Which Actigraphy Dimensions Predict Longitudinal Outcomes in Bipolar Disorders?
Abstract
:1. Introduction
- (1)
- Primary analyses: Time to mood recurrence.
- (1a)
- To utilize a survival analysis to examine whether dimensions of objective estimates of sleep quantity, sleep variability and/or circadian rhythmicity (obtained using principal component analysis of actigraphy estimates recorded during euthymia) could predict the time to new episode onset after adjusting for known clinical predictors of BD outcome;
- (1b)
- To determine which, if any, individual actigraphic parameters (derived from the sleep and/or circadian dimensions identified in the first survival analysis) were significantly associated with new episode onset.
- (2)
- Secondary analysis: Early mood recurrence.
2. Materials and Methods
2.1. Sample
- −
- Age ≥ 18 years,
- −
- DSM-IV diagnosis of BD type I or II according to the SCID (Structured Clinical Interview for DSM Disorders) [26];
- −
- −
- Willing and able to give written informed consent;
- −
- Currently treated with at least one mood stabilizer (lithium or anticonvulsants or atypical antipsychotics).
- −
- Exclusion criteria were:
- −
- Unable to undertake actigraphy monitoring for 21 consecutive days;
- −
- Current alcohol or substance misuse problems (excluding current tobacco use);
- −
- Inpatient treatment and/or any modification of mood stabilizer regime in the 3 months prior to the assessment;
- −
- Current employment involves nightwork or shiftwork;
- −
- Recent trans-meridian travel;
- −
- Currently diagnosed with a comorbid neurological or sleep disorder (narcolepsy, obstructive sleep apnea, restless leg syndrome) and/or being prescribed a non-psychotropic medication that can alter sleep and circadian rhythms (such as cortisone);
- −
- Any other mental or physical health problem that contradicted participation in the study.
2.2. Clinical Data
2.3. Actigraphy Recording
- (a)
- Sleep quality, as measured using the total sleep time (TST), sleep onset latency (SOL), sleep efficiency (SE), fragmentation index (FI) and time spent awake after sleep onset (WASO). We estimated the mean values for each sleep parameter.
- (b)
- Sleep variability, calculated as the within-individual variability in each of the sleep quantity parameters (using the standard deviation of each estimate over the recording period).
- (c)
