Magnetic Resonance Features of Liver Mucinous Colorectal Metastases: What the Radiologist Should Know
Abstract
:1. Introduction
2. Materials and Methods
2.1. MR Imaging Protocol
2.2. Images Analysis
- The maximum diameter of the lesions, in millimeters, on axial T1-W sequences, on axial T2-W sequence and in the portal phase of the contrast study.
- The signal intensity (SI) in T1 W, in T2-W, DWI sequences and the apparent diffusion coefficient (ADC) map.
- The presence and the type of contrast enhancement (CE) during the contrast study.
2.3. Reference Standard
2.4. Statistical Analysis
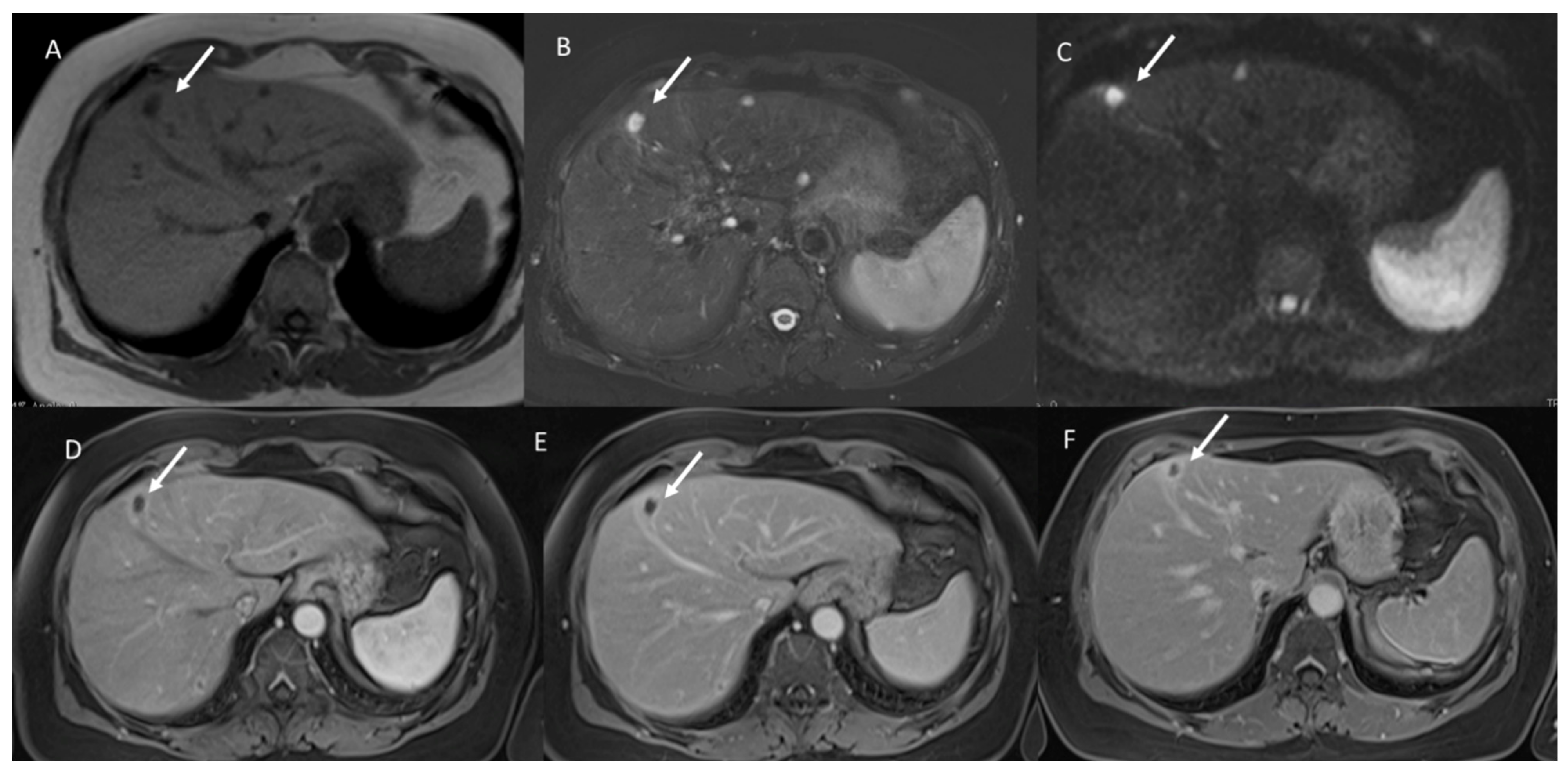
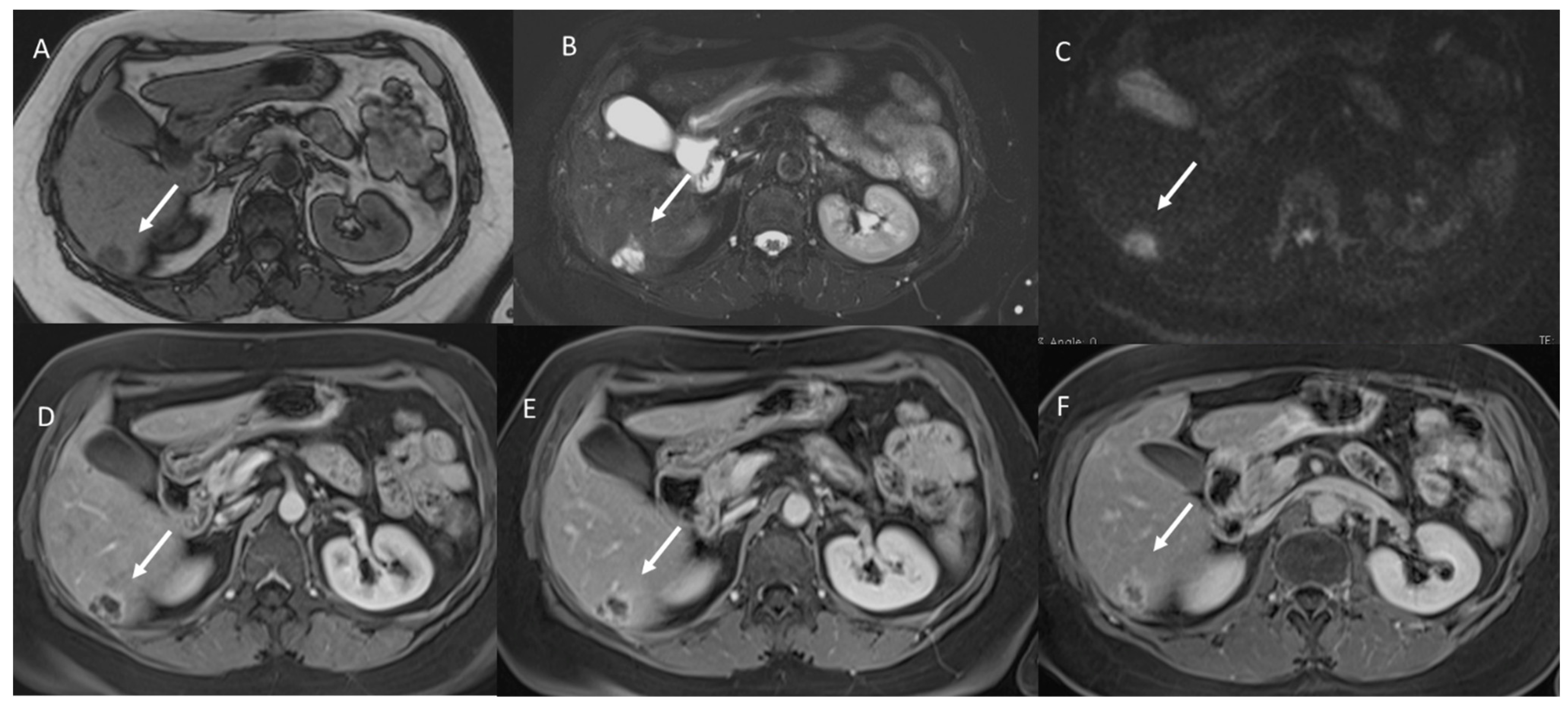
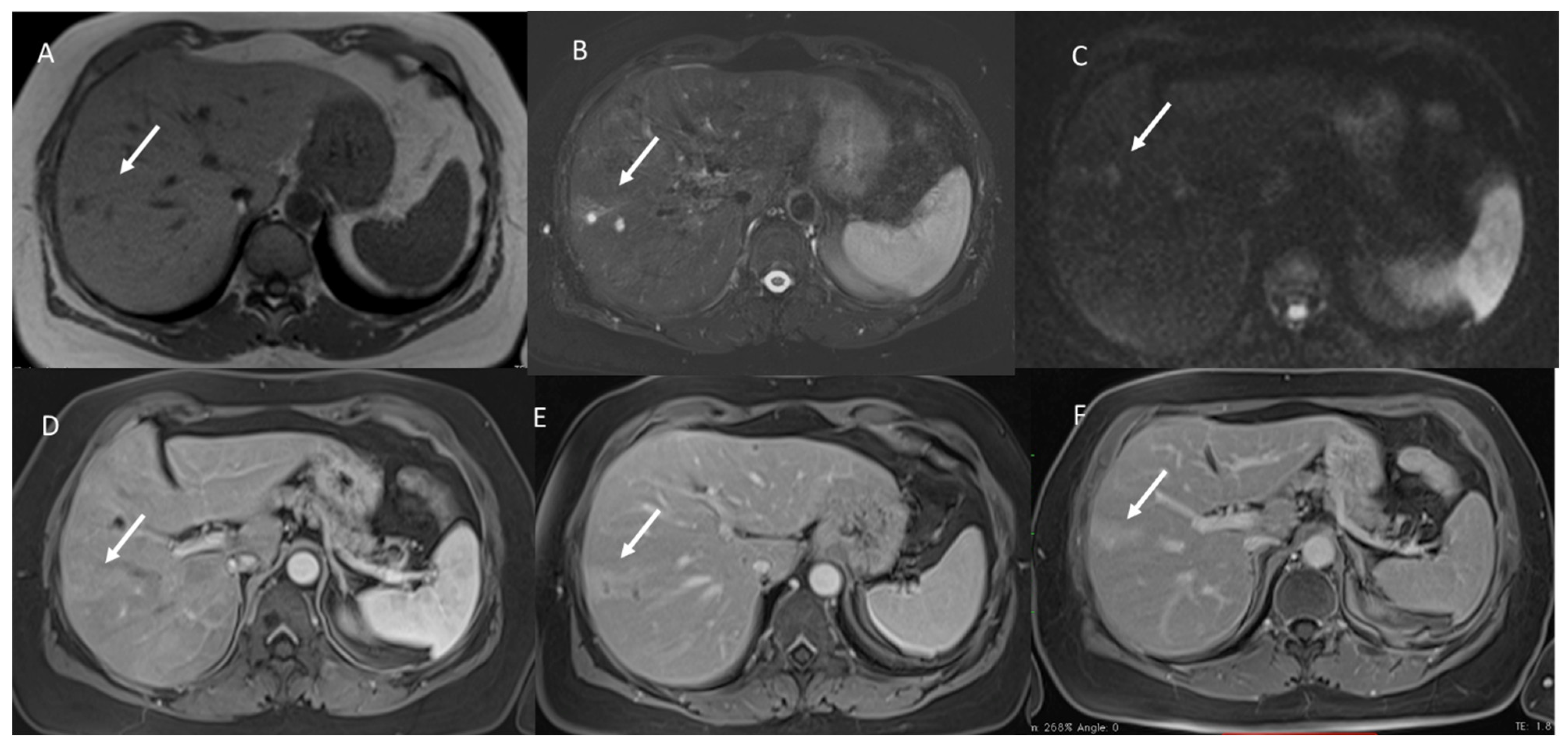
3. Results
3.1. T1-W Signal Intensity
3.2. T2-W Signal Intensity and Diffusion
3.3. Arterial Phase Appearance
3.4. Portal Phase Appearance
3.5. Equilibrium Phase Appearance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33, Erratum in CA Cancer J. Clin. 2021, 71, 359. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rega, D.; Pace, U.; Scala, D.; Chiodini, P.; Granata, V.; Fares Bucci, A.; Pecori, B.; Delrio, P. Treatment of splenic flexure colon cancer: A comparison of three different surgical procedures: Experience of a high volume cancer center. Sci. Rep. 2019, 9, 10953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schicchi, N.; Fogante, M.; Palumbo, P.; Agliata, G.; Pirani, P.E.; Di Cesare, E.; Giovagnoni, A. The sub-millisievert era in CTCA: The technical basis of the new radiation dose approach. Radiol. Med. 2020, 125, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Bandi, P.; Minihan, A.K.; Siegel, R.L.; Islami, F.; Nargis, N.; Jemal, A.; Fedewa, S.A. Updated Review of Major Cancer Risk Factors and Screening Test Use in the United States in 2018 and 2019, with a Focus on Smoking Cessation. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, Y.S.; Choi, J. Dosimetric analysis of the effects of a temporary tissue expander on the radiotherapy technique. Radiol. Med. 2021, 126, 437–444. [Google Scholar] [CrossRef]
- Crimì, F.; Capelli, G.; Spolverato, G.; Bao, Q.R.; Florio, A.; Rossi, S.M.; Cecchin, D.; Albertoni, L.; Campi, C.; Pucciarelli, S.; et al. MRI T2-weighted sequences-based texture analysis (TA) as a predictor of response to neoadjuvant chemo-radiotherapy (nCRT) in patients with locally advanced rectal cancer (LARC). Radiol. Med. 2020, 125, 1216–1224. [Google Scholar] [CrossRef]
