HHT-Related Epistaxis and Pregnancy—A Retrospective Survey and Recommendations for Management from an Otorhinolaryngology Perspective
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. General Pregnancy History
3.2. Awareness of the Disease
3.3. Patient Knowledge and Awareness
3.4. Screening
3.5. Epistaxis and Treatment
4. Discussion
4.1. Knowledge of Women with HHT about the Disease and Its Complications
4.2. Epistaxis during Pregnancy and Delivery in HHT Patients
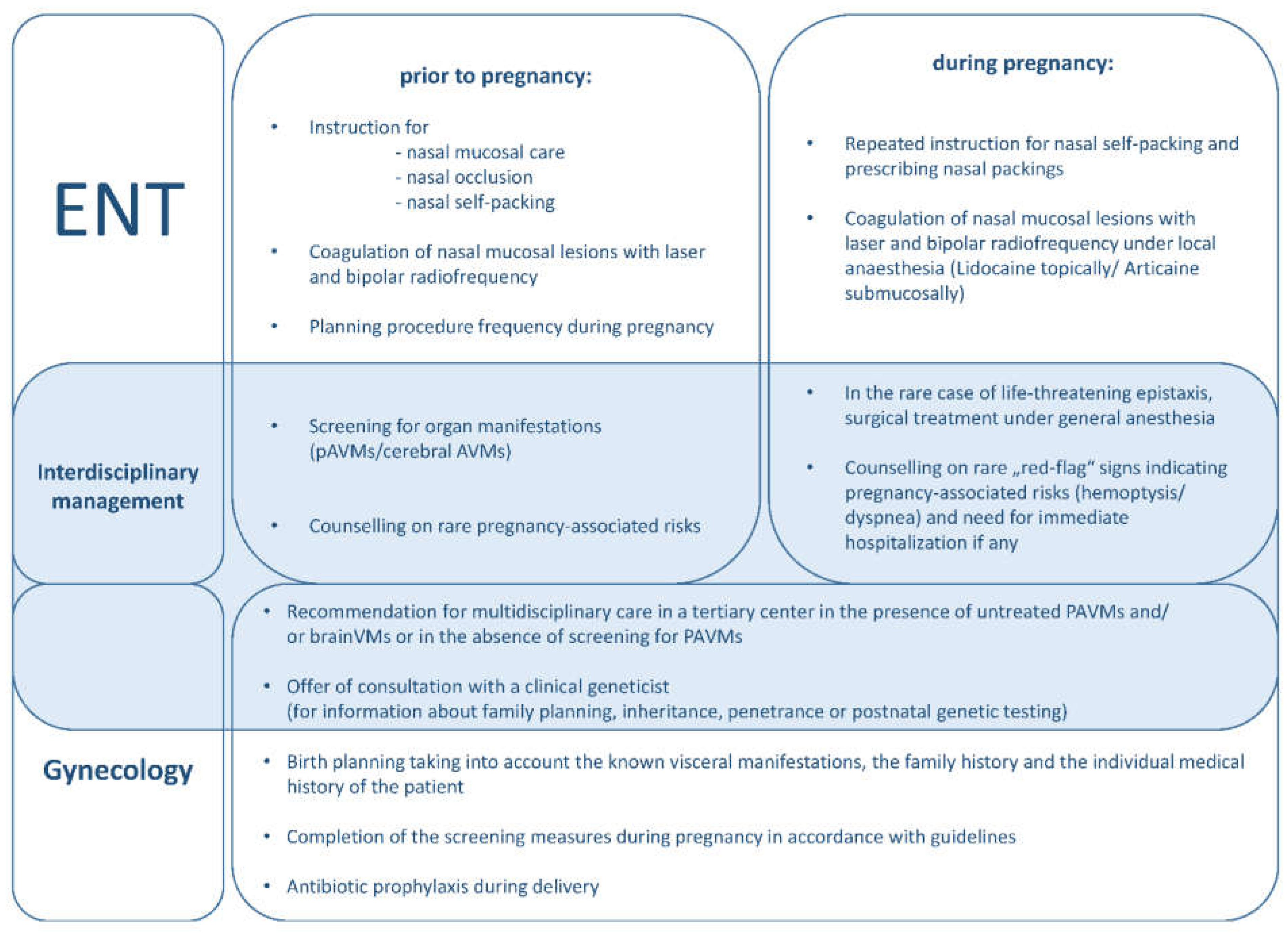
4.3. Recommendations for Management of Females with HHT from an Otorhinolaryngology Perspective
4.4. Future Research Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- 1.
- How many children do you have and how old were you at the time of each delivery?
- First delivery ı__ı__ı
- Second delivery ı__ı__ı
- Third delivery ı__ı__ı
- 2.
- When did you learn that you have HHT?
- ○
- before the onset of my first pregnancy
- ○
- before the onset of pregnancy number ı__ı
- ○
- during pregnancy number ı__ı
- ○
- after completion of all pregnancies
- 3.
- Has your illness influenced your family planning or desire to have children?
- ○
- no
- ○
- yes
- 4.
- Are you currently aware that HHT is linked to an increased likelihood of complications during pregnancy (for example, pulmonary bleeding, heart problems or increased risk of stroke)?
- ○
- no
- ○
- yes
- 4.1.
- If you answered “yes” to Question 4, were you aware before your pregnancy(ies)?
- ○
- no
- ○
- yes
- ○
- I have had multiple pregnancies. I was not aware before the first pregnancy, but was aware before subsequent pregnancy(ies).
- 5.
- If you answered either “yes” or “I have had multiple pregnancies.” to question 4.1, please answer the following question.
- ○
- general/primary care practitioner
- ○
- Ear-Nose-and-Throat doctor
- ○
- family members
- ○
- HHT support group
- ○