- Circadian rhythmicity was represented by the inter-day stability (IS; scores range from 0, indicative of a total lack of rhythm, to 1, indicative of perfectly stable rhythm), intra-day variability (IV; a measure of fragmentation of activity, with scores ranging from 0–2), L5 (average level of activity over the least active 5 h), M10 (average level of activity over the most active 10 h), L5 onset and M10 onset (onset of the least active 5 h and the most active 10 h), amplitude (Amp; the difference between M10 and L5 activity) and relative amplitude (RA; the difference between M10 and L5 activity divided by the sum of L5 and M10, with the reported ratio ranging from 0–1).
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Descriptive Analyses of the Sample
3.2. Principal Component Analyses of Actigraphy Variables
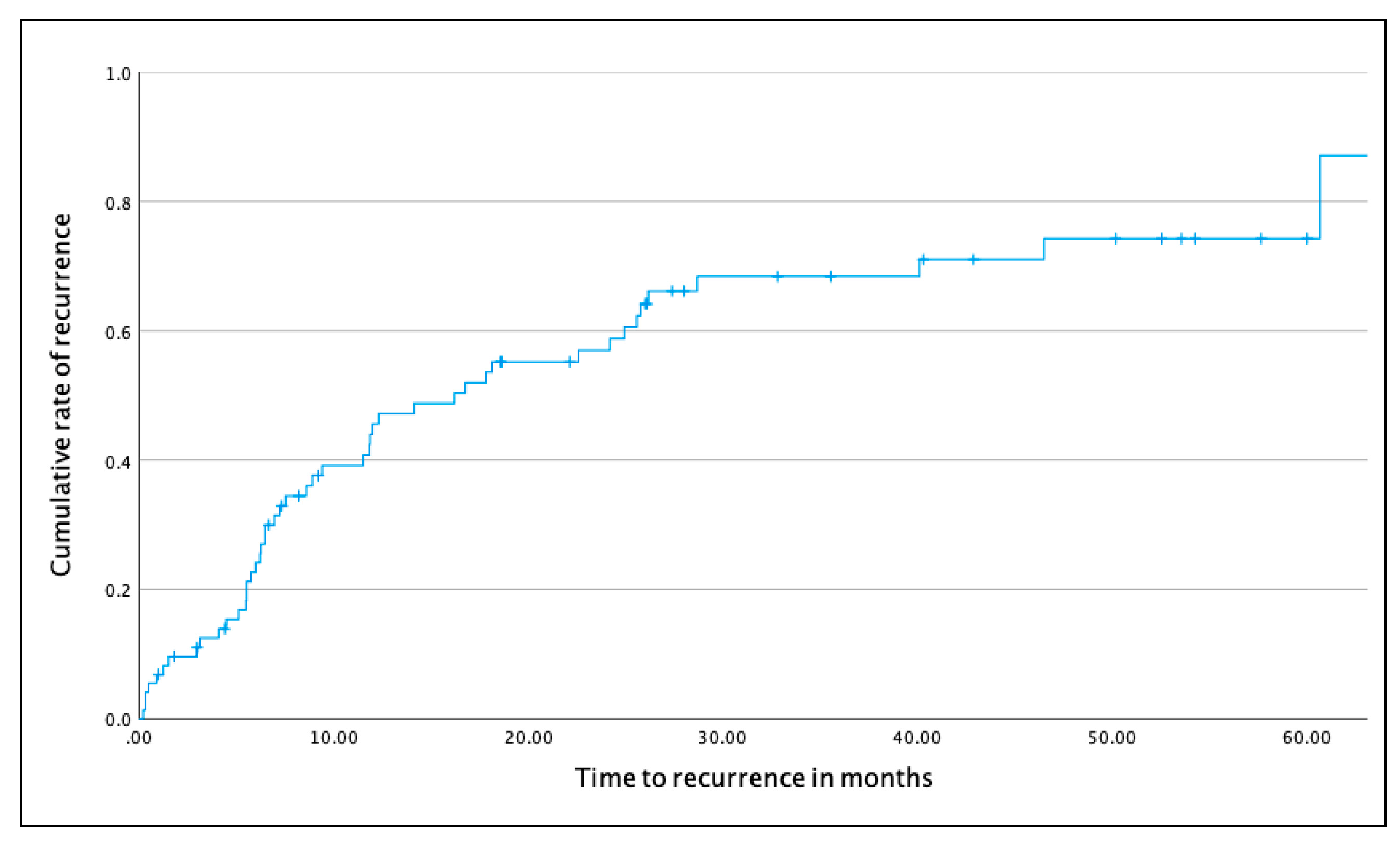
3.3. Survival Analyses
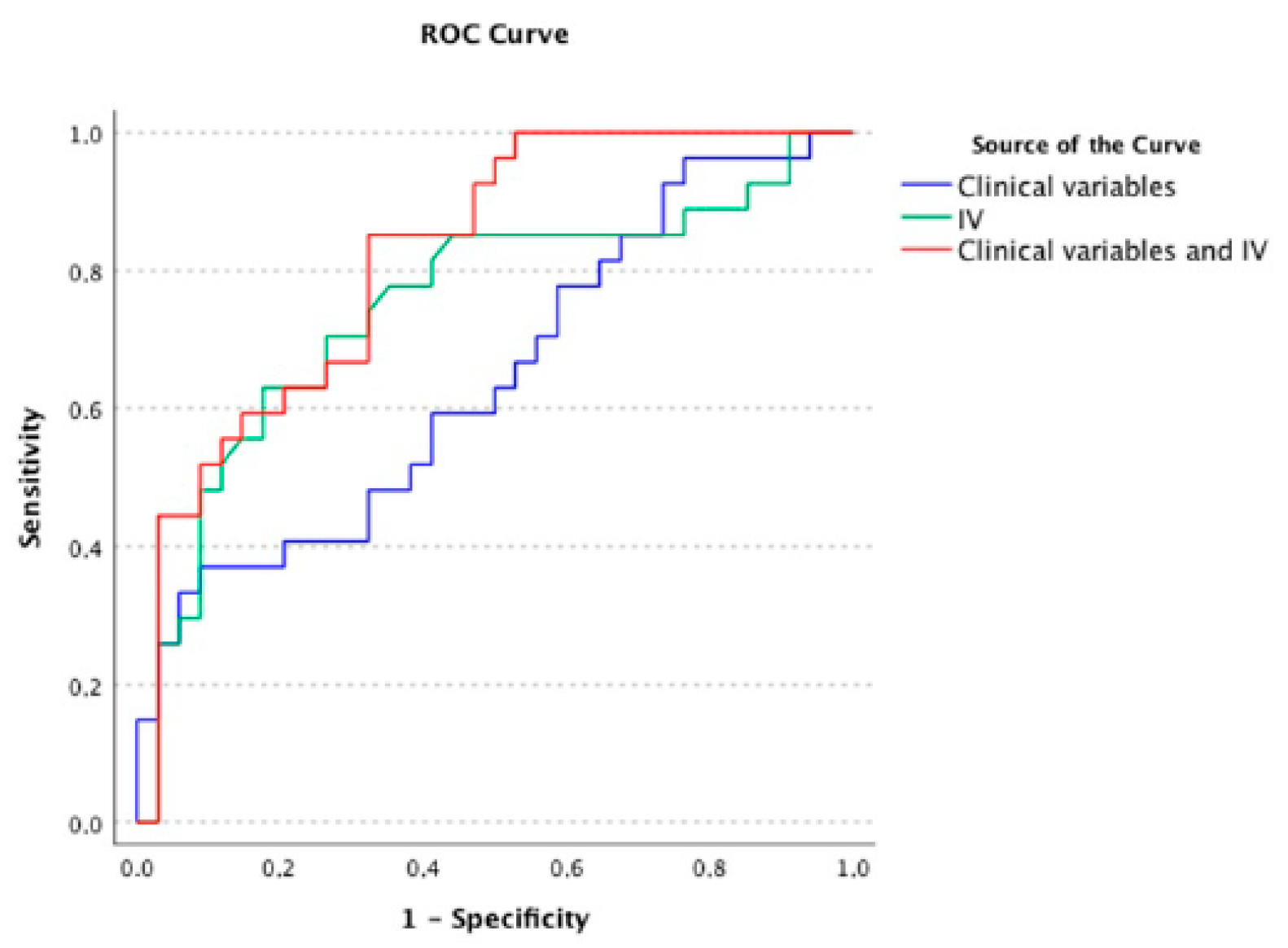
3.4. ROC Analyses of Early and Later Recurrence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tohen, M.; Zarate, C.A., Jr.; Hennen, J.; Khalsa, H.M.; Strakowski, S.M.; Gebre-Medhin, P.; Salvatore, P.; Baldessarini, R.J. The McLean-Harvard First-Episode Mania Study: Prediction of recovery and first recurrence. Am. J. Psychiatry 2003, 160, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, G.H.; Holtzman, J.N.; Lolich, M.; Ketter, T.A.; Baldessarini, R.J. Recurrence rates in bipolar disorder: Systematic comparison of long-term prospective, naturalistic studies versus randomized controlled trials. Eur. Neuropsychopharmacol. 2015, 25, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Radua, J.; Grunze, H.; Amann, B.L. Meta-Analysis of the Risk of Subsequent Mood Episodes in Bipolar Disorder. Psychother. Psychosom. 2017, 86, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, E.K.; Gatz, J.L.; Jacob, J.; Tohen, M. Predictors of relapse or recurrence in bipolar I disorder. J. Affect. Disord. 2012, 136, 733–739. [Google Scholar] [CrossRef]
- Etain, B.; Bellivier, F.; Olie, E.; Aouizerate, B.; Aubin, V.; Belzeaux, R.; Courtet, P.; Dubertret, C.; Schwan, R.; Roux, P.; et al. Clinical predictors of recurrences in bipolar disorders type 1 and 2: A FACE-BD longitudinal study. J. Psychiatr. Res. 2021, 134, 129–137. [Google Scholar] [CrossRef]
- Judd, L.L.; Schettler, P.J.; Akiskal, H.S.; Coryell, W.; Leon, A.C.; Maser, J.D.; Solomon, D.A. Residual symptom recovery from major affective episodes in bipolar disorders and rapid episode relapse/recurrence. Arch. Gen. Psychiatry 2008, 65, 386–394. [Google Scholar] [CrossRef] [Green Version]
- Tohen, M.; Wang, W.V.; Leboyer, M.; Jen, K.Y. Variables as mediators or moderators in predicting relapse to any type of mood episode in a bipolar maintenance study. J. Clin. Psychiatry 2012, 73, e913–e917. [Google Scholar] [CrossRef]