- Bertocchi, E.; Barugola, G.; Nicosia, L.; Mazzola, R.; Ricchetti, F.; Dell’Abate, P.; Alongi, F.; Ruffo, G. A comparative analysis between radiation dose intensification and conventional fractionation in neoadjuvant locally advanced rectal cancer: A monocentric prospective observational study. Radiol. Med. 2020, 125, 990–998. [Google Scholar] [CrossRef]
- Fornell-Perez, R.; Vivas-Escalona, V.; Aranda-Sanchez, J.; Gonzalez-Dominguez, M.C.; Rubio-Garcia, J.; Aleman-Flores, P.; Lozano-Rodriguez, A.; Porcel-De-Peralta, G.; Loro, J. Primary and post-chemoradiotherapy MRI detection of extramural venous invasion in rectal cancer: The role of diffusion-weighted imaging. Radiol. Med. 2020, 125, 522–530. [Google Scholar] [CrossRef]
- Cusumano, D.; Meijer, G.; Lenkowicz, J.; Chiloiro, G.; Boldrini, L.; Masciocchi, C.; Dinapoli, N.; Gatta, R.; Casà, C.; Damiani, A.; et al. A field strength independent MR radiomics model to predict pathological complete response in locally advanced rectal cancer. Radiol. Med. 2021, 126, 421–429. [Google Scholar] [CrossRef]
- Petrillo, A.; Fusco, R.; Petrillo, M.; Granata, V.; Delrio, P.; Bianco, F.; Pecori, B.; Botti, G.; Tatangelo, F.; Caracò, C.; et al. Standardized Index of Shape (DCE-MRI) and Standardized Uptake Value (PET/CT): Two quantitative approaches to discriminate chemo-radiotherapy locally advanced rectal cancer responders under a functional profile. Oncotarget 2017, 8, 8143–8153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granata, V.; Fusco, R.; Avallone, A.; Filice, F.; Tatangelo, F.; Piccirillo, M.; Grassi, R.; Izzo, F.; Petrillo, A. Critical analysis of the major and ancillary imaging features of LI-RADS on 127 proven HCCs evaluated with functional and morphological MRI: Lights and shadows. Oncotarget 2017, 8, 51224–51237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudsen, A.B.; Rutter, C.M.; Peterse, E.F.P.; Lietz, A.P.; Seguin, C.L.; Meester, R.G.S.; Perdue, L.A.; Lin, J.S.; Siegel, R.L.; Doria-Rose, V.P.; et al. Colorectal Cancer Screening. JAMA 2021, 325, 1998–2011. [Google Scholar] [CrossRef] [PubMed]