- Others: _______________________________________________________________
- 6.
- Did you have HHT-related examinations of the lungs, liver, brain, spine or gastrointestinal tract prior to or during your pregnancy(ies)?
- ○
- no
- ○
- yes
- ○
- Inspection of the lung (ultrasound/CT scan/pulmonary function test)
- ○
- Inspection of the liver (ultrasound)
- ○
- Inspection of the brain (MRI)
- ○
- Inspection of the spine (MRI)
- 7.
- Did you undergo any medical treatments before or during your pregnancy(ies) to reduce the likelihood of HHT-related complications (e.g., embolization of AV shunts of the lung, liver or brain)?
- ○
- no
- ○
- yes
- 8.
- Did you have nosebleeds during this pregnancy?
- ○
- no
- ○
- yes
- ○
- more intense
- ○
- same intensity
- ○
- less intense
- 9.
- Did your nosebleeds need to be treated during this pregnancy (more than the external use of a tissue?)?
- ○
- no
- ○
- yes
- ○
- Self-tamponade
- ○
- Tamponade via a health care professional
- ○
- cautery
- ○
- laser treatment
- ○
- cautery/laser treatment or embolization under general anesthesia
- 10.
- If you answered “yes” to Question 8, would you have been capable of treating your nosebleeds yourself (for example, by self-tamponade)?
- ○
- was not necessary
- ○
- no
- ○
- yes
- 11.
- Did you have nosebleeds during delivery?
- ○
- no
- ○
- yes
- ○
- Don’t know/cannot remember
- 12.
- Did you have to pack your nose during delivery?
- ○
- no
- ○
- yes
- ○
- Don’t know/cannot remember
- 13.
- We would now like to know how satisfied you were with your nosebleeds during pregnancy.
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ |
| 0 = completely unsatisfied; 10 = completely satisfied. | ||||||||||
- 14.
- We want to know how satisfied you were with your nosebleeds during delivery.
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ | ◯ |
| 0 = completely unsatisfied; 10 = completely satisfied. | ||||||||||
- 15.