- Tundo, A.; Musetti, L.; Benedetti, A.; Massimetti, E.; Pergentini, I.; Cambiali, E.; Dell′Osso, L. Predictors of recurrence during long-term treatment of bipolar I and II disorders. A 4 year prospective naturalistic study. J. Affect. Disord. 2018, 225, 123–128. [Google Scholar] [CrossRef]
- Treuer, T.; Tohen, M. Predicting the course and outcome of bipolar disorder: A review. Eur. Psychiatry 2010, 25, 328–333. [Google Scholar] [CrossRef]
- Vieta, E.; Langosch, J.M.; Figueira, M.L.; Souery, D.; Blasco-Colmenares, E.; Medina, E.; Moreno-Manzanaro, M.; Gonzalez, M.A.; Bellivier, F. Clinical management and burden of bipolar disorder: Results from a multinational longitudinal study (WAVE-bd). Int. J. Neuropsychopharmacol. 2013, 16, 1719–1732. [Google Scholar] [CrossRef] [Green Version]
- Frey, B.N.; Andreazza, A.C.; Houenou, J.; Jamain, S.; Goldstein, B.I.; Frye, M.A.; Leboyer, M.; Berk, M.; Malhi, G.S.; Lopez-Jaramillo, C.; et al. Biomarkers in bipolar disorder: A positional paper from the International Society for Bipolar Disorders Biomarkers Task Force. Aust. N. Z. J. Psychiatry 2013, 47, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.L.; Colpo, G.D.; Fries, G.R.; Bauer, I.E.; Selvaraj, S. Biomarkers for bipolar disorder: Current status and challenges ahead. Expert Rev. Neurother. 2019, 19, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Murray, G.; Gottlieb, J.; Hidalgo, M.P.; Etain, B.; Ritter, P.; Skene, D.J.; Garbazza, C.; Bullock, B.; Merikangas, K.; Zipunnikov, V.; et al. Measuring circadian function in bipolar disorders: Empirical and conceptual review of physiological, actigraphic, and self-report approaches. Bipolar Disord. 2020, 22, 693–710. [Google Scholar] [CrossRef]
- Murray, G.; Harvey, A. Circadian rhythms and sleep in bipolar disorder. Bipolar Disord. 2010, 12, 459–472. [Google Scholar] [CrossRef] [Green Version]
- McClung, C.A. How might circadian rhythms control mood? Let me count the ways. Biol. Psychiatry 2013, 74, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Meyrel, M.; Scott, J.; Etain, B. Chronotypes and circadian rest-activity rhythms in bipolar disorders: A meta-analysis of self- and observer rating scales. Bipolar Disord. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sylvia, L.G.; Dupuy, J.M.; Ostacher, M.J.; Cowperthwait, C.M.; Hay, A.C.; Sachs, G.S.; Nierenberg, A.A.; Perlis, R.H. Sleep disturbance in euthymic bipolar patients. J. Psychopharmacol. 2012, 26, 1108–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cretu, J.B.; Culver, J.L.; Goffin, K.C.; Shah, S.; Ketter, T.A. Sleep, residual mood symptoms, and time to relapse in recovered patients with bipolar disorder. J. Affect. Disord. 2016, 190, 162–166. [Google Scholar] [CrossRef]