- Petrillo, A.; Fusco, R.; Granata, V.; Filice, S.; Sansone, M.; Rega, D.; Delrio, P.; Bianco, F.; Romano, G.M.; Tatangelo, F.; et al. Assessing response to neo-adjuvant therapy in locally advanced rectal cancer using Intra-voxel Incoherent Motion modelling by DWI data and Standardized Index of Shape from DCE-MRI. Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Fusco, R.; Granata, V.; Sansone, M.; Rega, D.; Delrio, P.; Tatangelo, F.; Romano, C.; Avallone, A.; Pupo, D.; Giordano, M.; et al. Validation of the standardized index of shape tool to analyze DCE-MRI data in the assessment of neo-adjuvant therapy in locally advanced rectal cancer. Radiol. Med. 2021, 126, 1044–1054. [Google Scholar] [CrossRef]
- Granata, V.; Petrillo, M.; Fusco, R.; Setola, S.V.; De Lutio Di Castelguidone, E.; Catalano, O.; Piccirillo, M.; Albino, V.; Izzo, F.; Petrillo, A. Surveillance of HCC patients after liver RFA: Role of MRI with hepatospecific contrast versus three-phase CT scan—Experience of high volume oncologic institute. Gastroenterol. Res. Pract. 2013, 2013, 469097. [Google Scholar] [CrossRef]
- Rees, M.; Tekkis, P.P.; Welsh, F.K.; O’Rourke, T.; John, T.G. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann. Surg. 2008, 247, 125–135. [Google Scholar] [CrossRef]
- Abdalla, E.K.; Vauthey, J.-N.; Ellis, L.M.; Ellis, V.; Pollock, R.; Broglio, K.R.; Hess, K.; Curley, S.A. Recurrence and Outcomes Following Hepatic Resection, Radiofrequency Ablation, and Combined Resection/Ablation for Colorectal Liver Metastases. Ann. Surg. 2004, 239, 818–827. [Google Scholar] [CrossRef]
- Viganò, L.; Capussotti, L.; Lapointe, R.; Barroso, E.; Hubert, C.; Giuliante, F.; Ijzermans, J.N.M.; Mirza, D.F.; Elias, D.; Adam, R. Early Recurrence After Liver Resection for Colorectal Metastases: Risk Factors, Prognosis, and Treatment. A LiverMetSurvey-Based Study of 6025 Patients. Ann. Surg. Oncol. 2014, 21, 1276–1286. [Google Scholar] [CrossRef]
- Reynolds, I.S.; Furney, S.J.; Kay, E.W.; McNamara, D.A.; Prehn, J.H.M.; Burke, J.P. Meta-analysis of the molecular associations of mucinous colorectal cancer. Br. J. Surg. 2019, 106, 682–691. [Google Scholar] [CrossRef]
- Reynolds, I.S.; O’Connell, E.; Fichtner, M.; McNamara, D.A.; Kay, E.W.; Prehn, J.H.M.; Furney, S.J.; Burke, J.P. Mucinous adenocarcinoma is a pharmacogenomically distinct subtype of colorectal cancer. Pharm. J. 2020, 20, 524–532. [Google Scholar] [CrossRef] [PubMed]
- McCawley, N.; Clancy, C.; O’Neill, B.D.P.; Deasy, J.; McNamara, D.A.; Burke, J.P. Mucinous Rectal Adenocarcinoma Is Associated with a Poor Response to Neoadjuvant Chemoradiotherapy: A Systematic Review and Meta-analysis. Dis. Colon Rectum 2016, 59, 1200–1208. [Google Scholar] [CrossRef]
