0.93 b |
| Satisfaction with surgery | 0.26 b | ||
| very satisfied | 21 (39.6%) | 16 (26.2%) | |
| satisfied | 11 (20.7%) | 22 (36.1%) | |
| neutral | 6 (11.3%) | 8 (13.1%) | |
| unsatisfied | 14 (26.4%) | 12 (20%) | |
| very unsatisfied | 1 (1.9%) | 3 (5%) | |
| Willingness to suggest such surgery to a friend with a similar problem | 0.98 b | ||
| yes, I would | 31 (58.5%) | 34 (55.7%) | |
| rather yes | 5 (9.4%) | 6 (10%) | |
| I am not sure | 5 (9.4%) | 5 (8.2%) | |
| rather not | 3 (5.7%) | 3 (5%) | |
| no | 9 (17%) | 13 (21.3%) | |
| PGI-I | 0.08 b | ||
| very much better | 21 (39.6%) | 20 (32.8%) | |
| much better | 7 (13.2%) | 16 (26.2%) | |
| a little better | 12 (22.6%) | 8 (13.1%) | |
| same as before surgery | 10 (18.9%) | 13 (21.3%) | |
| a bit worse | 0 | 4 (6.5%) | |
| much worse | 3 (5.7%) | 0 | |
| very much worse | 0 | 0 |
| LP (n = 53) | SSLF (n = 61) | Comparison of Change between Groups | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Change | p-Value | Pre | Post | Change | p-Value | p-Value | |
| ISI | 2.0 ± 2.0 | 1.6 ± 2.1 | −0.5 ± 2.1 | 0.11 b | 1.4 ± 1.9 | 1.5 ± 2.1 | 0.1 ± 2.0 | 0.8 b | 0.18 a |
| EPIQ#35 | 8.8 ± 1.9 | 5.5 ± 3.3 | −3.8 ± 3.3 | <0.001 b | 8.4 ± 2.2 | 5.6 ± 3.2 | −2.8 ± 3.2 | <0.001 b | 0.26 a |
| PFDI-20 | |||||||||
| POPDI-6 | 51.7 ± 22.6 | 20.8 ± 22.5 | −20.8 ± 22.8 | <0.001 b | 52.5 ± 21.3 | 21.1 ± 24.5 | −15.4 ± 30.0 | <0.001 b | 0.28 a |
| CRADI-8 | 22.2 ± 19.9 | 16.5 ± 18.8 | −5.8 ± 16.9 | 0.02 b | 23.1 ± 21.3 | 17.3 ± 2.0 | −5.8 ± 22.0 | 0.04 b | 0.99 a |
| UDI-6 | 46.5 ± 26.0 | 25.6 ± 25.2 | −31.0 ± 21.0 | <0.001 b | 40.2 ± 27.0 | 24.9 ± 25.5 | −31.3 ± 28.5 | <0.001 b | 0.92 a |
| Total score | 120.3 ± 60.0 | 62.8 ± 57.3 | −57.5 ± 51.4 | <0.001 b | 115.7 ± 54.7 | 63.2 ± 56.0 | −52.5 ± 64.8 | <0.001 b | 0.65 a |
| PFIQ-7 | |||||||||
| UIQ-7 | 27.8 ± 29.5 | 21.2 ± 28.2 | −6.6 ± 32.5 | 0.14 b | 26.5 ± 30.0 | 19.5 ± 28.2 | −7.0 ± 32.1 | 0.09 b | 0.95 a |
| CRAIQ-7 | 11.6 ± 23.4 | 11.3 ± 20.6 | −9.6 ± 31.8 | 0.09 b | 13.0 ± 20.5 | 9.0 ± 17.4 | −6.6 ± 31.4 | 0.15 b | 0.61 a |
| POPIQ-7 | 31.6 ± 24.0 | 16.1 ± 23.5 | −10.4 ± 33.1 | <0.001 b | 37.5 ± 25.7 | 14.4 ± 23.3 | −18.0 ± 35.3 | <0.001 b | 0.24 a |
| Total score | 71.0 ± 68.1 | 48.6 ± 64.2 | −7.4 ± 90.1 | 0.04 b | 77.0 ± 64.2 | 43.0 ± 54.3 | −18.5 ± 89.4 | <0.001 b | 0.51 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymczak, P.; Grzybowska, M.E.; Sawicki, S.; Futyma, K.; Wydra, D.G. Perioperative and Long-Term Anatomical and Subjective Outcomes of Laparoscopic Pectopexy and Sacrospinous Ligament Suspension for POP-Q Stages II–IV Apical Prolapse. J. Clin. Med. 2022, 11, 2215. https://doi.org/10.3390/jcm11082215
Szymczak P, Grzybowska ME, Sawicki S, Futyma K, Wydra DG. Perioperative and Long-Term Anatomical and Subjective Outcomes of Laparoscopic Pectopexy and Sacrospinous Ligament Suspension for POP-Q Stages II–IV Apical Prolapse. Journal of Clinical Medicine. 2022; 11(8):2215. https://doi.org/10.3390/jcm11082215
Chicago/Turabian StyleSzymczak, Paulina, Magdalena Emilia Grzybowska, Sambor Sawicki, Konrad Futyma, and Dariusz Grzegorz Wydra. 2022. "Perioperative and Long-Term Anatomical and Subjective Outcomes of Laparoscopic Pectopexy and Sacrospinous Ligament Suspension for POP-Q Stages II–IV Apical Prolapse" Journal of Clinical Medicine 11, no. 8: 2215. https://doi.org/10.3390/jcm11082215
APA StyleSzymczak, P., Grzybowska, M. E., Sawicki, S., Futyma, K., & Wydra, D. G. (2022). Perioperative and Long-Term Anatomical and Subjective Outcomes of Laparoscopic Pectopexy and Sacrospinous Ligament Suspension for POP-Q Stages II–IV Apical Prolapse. Journal of Clinical Medicine, 11(8), 2215. https://doi.org/10.3390/jcm11082215