- Kaplan, K.A.; McGlinchey, E.L.; Soehner, A.; Gershon, A.; Talbot, L.S.; Eidelman, P.; Gruber, J.; Harvey, A.G. Hypersomnia subtypes, sleep and relapse in bipolar disorder. Psychol. Med. 2015, 45, 1751–1763. [Google Scholar] [CrossRef] [Green Version]
- Saunders, E.F.; Fernandez-Mendoza, J.; Kamali, M.; Assari, S.; McInnis, M.G. The effect of poor sleep quality on mood outcome differs between men and women: A longitudinal study of bipolar disorder. J. Affect. Disord. 2015, 180, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Takaesu, Y.; Inoue, Y.; Ono, K.; Murakoshi, A.; Futenma, K.; Komada, Y.; Inoue, T. Circadian Rhythm Sleep-Wake Disorders Predict Shorter Time to Relapse of Mood Episodes in Euthymic Patients With Bipolar Disorder: A Prospective 48-Week Study. J. Clin. Psychiatry 2018, 79, 17m11565. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.; Colom, F.; Young, A.; Bellivier, F.; Etain, B. An evidence map of actigraphy studies exploring longitudinal associations between rest-activity rhythms and course and outcome of bipolar disorders. Int. J. Bipolar Disord. 2020, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Esaki, Y.; Obayashi, K.; Saeki, K.; Fujita, K.; Iwata, N.; Kitajima, T. Association between circadian activity rhythms and mood episode relapse in bipolar disorder: A 12-month prospective cohort study. Transl. Psychiatry 2021, 11, 525. [Google Scholar] [CrossRef] [PubMed]
- Geoffroy, P.A.; Scott, J.; Boudebesse, C.; Lajnef, M.; Henry, C.; Leboyer, M.; Bellivier, F.; Etain, B. Sleep in patients with remitted bipolar disorders: A meta-analysis of actigraphy studies. Acta. Psychiatr. Scand. 2015, 131, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Martin, J.L.; Blackwell, T.; Buenaver, L.; Liu, L.; Meltzer, L.J.; Sadeh, A.; Spira, A.P.; Taylor, D.J. The SBSM Guide to Actigraphy Monitoring: Clinical and Research Applications. Behav. Sleep Med. 2015, 13 (Suppl. 1), S4–S38. [Google Scholar] [CrossRef] [PubMed]
- First, M.; Spitzer, R.; Gibbon, M.; William, J. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P); New York State Psychiatric Institute: New York, NY, USA, 1995. [Google Scholar]