- Petralia, G.; Zugni, F.; Summers, P.E.; Colombo, A.; Pricolo, P.; Grazioli, L.; Colagrande, S.; Giovagnoni, A.; Padhani, A.R. Italian Working Group on Magnetic Resonance. Whole-body magnetic resonance imaging (WB-MRI) for cancer screening: Recommendations for use. Radiol. Med. 2021, 126, 1434–1450. [Google Scholar] [CrossRef] [PubMed]
- Petralia, G.; Summers, P.E.; Agostini, A.; Ambrosini, R.; Cianci, R.; Cristel, G.; Calistri, L.; Colagrande, S. Dynamic contrast-enhanced MRI in oncology: How we do it. Radiol. Med. 2020, 125, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Belli, A.; Ottaiano, A.; Nasti, G.; La Porta, M.; Danti, G.; Cappabianca, S.; et al. Intrahepatic cholangiocarcinoma and its differential diagnosis at MRI: How radiologist should assess MR features. Radiol. Med. 2021, 126, 1584–1600. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Bicchierai, G.; Fusco, R.; Cozzi, D.; Grazzini, G.; Danti, G.; De Muzio, F.; Maggialetti, N.; Smorchkova, O.; D’Elia, M.; et al. Diagnostic protocols in oncology: Workup and treatment planning. Part 2: Abbreviated MR protocol. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6499–6528. [Google Scholar] [CrossRef] [PubMed]
- Gurgitano, M.; Angileri, S.A.; Rodà, G.M.; Liguori, A.; Pandolfi, M.; Ierardi, A.M.; Wood, B.J.; Carrafiello, G. Interventional Radiology ex-machina: Impact of Artificial Intelligence on practice. Radiol. Med. 2021, 126, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Barretta, M.L.; Picone, C.; Avallone, A.; Belli, A.; Patrone, R.; Ferrante, M.; Cozzi, D.; Grassi, R.; et al. Radiomics in hepatic metastasis by colorectal cancer. Infect. Agents Cancer 2021, 16, 39. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; Catalano, O.; Piccirillo, M.; Palaia, R.; Nasti, G.; Petrillo, A.; Izzo, F. A radiologist’s point of view in the presurgical and intraoperative setting of colorectal liver metastases. Future Oncol. 2018, 14, 2189–2206. [Google Scholar] [CrossRef]
- Mathew, R.P.; Sam, M.; Raubenheimer, M.; Patel, V.; Low, G. Hepatic hemangiomas: The various imaging avatars and its mimickers. Radiol. Med. 2020, 125, 801–815. [Google Scholar] [CrossRef]
