Isolation and Characterisation of Quercitrin as a Potent Anti-Sickle Cell Anaemia Agent from Alchornea cordifolia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection of Plant Samples
2.3. Blood Sampling
2.4. Extraction of A. cordifolia Leaves
2.5. Bioactivity-Guided Purification of MeOH Extract
2.6. Ultra High-Performance Liquid Chromatography–High Resolution Mass Spectrometry (UHPLC-HRMS)
2.7. Nuclear Magnetic Resonance
2.8. In Vitro Sickling-Inhibition and Reversibility Assays
2.9. Erythrocyte Leakage Assay
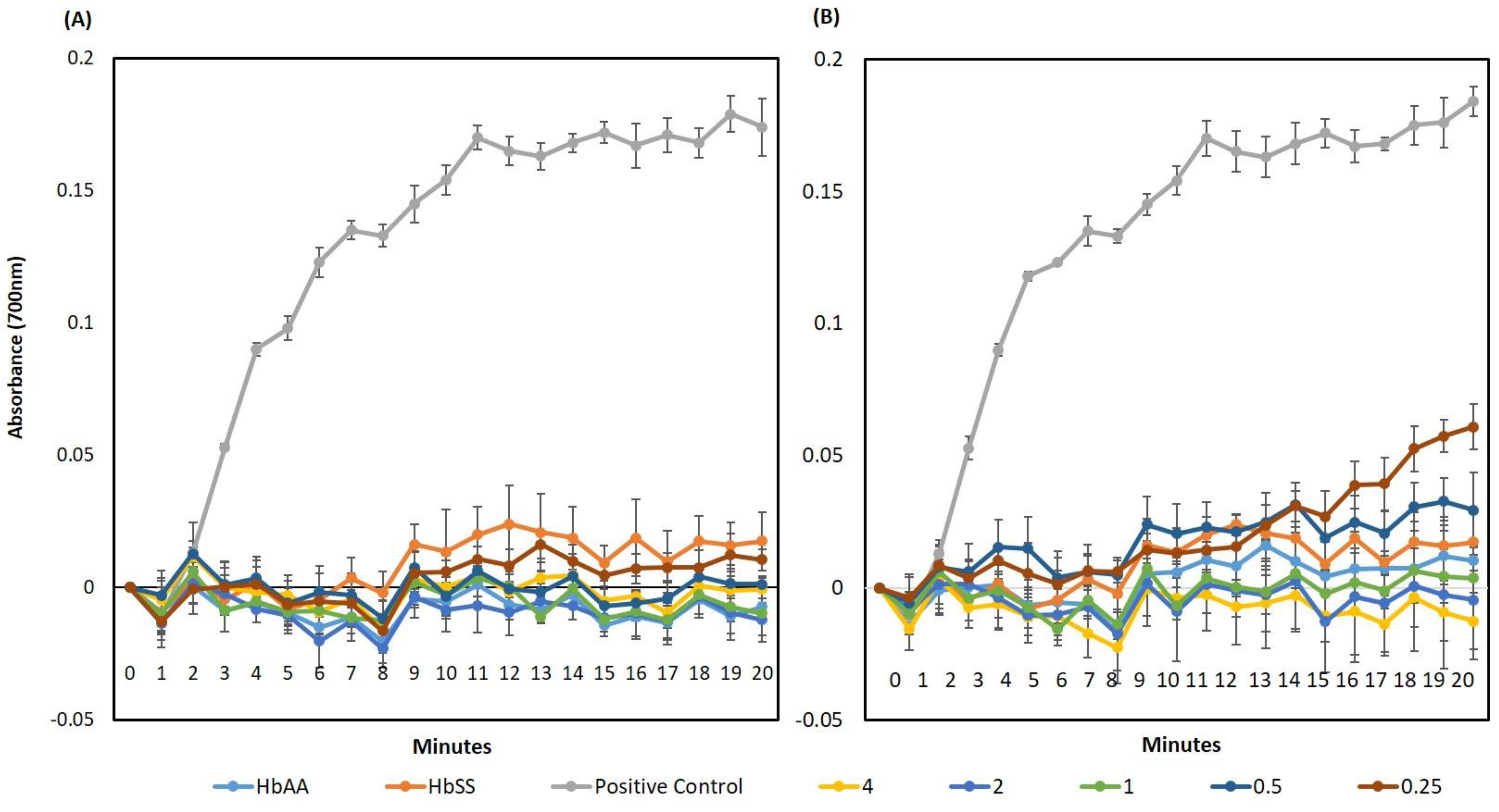
2.10. Hb Polymerisation Assay
2.11. Scanning Electron Microscopy (SEM)
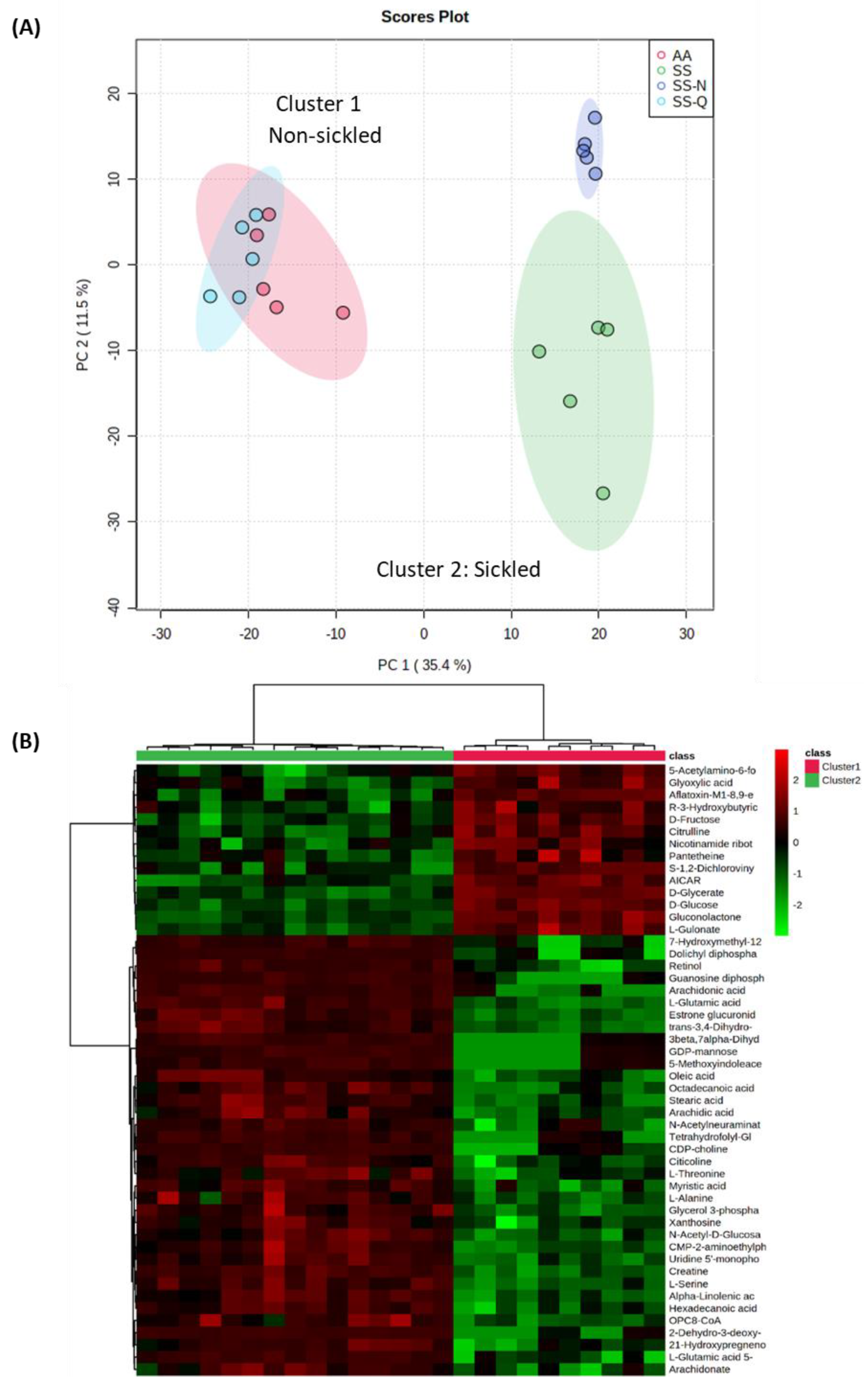
2.12. Metabolomic Analyses
2.13. Statistical Analysis
3. Results
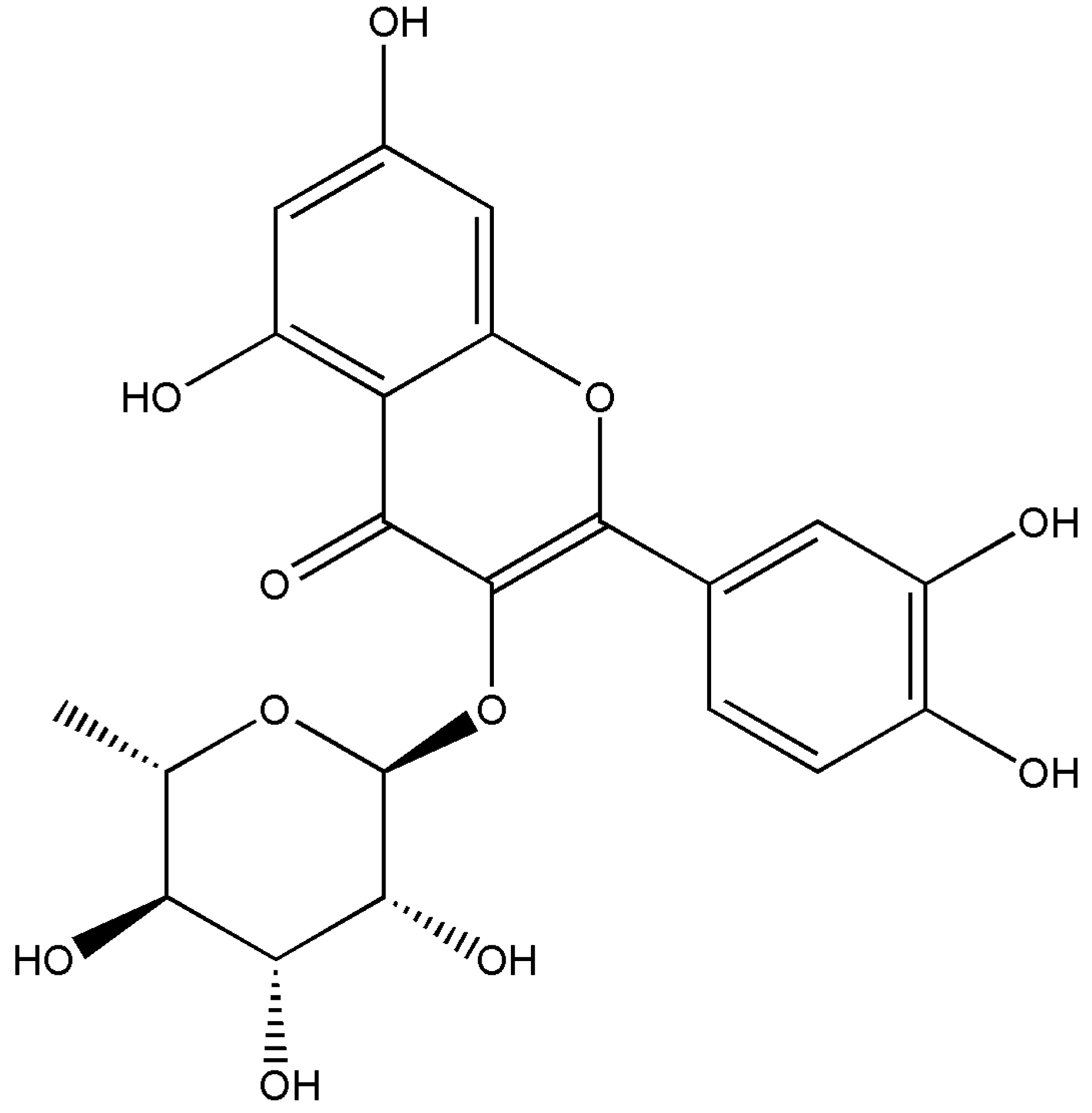