- Montgomery, S.A.; Asberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry J. Ment. Sci. 1979, 134, 382–389. [Google Scholar] [CrossRef]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A rating scale for mania: Reliability, validity and sensitivity. Br. J. Psychiatry J. Ment. Sci. 1978, 133, 429–435. [Google Scholar] [CrossRef]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann. Intern. Med. 1999, 131, 485–491. [Google Scholar] [CrossRef]
- Krane-Gartiser, K.; Scott, J.; Nevoret, C.; Benard, V.; Benizri, C.; Brochard, H.; Geoffroy, P.A.; Katsahian, S.; Maruani, J.; Yeim, S.; et al. Which actigraphic variables optimally characterize the sleep-wake cycle of individuals with bipolar disorders? Acta. Psychiatr. Scand. 2019, 139, 269–279. [Google Scholar] [CrossRef]
- Klein, E.; Lavie, P.; Meiraz, R.; Sadeh, A.; Lenox, R.H. Increased motor activity and recurrent manic episodes: Predictors of rapid relapse in remitted bipolar disorder patients after lithium discontinuation. Biol. Psychiatry 1992, 31, 279–284. [Google Scholar] [CrossRef]
- Klein, E.; Mairaz, R.; Pascal, M.; Hefez, A.; Lavie, P. Discontinuation of lithium treatment in remitted bipolar patients: Relationship between clinical outcome and changes in sleep-wake cycles. J. Nerv. Ment. Dis. 1991, 179, 499–501. [Google Scholar] [CrossRef] [PubMed]
- Pagani, L.; St Clair, P.A.; Teshiba, T.M.; Service, S.K.; Fears, S.C.; Araya, C.; Araya, X.; Bejarano, J.; Ramirez, M.; Castrillon, G.; et al. Genetic contributions to circadian activity rhythm and sleep pattern phenotypes in pedigrees segregating for severe bipolar disorder. Proc. Natl. Acad. Sci. USA 2016, 113, E754–E761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyall, L.M.; Wyse, C.A.; Graham, N.; Ferguson, A.; Lyall, D.M.; Cullen, B.; Celis Morales, C.A.; Biello, S.M.; Mackay, D.; Ward, J.; et al. Association of disrupted circadian rhythmicity with mood disorders, subjective wellbeing, and cognitive function: A cross-sectional study of 91 105 participants from the UK Biobank. Lancet Psychiatry 2018, 5, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Meyer, N.; Faulkner, S.M.; McCutcheon, R.A.; Pillinger, T.; Dijk, D.J.; MacCabe, J.H. Sleep and Circadian Rhythm Disturbance in Remitted Schizophrenia and Bipolar Disorder: A Systematic Review and Meta-analysis. Schizophr Bull. 2020, 46, 1126–1143. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M. High Ceiling or Low Threshold? Accuracy of a Diagnostic Test. In Making Sense of Medical Statistics: A Bite Sized Visual Guide; Cambridge University Press: Cambridge, UK, 2021; pp. 141–154. [Google Scholar]