- Michallek, F.; Genske, U.; Niehues, S.M.; Hamm, B.; Jahnke, P. Deep learning reconstruction improves radiomics feature stability and discriminative power in abdominal CT imaging: A phantom study. Eur. Radiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Rabe, E.; Cioni, D.; Baglietto, L.; Fornili, M.; Gabelloni, M.; Neri, E. Can the computed tomography texture analysis of colorectal liver metastases predict the response to first-line cytotoxic chemotherapy? World J. Hepatol. 2022, 14, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Kelahan, L.C.; Kim, D.; Soliman, M.; Avery, R.J.; Savas, H.; Agrawal, R.; Magnetta, M.; Liu, B.P.; Velichko, Y.S. Role of hepatic metastatic lesion size on inter-reader reproducibility of CT-based radiomics features. Eur. Radiol. 2022. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Catalano, O.; Avallone, A.; Palaia, R.; Botti, G.; Tatangelo, F.; Granata, F.; Cascella, M.; Izzo, F.; et al. Diagnostic accuracy of magnetic resonance, computed tomography and contrast enhanced ultrasound in radiological multimodality assessment of peribiliary liver metastases. PLoS ONE 2017, 12, e0179951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granata, V.; Fusco, R.; Catalano, O.; Filice, S.; Amato, D.M.; Nasti, G.; Avallone, A.; Izzo, F.; Petrillo, A. Early Assessment of Colorectal Cancer Patients with Liver Metastases Treated with Antiangiogenic Drugs: The Role of Intravoxel Incoherent Motion in Diffusion-Weighted Imaging. PLoS ONE 2015, 10, e0142876. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Zhong, Y.; Zhang, K.; Kong, H.; Yu, L.; Chen, Y.; Bai, Y.; Zhu, Z.; Yang, Y.; et al. Novel and Specific MRI Features Indicate the Clinical Features of Patients with Rare Hepatic Tumor Epithelioid Hemangioendothelioma. Front. Oncol. 2022, 12, 729177. [Google Scholar] [CrossRef]
- Yang, H.; Tan, S.; Qiao, J.; Xu, Y.; Gui, Z.; Meng, Y.; Dong, B.; Peng, G.; Ibhagui, O.Y.; Qian, W.; et al. Non-invasive detection and complementary diagnostic of liver metastases via chemokine receptor 4 imaging. Cancer Gene Ther. 2022. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Petrillo, A. Additional Considerations on Use of Abbreviated Liver MRI in Patients with Colorectal Liver Metastases. Am. J. Roentgenol. 2021, 217, W1. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, G.; Zhang, J.; Xu, C.; Zhu, F.; Xu, P. DCE-MRI based radiomics nomogram for preoperatively differentiating combined hepatocellular-cholangiocarcinoma from mass-forming intrahepatic cholangiocarcinoma. Eur. Radiol. 2022. [Google Scholar] [CrossRef]