- What kind of birth/births did you have? Specify the number:
- ı__ı time/s spontaneous delivery with epidural anesthesia
- ı__ı time/s spontaneous delivery without epidural anesthesia
- ı__ı time/s c- section with epidural anesthesia
- ı__ı time/s c- section under general anesthesia
- 16.
- Would you have found medical advice on HHT during your pregnancy(ies) helpful?
- ○
- no
- ○
- yes
- ○
- I don’t know
- 17.
- How old are you now? ______________________years
References
- Dakeishi, M.; Shioya, T.; Wada, Y.; Shindo, T.; Otaka, K.; Manabe, M.; Nozaki, J.-I.; Inoue, S.; Koizumi, A. Genetic epidemiology of hereditary hemorrhagic telangiectasia in a local community in the northern part of Japan. Hum. Mutat. 2002, 19, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Faughnan, M.E.; Palda, V.A.; Garcia-Tsao, G.; Geisthoff, U.W.; McDonald, J.; Proctor, D.D.; Spears, J.; Brown, D.H.; Buscarini, E.; Chesnutt, M.S.; et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J. Med. Genet. 2011, 48, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Shovlin, C.L.; Guttmacher, A.E.; Buscarini, E.; Faughnan, M.E.; Hyland, R.H.; Westermann, C.J.; Kjeldsen, A.D.; Plauchu, H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am. J. Med Genet. 2000, 91, 66–67. [Google Scholar] [CrossRef]
- Plauchu, H.; De Chadarévian, J.-P.; Bideau, A.; Robert, J.-M. Age-related clinical profile of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am. J. Med. Genet. 1989, 32, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Assar, O.S.; Friedman, C.M.; White, R.I.J. The natural history of epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope. 1991, 101, 977–980. [Google Scholar] [CrossRef]
- Latino, G.A.; Brown, D.; Glazier, R.H.; Weyman, J.T.; Faughnan, M.E. Targeting under-diagnosis in hereditary hemorrhagic telangiectasia: A model approach for rare diseases? Orphanet J. Rare Dis. 2014, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Pierucci, P.; Lenato, G.M.; Suppressa, P.; Lastella, P.; Triggiani, V.; Valerio, R.; Comelli, M.; Salvante, D.; Stella, A.; Resta, N.; et al. A long diagnostic delay in patients with Hereditary Haemorrhagic Telangiectasia: A questionnaire-based retrospective study. Orphanet J. Rare Dis. 2012, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Dupuis, O.; Delagrange, L.; Dupuis-Girod, S. Hereditary haemorrhagic telangiectasia and pregnancy: A review of the literature. Orphanet J. Rare Dis. 2020, 15, 5. [Google Scholar] [CrossRef] [Green Version]