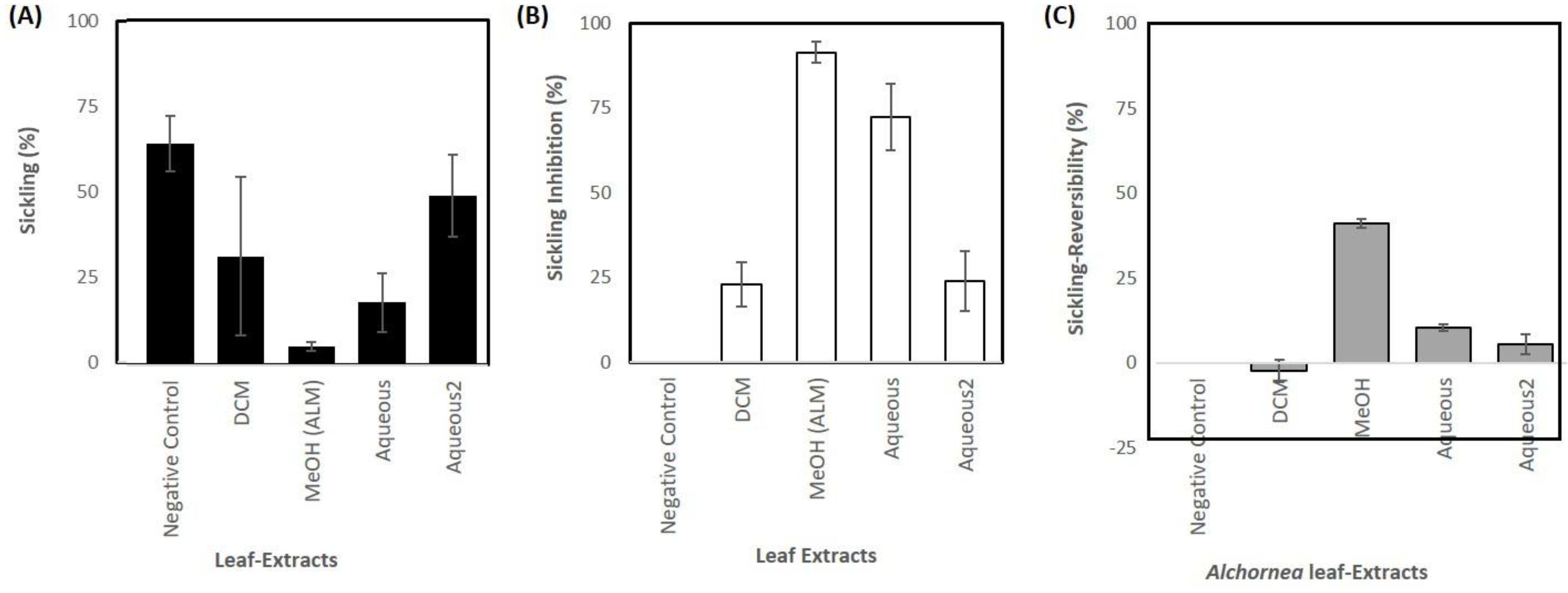
3.1. Sample Extraction and Anti-Sickling Activity of A. cordifolia Crude Extracts
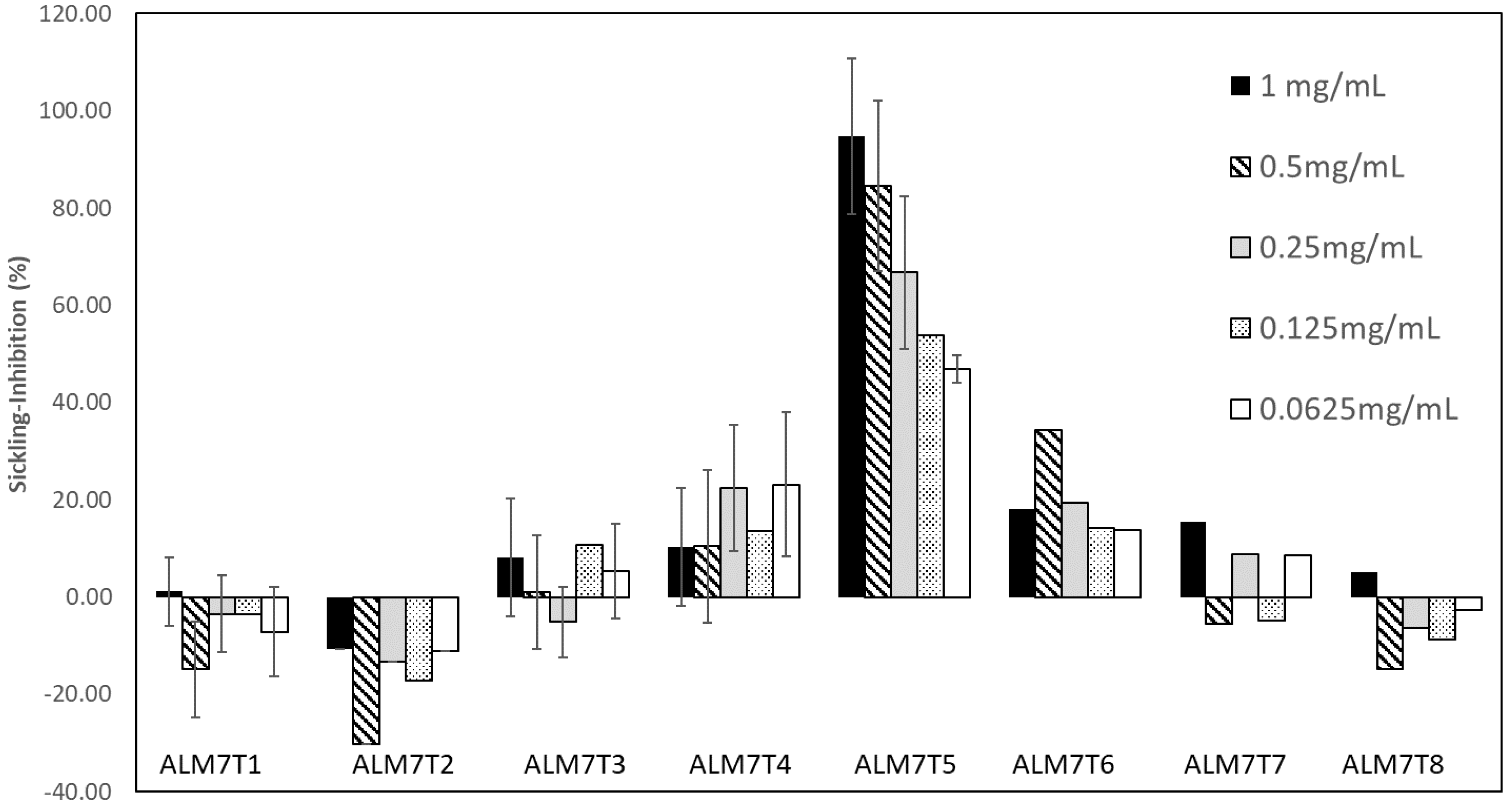
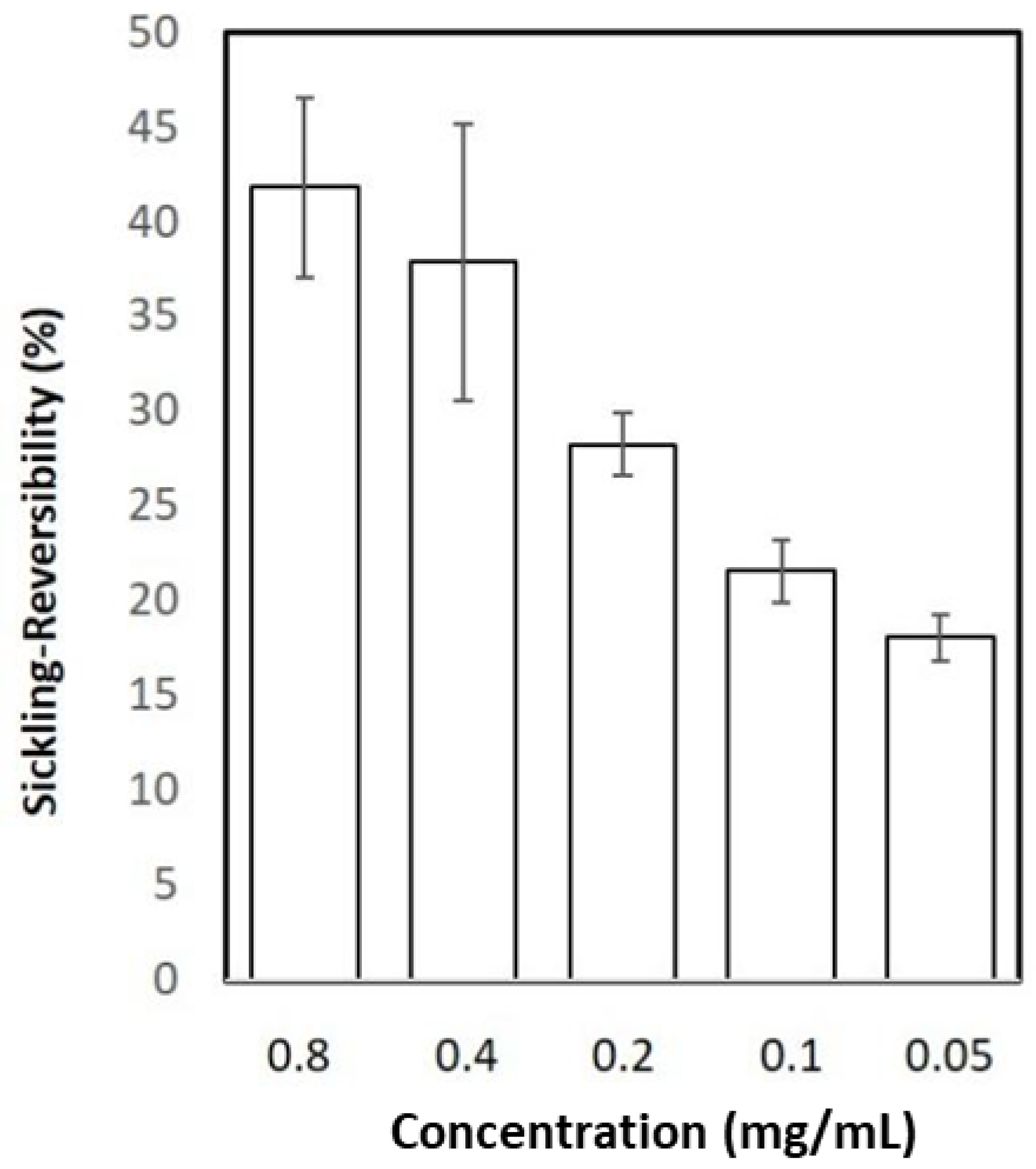
3.2. Isolation of the Anti-Sickling Bioactives in the A. cordifolia Methanolic Extract
3.3. Assessing the Impact on Quercitrin on HbSS Sickling Using Metabolomics
4. Discussion
4.1. Inhibition of HbS Polymerisation: An Important Mode of Action for Quercitrin
4.2. Insights into the Mechanism of Quercitrin Effects Using Metabolomics Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roucher, C.; Rogier, C.; Dieye-Ba, F.; Sokhna, C.; Tall, A.; Trape, J.-F. Changing malaria epidemiology and diagnostic criteria for Plasmodium falciparum clinical malaria. PLoS ONE 2012, 