- Esposito, A.; Buscarino, V.; Raciti, D.; Casiraghi, E.; Manini, M.; Biondetti, P.; Forzenigo, L. Characterization of liver nodules in patients with chronic liver disease by MRI: Performance of the Liver Imaging Reporting and Data System (LI-RADS v.2018) scale and its comparison with the Likert scale. Radiol. Med. 2020, 125, 15–23. [Google Scholar] [CrossRef]
- Bozkurt, M.; Eldem, G.; Bozbulut, U.B.; Bozkurt, M.F.; Kılıçkap, S.; Peynircioğlu, B.; Çil, B.; Ergün, E.L.; Volkan-Salanci, B. Factors affecting the response to Y-90 microsphere therapy in the cholangiocarcinoma patients. Radiol. Med. 2021, 126, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.; Choi, J.A.; Choi, J.M.; Cho, E.S.; Kim, J.H.; Chung, J.J.; Yu, J.S. Sclerotic changes of cavernous hemangioma in the cirrhotic liver: Long-term follow-up using dynamic contrast-enhanced computed tomography. Radiol. Med. 2020, 125, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Avallone, A.; Cassata, A.; Palaia, R.; Delrio, P.; Grassi, R.; Tatangelo, F.; Grazzini, G.; Izzo, F.; et al. Abbreviated MRI protocol for colorectal liver metastases: How the radiologist could work in pre surgical setting. PLoS ONE 2020, 15, e0241431. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Catalano, O.; Fusco, R.; Tatangelo, F.; Rega, D.; Nasti, G.; Avallone, A.; Piccirillo, M.; Izzo, F.; Petrillo, A. The target sign in colorectal liver metastases: An atypical Gd-EOB-DTPA “uptake” on the hepatobiliary phase of MR imaging. Abdom. Imaging 2015, 40, 2364–2371. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, P.; Donati, F.; Cervelli, R.; Pacciardi, F.; Tarantini, G.; Castagna, M.; Urbani, L.; Lencioni, R. Colorectal liver metastases: ADC as an imaging biomarker of tumor behavior and therapeutic response. Eur. J. Radiol. 2021, 137, 109609. [Google Scholar] [CrossRef]
- Liu, L.-H.; Zhou, G.-F.; Lv, H.; Wang, Z.-C.; Rao, S.-X.; Zeng, M.-S. Identifying response in colorectal liver metastases treated with bevacizumab: Development of RECIST by combining contrast-enhanced and diffusion-weighted MRI. Eur. Radiol. 2021, 31, 5640–5649. [Google Scholar] [CrossRef]
- American College of Radiology. (2018) CT/MRI LI-RADS, Version 2018. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018 (accessed on 1 November 2019).