- Vancampfort, D.; Firth, J.; Schuch, F.B.; Rosenbaum, S.; Mugisha, J.; Hallgren, M.; Probst, M.; Ward, P.B.; Gaughran, F.; De Hert, M.; et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: A global systematic review and meta-analysis. World Psychiatry 2017, 16, 308–315. [Google Scholar] [CrossRef]
- Tanaka, T.; Kokubo, K.; Iwasa, K.; Sawa, K.; Yamada, N.; Komori, M. Intraday Activity Levels May Better Reflect the Differences Between Major Depressive Disorder and Bipolar Disorder Than Average Daily Activity Levels. Front Psychol. 2018, 9, 2314. [Google Scholar] [CrossRef]
- Cardoso Tde, A.; Campos Mondin, T.; Reyes, A.N.; Zeni, C.P.; Souza, L.D.; da Silva, R.A.; Jansen, K. Biological Rhythm and Bipolar Disorder: Twelve-Month Follow-Up of a Randomized Clinical Trial. J. Nerv. Ment. Dis. 2015, 203, 792–797. [Google Scholar] [CrossRef]
- Bouwkamp, C.G.; de Kruiff, M.E.; van Troost, T.M.; Snippe, D.; Blom, M.J.; de Winter, R.F.; Judith Haffmans, P.M. Interpersonal and social rhythm group therapy for patients with bipolar disorder. Int. J. Group Psychother. 2013, 63, 97–115. [Google Scholar] [CrossRef]
- Xu, N.; Shinohara, K.; Saunders, K.E.A.; Geddes, J.R.; Cipriani, A. Effect of lithium on circadian rhythm in bipolar disorder: A systematic review and meta-analysis. Bipolar Disord. 2021, 23, 445–453. [Google Scholar] [CrossRef]
- Moreira, J.; Geoffroy, P.A. Lithium and bipolar disorder: Impacts from molecular to behavioural circadian rhythms. Chronobiol. Int. 2016, 33, 351–373. [Google Scholar] [CrossRef]
- Kripke, D.F.; Mullaney, D.J.; Atkinson, M.; Wolf, S. Circadian rhythm disorders in manic-depressives. Biol. Psychiatry 1978, 13, 335–351. [Google Scholar] [PubMed]
- Hwang, J.Y.; Choi, J.W.; Kang, S.G.; Hwang, S.H.; Kim, S.J.; Lee, Y.J. Comparison of the Effects of Quetiapine XR and Lithium Monotherapy on Actigraphy-Measured Circadian Parameters in Patients With Bipolar II Depression. J. Clin. Psychopharmacol. 2017, 37, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Etain, B.; Meyrel, M.; Hennion, V.; Bellivier, F.; Scott, J. Can actigraphy be used to define lithium response dimensions in bipolar disorders? J. Affect. Disord. 2021, 283, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.; Hennion, V.; Meyrel, M.; Bellivier, F.; Etain, B. An ecological study of objective rest-activity markers of lithium response in bipolar-I-disorder. Psychol. Med. 2020, 1–9. [Google Scholar] [CrossRef]
| Variables | N | % | Median | IQR |
|---|---|---|---|---|
| Females | 41 | 59.4 | ||
| Current age | 42 | 34–55 | ||
| BD type (type 1) | 54 | 78.3 | ||
| Density of mood episodes (N/year) | 0.52 | 0.3–0.9 | ||
| MADRS | 2 | 0–3.5 | ||
| YMRS | 1 | 0–2 | ||