- Silversides, C.K.; Colman, J.M. Physiological Changes in Pregnancy. In Heart Disease in Pregnancy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 6–17. [Google Scholar] [CrossRef]
- Shovlin, C.L.; Sodhi, V.; McCarthy, A.; Lasjaunias, P.; Jackson, J.E.; Sheppard, M.N. Estimates of maternal risks of pregnancy for women with hereditary haemorrhagic telangiectasia (Osler-Weber-Rendu syndrome): Suggested approach for obstetric services. BJOG 2008, 115, 1108–1115. [Google Scholar] [CrossRef] [Green Version]
- de Gussem, E.M.; Lausman, A.Y.; Beder, A.J.; Edwards, C.P.; Blanker, M.H.; Terbrugge, K.G.; Mager, J.J.; Faughnan, M.E. Outcomes of pregnancy in women with hereditary hemorrhagic telangiectasia. Obstet. Gynecol. 2014, 123, 514–520. [Google Scholar] [CrossRef] [Green Version]
- Faughnan, M.E.; Mager, J.J.; Hetts, S.W.; Palda, V.A.; Lang-Robertson, K.; Buscarini, E.; Deslandres, E.; Kasthuri, R.S.; Lausman, A.; Poetker, D.; et al. Second International Guidelines for the Diagnosis and Management of Hereditary Hemorrhagic Telangiectasia. Ann. Intern. Med. 2020, 173, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Giambanco, L.; Iannone, V.; Borriello, M.; Scibilia, G.; Scollo, P. The way a nose could affect pregnancy: Severe and recurrent epistaxis. Pan Afr. Med J. 2019, 34, 49. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, M.G.; Derme, M.; Salerno, L.; Morrocchi, E.; Pecorini, F.; Porpora, M.G.; Brunelli, R. Management of Severe Epistaxis during Pregnancy: A Case Report and Review of the Literature. Case Rep. Obstet. Gynecol. 2019, 2019, 5825309. [Google Scholar] [CrossRef] [PubMed]
- Wirsching, K.E.C.; Kühnel, T.S. Update on Clinical Strategies in Hereditary Hemorrhagic Telangiectasia from an ENT Point of View. Clin. Exp. Otorhinolaryngol. 2017, 10, 153–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirsching, K.E.C.; Haubner, F.; Kühnel, T.S. Influence of temporary nasal occlusion (tNO) on epistaxis frequency in patients with hereditary hemorrhagic telangiectasia (HHT). Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Bertlich, M.; Kashani, F.; Weiss, B.G.; Wiebringhaus, R.; Ihler, F.; Freytag, S.; Gires, O.; Kühnel, T.; Haubner, F. Safety and Efficacy of Blue Light Laser Treatment in Hereditary Hemorrhagic Telangiectasia. Lasers Surg. Med. 2021, 53, 309–315. [Google Scholar] [CrossRef]