- Colagrande, S.; Centi, N.; Galdiero, R.; Ragozzino, A. Transient Hepatic Intensity Differences: Part 1, Those Associated with Focal Lesions. Am. J. Roentgenol. 2007, 188, 154–159. [Google Scholar] [CrossRef]
- Paulatto, L.; Burgio, M.D.; Sartoris, R.; Beaufrère, A.; Cauchy, F.; Paradis, V.; Vilgrain, V.; Ronot, M. Colorectal liver metastases: Radiopathological correlation. Insights Imaging 2020, 11, 99. [Google Scholar] [CrossRef]
- Aoki, K.; Takayasu, K.; Muramatsu, Y.; Moriyama, N.; Matsue, H.; Yamada, T. Liver metastases of mucinous colorectal carcinoma: Clinico-radiological study of six cases. Nihon Igaku Hoshasen Gakkai Zasshi. Nippon Acta Radiol. 1990, 50, 1513–1518. [Google Scholar]
- Lee, J.E.; Kim, S.H.; Lee, S.; Choi, S.-Y.; Hwang, J.A.; Woo, S.-Y. Differentiating metastatic mucinous colorectal adenocarcinomas from simple cysts of the liver using contrast-enhanced and diffusion-weighted MRI. Br. J. Radiol. 2018, 91, 20180303. [Google Scholar] [CrossRef] [Green Version]
- Lacout, A.; El Hajjam, M.; Julie, C.; Lacombe, P.; Pelage, J.P. Liver metastasis of a mucinous colonic carcinoma mimicking a haemangioma in T2-weighted sequences. J. Med. Imaging Radiat. Oncol. 2008, 52, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Santone, A.; Brunese, M.C.; Donnarumma, F.; Guerriero, P.; Mercaldo, F.; Reginelli, A.; Miele, V.; Giovagnoni, A.; Brunese, L. Radiomic features for prostate cancer grade detection through formal verification. Radiol. Med. 2021, 126, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Costa, M.; Picone, C.; Cozzi, D.; Moroni, C.; La Casella, G.; Montanino, A.; Monti, R.; Mazzoni, F.; et al. Preliminary Report on Computed Tomography Radiomics Features as Biomarkers to Immunotherapy Selection in Lung Adenocarcinoma Patients. Cancers 2021, 13, 3992. [Google Scholar] [CrossRef] [PubMed]
- Agazzi, G.M.; Ravanelli, M.; Roca, E.; Medicina, D.; Balzarini, P.; Pessina, C.; Vermi, W.; Berruti, A.; Maroldi, R.; Farina, D. CT texture analysis for prediction of EGFR mutational status and ALK rearrangement in patients with non-small cell lung cancer. Radiol. Med. 2021, 126, 786–794. [Google Scholar] [CrossRef]
- Fusco, R.; Granata, V.; Mazzei, M.A.; Di Meglio, N.; Del Roscio, D.; Moroni, C.; Monti, R.; Cappabianca, C.; Picone, C.; Neri, E.; et al. Quantitative imaging decision support (QIDSTM) tool consistency evaluation and radiomic analysis by means of 594 metrics in lung carcinoma on chest CT scan. Cancer Control 2021, 28, 1073274820985786. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; De Stefano, A.; Ottaiano, A.; Sbordone, C.; Brunese, L.; Izzo, F.; Petrillo, A. Radiomics-Derived Data by Contrast Enhanced Magnetic Resonance in RAS Mutations Detection in Colorectal Liver Metastases. Cancers 2021, 13, 453. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Risi, C.; Ottaiano, A.; Avallone, A.; De Stefano, A.; Grimm, R.; Grassi, R.; Brunese, L.; Izzo, F.; et al. Diffusion-Weighted MRI and Diffusion Kurtosis Imaging to Detect RAS Mutation in Colorectal Liver Metastasis. Cancers 2020, 12, 2420. [Google Scholar] [CrossRef]
- Kirienko, M.; Ninatti, G.; Cozzi, L.; Voulaz, E.; Gennaro, N.; Barajon, I.; Ricci, F.; Carlo-Stella, C.; Zucali, P.; Sollini, M.; et al. Computed tomography (CT)-derived radiomic features differentiate prevascular mediastinum masses as thymic neoplasms versus lymphomas. Radiol. Med. 2020, 125, 951–960. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, L.; Li, G.; Zhang, X.; Ren, J.; Shi, Z.; Li, J.; Yu, S. Computed tomography-based radiomics model for discriminating the risk stratification of gastrointestinal stromal tumors. Radiol. Med. 2020, 125, 465–473. [Google Scholar] [CrossRef]
- Van Assen, M.; Muscogiuri, G.; Caruso, D.; Lee, S.J.; Laghi, A.; De Cecco, C.N. Artificial intelligence in cardiac radiology. Radiol Med. 2020, 125, 1186–1199. [Google Scholar] [CrossRef]