7, e46188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundd, P.; Gladwin, M.T.; Novelli, E.M. Pathophysiology of Sickle Cell Disease. Annu. Rev. Pathol. 2019, 14, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Gibson, J.S. Biomarkers in sickle cell disease. Br. J. Haematol. 2012, 156, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, S.; Telford, J.; Crepeau, R. Structure of Fibers of Sickle Cell Hemoglobin. Proc. Natl. Acad. Sci. USA 1973, 70, 1104–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brugnara, C. Erythrocyte dehydration in pathophysiology and treatment of sickle cell disease. Curr. Opin. Hematol. 1995, 2, 132–138. [Google Scholar] [CrossRef]
- Odièvre, M.H.; Verger, E.; Silva-Pinto, A.C.; Elion, J. Pathophysiological insights in sickle cell disease. Indian J. Med. Res. 2011, 134, 532–537. [Google Scholar]
- Friedman, M.T.; West, K.A.; Bizargity, P. The Perils of Transfusing the Sickle Cell Patient, in Immunohematology and Transfusion Medicine: A Case Study Approach; Springer International Publishing: Cham, Germany, 2016; pp. 115–121. [Google Scholar]
- Piccin, A.; Murphy, C.; Eakins, E.; Rondinelli, M.B.; Daves, M.; Vecchiato, C.; Wolf, D.; Mc Mahon, C.; Smith, O.P. Insight into the complex pathophysiology of sickle cell anaemia and possible treatment. Eur. J. Haematol. 2019, 102, 319–330. [Google Scholar] [CrossRef]
- Carden, M.A.; Tanabe, P.; Glassberg, J.A. Intravenous Fluid Boluses Are Commonly Administered to Adults with Sickle Cell Disease and Vaso-Occlusive Pain. Blood 2019, 134, 4839. [Google Scholar] [CrossRef]