- Ouanounou, A.; Haas, D.A. Drug therapy during pregnancy: Implications for dental practice. Br. Dent. J. 2016, 220, 413–417. [Google Scholar] [CrossRef]
- Seebauer, C.T.; Freigang, V.; Schwan, F.E.; Fischer, R.; Bohr, C.; Kühnel, T.S.; Andorfer, K.E.C. Hereditary Hemorrhagic Telangiectasia: Success of the Osler Calendar for Documentation of Treatment and Course of Disease. J. Clin. Med. 2021, 10, 4720. [Google Scholar] [CrossRef]
- Gaillard, S.; Dupuis-Girod, S.; Boutitie, F.; Rivière, S.; Morinière, S.; Hatron, P.-Y.; Manfredi, G.; Kaminsky, P.; Capitaine, A.-L.; Roy, P.; et al. Tranexamic acid for epistaxis in hereditary hemorrhagic telangiectasia patients: A European cross-over controlled trial in a rare disease. J. Thromb. Haemost. 2014, 12, 1494–1502. [Google Scholar] [CrossRef]
- Albiñana, V.; Cuesta, A.M.; De Rojas, P.I.; Gallardo-Vara, E.; Recio-Poveda, L.; Bernabéu, C.; Botella, L.M. Review of Pharmacological Strategies with Repurposed Drugs for Hereditary Hemorrhagic Telangiectasia Related Bleeding. J. Clin. Med. 2020, 9, 1766. [Google Scholar] [CrossRef]
- Peitsidis, P.; Kadir, R.A. Antifibrinolytic therapy with tranexamic acid in pregnancy and postpartum. Expert Opin. Pharmacother. 2011, 12, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Shakur-Still, H.; Roberts, I.; Fawole, B.; Chaudhri, R.; El-Sheikh, M.; Akintan, A.; Qureshi, Z.; Kidanto, H.; Vwalika, B.; Abdulkadir, A.; et al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): An international, randomised, double-blind, placebo-controlled trial. Lancet 2017, 389, 2105–2116. [Google Scholar] [CrossRef] [Green Version]
- Shovlin, C.L.; Sulaiman, N.L.; Govani, F.S.; Jackson, J.E.; Begbie, M.E. Elevated factor VIII in hereditary haemorrhagic telangiectasia (HHT): Association with venous thromboembolism. Thromb. Haemost. 2007, 98, 1031–1039. [Google Scholar]
- Droege, F.; Lueb, C.; Thangavelu, K.; Stuck, B.A.; Lang, S.; Geisthoff, U. Nasal self-packing for epistaxis in Hereditary Hemorrhagic Telangiectasia increases quality of life. Rhinology 2019, 57, 231–239. [Google Scholar] [CrossRef]
- Williams, M.D.; Wheby, M.S. Anemia in pregnancy. Med Clin. N. Am. 1992, 76, 631–647. [Google Scholar] [CrossRef]