- Scapicchio, C.; Gabelloni, M.; Barucci, A.; Cioni, D.; Saba, L.; Neri, E. A deep look into radiomics. Radiol. Med. 2021, 126, 1296–1311. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, G.; Mori, M.; Panzeri, M.M.; Barbera, M.; Palumbo, D.; Sini, C.; Muffatti, F.; Andreasi, V.; Steidler, S.; Doglioni, C.; et al. CT-derived radiomic features to discriminate histologic characteristics of pancreatic neuroendocrine tumors. Radiol. Med. 2021, 126, 745–760. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Grassi, R.; Grassi, F.; Ottaiano, A.; Nasti, G.; Tatangelo, F.; et al. Radiomics textural features by MR imaging to assess clinical outcomes following liver resection in colorectal liver metastases. Radiol. Med. 2022. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Ottaiano, A.; Nasti, G.; Grassi, R.; Pilone, V.; et al. EOB-MR Based Radiomics Analysis to Assess Clinical Outcomes following Liver Resection in Colorectal Liver Metastases. Cancers 2022, 14, 1239. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; De Muzio, F.; Aversana, F.D.; Cutolo, C.; Faggioni, L.; Miele, V.; Izzo, F.; Petrillo, A. CT-Based Radiomics Analysis to Predict Histopathological Outcomes Following Liver Resection in Colorectal Liver Metastases. Cancers 2022, 14, 1648. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, I.S.; Cromwell, P.M.; Ryan, É.J.; McGrath, E.; Kennelly, R.; Ryan, R.; Swan, N.; Sheahan, K.; Winter, D.C.; Hoti, E. An Analysis of Clinicopathological Outcomes and the Utility of Preoperative MRI for Patients Undergoing Resection of Mucinous and Non-Mucinous Colorectal Cancer Liver Metastases. Front. Oncol. 2022, 12, 821159. [Google Scholar] [CrossRef]
| Patient Description | Numbers (%)/Range |
|---|---|
| Gender | Women 19 (36.5%) |
| Men 33 (63.4%) | |
| Age | 63 years; range: 37–82 years |
| Primary cancer site | |
| Colon | 39 (75%) |
| Non mucinous type | 26 (66.7% of colon cancer patients) |
| Mucinous type | 13 (33.3% of colon cancer patients) |
| Rectum | 13 (25%) |
| Non mucinous type | 9 (69.2% of rectal cancer patients) |
| Mucinous type | 4 (30.8% of rectal cancer patients) |
| Hepatic metastases | |
| Non-mucinous type | 35 patients (67.3%) (12 women; 23 men); 118 metastases assessed |
| Mucinous type | 17 patients (32.7%) (7 women; 10 men); 39 metastases assessed |
| Patients with single nodule | 22 (64.2%) |
| Patients with multiple nodules | 30(35.8%)/range: 2–14 metastases for mucinous type 2–16 metastases for non-mucinous type |
| Nodule size (mm) | mean size 36.4 mm; range 7–63 mm |
| Growth pattern on histopathology | |
| Mucinous type | 25 pushing or capsulated 14 infiltrative |
| Non-mucinous type | 35 pushing or capsulated 83 infiltrative |
| Recurrence | 12 patients (3 mucinous patients) medium follow-up 6 months |
| Control Study Group B | |
| Gender | Women 12 (60%) |
| Men 8 (40%) | |
| Age | 55 years; range: 27–68 years |
| Hemangioma size (mm) | mean size 25 mm; range 8–43 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Belli, A.; Romano, C.; Ottaiano, A.; Nasti, G.; et al. Magnetic Resonance Features of Liver Mucinous Colorectal Metastases: What the Radiologist Should Know. J. Clin. Med. 2022, 11, 2221. https://doi.org/10.3390/jcm11082221
Granata V, Fusco R, De Muzio F, Cutolo C, Setola SV, Dell’Aversana F, Belli A, Romano C, Ottaiano A, Nasti G, et al. Magnetic Resonance Features of Liver Mucinous Colorectal Metastases: What the Radiologist Should Know. Journal of Clinical Medicine. 2022; 11(8):2221. https://doi.org/10.3390/jcm11082221
Chicago/Turabian StyleGranata, Vincenza, Roberta Fusco, Federica De Muzio, Carmen Cutolo, Sergio Venanzio Setola, Federica Dell’Aversana, Andrea Belli, Carmela Romano, Alessandro Ottaiano, Guglielmo Nasti, and et al. 2022. "Magnetic Resonance Features of Liver Mucinous Colorectal Metastases: What the Radiologist Should Know" Journal of Clinical Medicine 11, no. 8: 2221. https://doi.org/10.3390/jcm11082221
APA StyleGranata, V., Fusco, R., De Muzio, F., Cutolo, C., Setola, S. V., Dell’Aversana, F., Belli, A., Romano, C., Ottaiano, A., Nasti, G., Avallone, A., Miele, V., Tatangelo, F., Petrillo, A., & Izzo, F. (2022). Magnetic Resonance Features of Liver Mucinous Colorectal Metastases: What the Radiologist Should Know. Journal of Clinical Medicine, 11(8), 2221. https://doi.org/10.3390/jcm11082221