| Current tobacco use | 31 | 44.9 | ||
| High risk of OSA | 15 | 21.7 | ||
| BMI (kg/m2) | 24.7 | 22.7–27.9 | ||
| Mood stabilizer monotherapy * | 28 | 40.6 |
| Variables | Median | IQR |
|---|---|---|
| Sleep variables | ||
| TST (min) | 482 | 444–525 |
| WASO (min) | 52 | 33–66 |
| SOL (min) | 12 | 8–19 |
| SE (%) | 85 | 82–88 |
| FI | 30 | 24–37 |
| Sleep variables’ variability | ||
| SD TST (min) | 87 | 67–108 |
| SD WASO (min) | 19.32 | 12.91–28.35 |
| SD SOL (min) | 14.77 | 8.57–28.15 |
| SD SE | 6.24 | 3.47–9.51 |
| SD FI | 9.19 | 7.58–13.11 |
| Circadian rhythms | ||
| IS (range: 0–1) | 0.46 | 0.37–0.54 |
| IV (range: 0–2) | 0.83 | 0.69–0.92 |
| M10 onset (h: min) | 9:00 | 8–11 |
| L5 onset (h: min) | 1:00 | 0–2 |
| M10 | 15,132 | 12,195–20,319 |
| L5 | 890 | 582–1343 |
| Amplitude | 14,085 | 11,207–18,939 |
| Relative amplitude (range: 0–1) | 0.89 | 0.84–0.93 |
| Variables | Beta | SE | Wald | df | p | HR ** | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|---|---|---|
| Age | 0.027 | 0.016 | 2.904 | 1 | 0.09 | 1.03 | 0.99 | 1.06 |
| Type BD | −0.189 | 0.421 | 0.201 | 1 | 0.65 | 0.83 | 0.36 | 1.89 |
| MS polytherapy | 0.821 | 0.335 | 6.020 | 1 | 0.014 | 2.27 | 1.18 | 4.38 |
| Density mood episodes * | 1.759 | 0.731 | 5.801 | 1 | 0.016 | 5.80 | 1.39 | 24.28 |
| BMI | −0.151 | 0.053 | 7.938 | 1 | 0.005 | 0.86 | 0.78 | 0.95 |
| SQ2 | −0.381 | 0.201 | 3.597 | 1 | 0.06 | 0.68 | 0.46 | 1.01 |
| CR1 | −0.412 | 0.158 | 6.804 | 1 | 0.009 | 0.66 | 0.49 | 0.90 |
| CR2 | −0.353 | 0.203 | 3.036 | 1 | 0.08 | 0.70 | 0.47 | 1.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrand, L.; Hennion, V.; Godin, O.; Bellivier, F.; Scott, J.; Etain, B. Which Actigraphy Dimensions Predict Longitudinal Outcomes in Bipolar Disorders? J. Clin. Med. 2022, 11, 2204. https://doi.org/10.3390/jcm11082204
Ferrand L, Hennion V, Godin O, Bellivier F, Scott J, Etain B. Which Actigraphy Dimensions Predict Longitudinal Outcomes in Bipolar Disorders? Journal of Clinical Medicine. 2022; 11(8):2204. https://doi.org/10.3390/jcm11082204
Chicago/Turabian StyleFerrand, Lisa, Vincent Hennion, Ophelia Godin, Frank Bellivier, Jan Scott, and Bruno Etain. 2022. "Which Actigraphy Dimensions Predict Longitudinal Outcomes in Bipolar Disorders?" Journal of Clinical Medicine 11, no. 8: 2204. https://doi.org/10.3390/jcm11082204
APA StyleFerrand, L., Hennion, V., Godin, O., Bellivier, F., Scott, J., & Etain, B. (2022). Which Actigraphy Dimensions Predict Longitudinal Outcomes in Bipolar Disorders? Journal of Clinical Medicine, 11(8), 2204. https://doi.org/10.3390/jcm11082204