- Shovlin, C.L.; Buscarini, E.; Kjeldsen, A.D.; Mager, H.J.; Sabba, C.; Droege, F.; Geisthoff, U.; Ugolini, S.; Dupuis-Girod, S. European Reference Network for Rare Vascular Diseases (VASCERN) Outcome Measures for Hereditary Haemorrhagic Telangiectasia (HHT). Orphanet. J. Rare Dis. 2018, 13, 136. [Google Scholar] [CrossRef]
- Mora-Luján, J.M.; Iriarte, A.; Alba, E.; Sánchez-Corral, M.A.; Cerdà, P.; Cruellas, F.; Ordi, Q.; Corbella, X.; Ribas, J.; Castellote, J.; et al. Gender differences in hereditary hemorrhagic telangiectasia severity. Orphanet J. Rare Dis. 2020, 15, 63. [Google Scholar] [CrossRef] [Green Version]

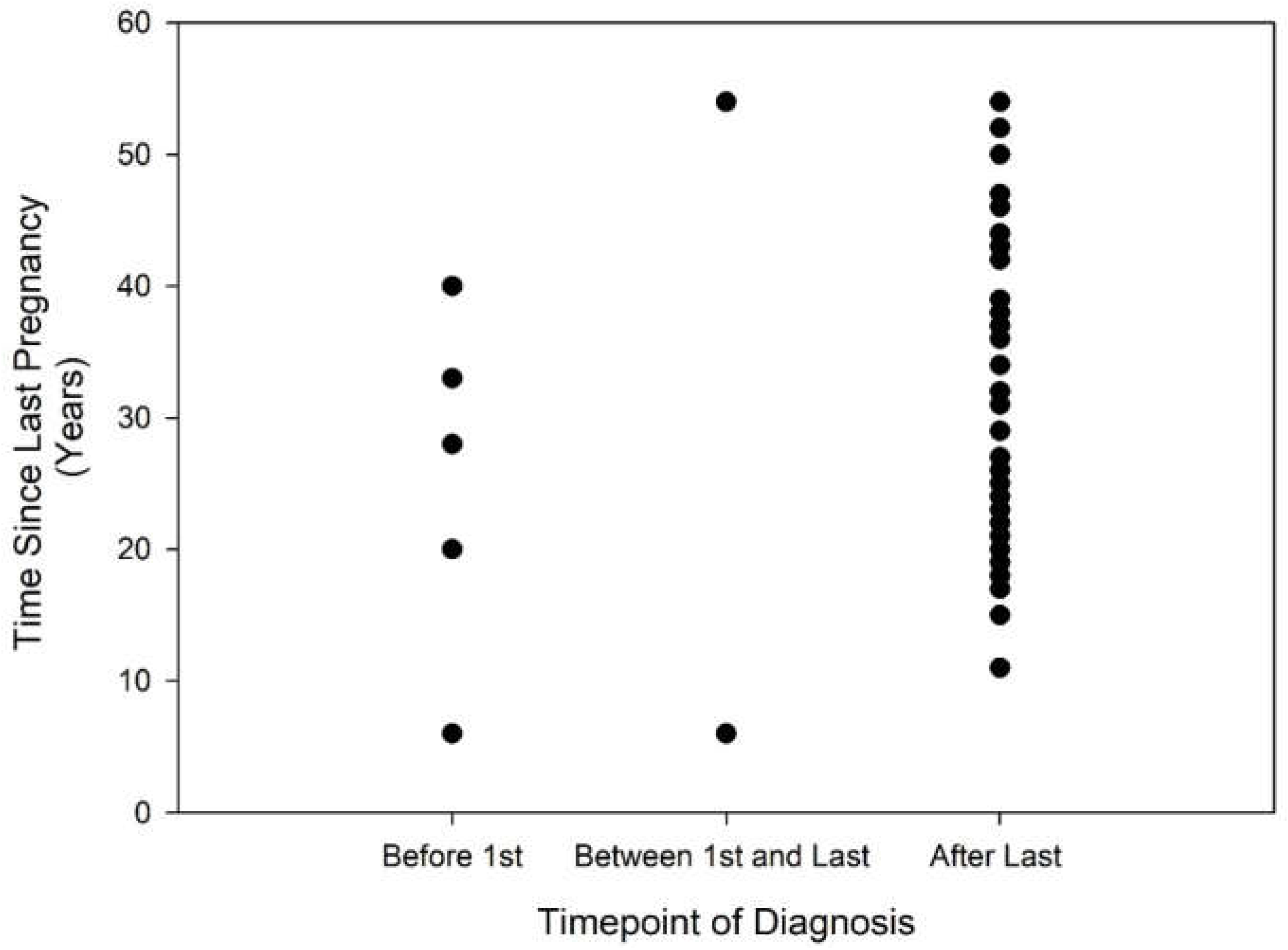
| All Respondents n = 45 | Age at Survey < 60 Years | Age at Survey > 60 Years | |
|---|---|---|---|
| Prior to first pregnancy | 5 (11.1%) | 3 (13.6%) | 2 (8.6%) |
| Between first and last pregnancy | 2 (4.4%) | 1 (4.5%) | 1 (4.3%) |
| After last pregnancy | 38 (84.4%) | 18 (81.8%) | 20 (86.9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andorfer, K.E.C.; Seebauer, C.T.; Dienemann, C.; Marcrum, S.C.; Fischer, R.; Bohr, C.; Kühnel, T.S. HHT-Related Epistaxis and Pregnancy—A Retrospective Survey and Recommendations for Management from an Otorhinolaryngology Perspective. J. Clin. Med. 2022, 11, 2178. https://doi.org/10.3390/jcm11082178
Andorfer KEC, Seebauer CT, Dienemann C, Marcrum SC, Fischer R, Bohr C, Kühnel TS. HHT-Related Epistaxis and Pregnancy—A Retrospective Survey and Recommendations for Management from an Otorhinolaryngology Perspective. Journal of Clinical Medicine. 2022; 11(8):2178. https://doi.org/10.3390/jcm11082178
Chicago/Turabian StyleAndorfer, Kornelia E. C., Caroline T. Seebauer, Carolin Dienemann, Steven C. Marcrum, René Fischer, Christopher Bohr, and Thomas S. Kühnel. 2022. "HHT-Related Epistaxis and Pregnancy—A Retrospective Survey and Recommendations for Management from an Otorhinolaryngology Perspective" Journal of Clinical Medicine 11, no. 8: 2178. https://doi.org/10.3390/jcm11082178
APA StyleAndorfer, K. E. C., Seebauer, C. T., Dienemann, C., Marcrum, S. C., Fischer, R., Bohr, C., & Kühnel, T. S. (2022). HHT-Related Epistaxis and Pregnancy—A Retrospective Survey and Recommendations for Management from an Otorhinolaryngology Perspective. Journal of Clinical Medicine, 11(8), 2178. https://doi.org/10.3390/jcm11082178