- Puri, L.; Nottage, K.A.; Hankins, J.S.; Anghelescu, D.L. State of the Art Management of Acute Vaso-occlusive Pain in Sickle Cell Disease. Paediatr. Drugs 2018, 20, 29–42. [Google Scholar] [CrossRef]
- Abdulmalik, O.; Safo, M.K.; Chen, Q.; Yang, J.; Brugnara, C.; Ohene-Frempong, K.; Abraham, D.J.; Asakura, T. 5-hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. Br. J. Haematol. 2005, 128, 552–561. [Google Scholar] [CrossRef]
- Omar, A.M.; David, T.; Pagare, P.P.; Ghatge, M.S.; Chen, Q.; Mehta, A.; Zhang, Y.; Abdulmalik, O.; Naghi, A.H.; El-Araby, M.E.; et al. Structural modification of azolylacryloyl derivatives yields a novel class of covalent modifiers of hemoglobin as potential antisickling agents. Medchemcomm 2019, 10, 1900–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federal Drug Agency. FDA Approves Voxelotor for Sickle Cell Disease. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-voxelotor-sickle-cell-disease (accessed on 13 October 2019).
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef]
- Best, P.J.; Daoud, M.S.; Pittelkow, M.R.; Petitt, R.M. Hydroxyurea-induced leg ulceration in 14 patients. Ann. Intern. Med. 1998, 128, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.J.; Piel, F.B.; Reid, C.D.; Gaston, M.H.; Ohene-Frempong, K.; Krishnamurti, L.; Smith, W.R.; Panepinto, J.A.; Weatherall, D.J.; Costa, F.F.; et al. Sickle cell disease. Nat. Rev. Dis. Primers 2018, 4, 18010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imaga, N. The use of phytomedicines as effective therapeutic agents in sickle cell anemia. Sci. Res. Essays 2010, 5, 3803–3807. [Google Scholar]
- Odunlade, O.C.; Adeodu, O.; Owa, J.; Obuotor, E. Iron overload in steady state, non-chronically transfused children with sickle cell anaemia in Ile-Ife, Nigeria. Pediatric Hematol. Oncol. J. 2017, 2, 35–38. [Google Scholar] [CrossRef]
- Oyewole, O.I.; Malomo, S.; Adebayo, J. Comparative studies on antisickling properties of thiocyanate, tellurite and hydroxyurea. Pak. J. Med. Sci. 2008, 24, 18–22. [Google Scholar]
- Akojie, F.O.; Fung, L.W. Antisickling activity of hydroxybenzoic acids in Cajanus cajan. Planta Med. 1992, 58, 317–320. [Google Scholar] [CrossRef]
- Sofowora, E.A.; Isaac-Sodeye, W.A.; Ogunkoya, L.O. Isolation and characterisation of an antisickling agent from Fagara zanthoxyloides root. Lloydia 1975, 38, 169–171. [Google Scholar]
- Tshibangu, D.S.T. Phytochemical and anti-drepanocytosis studies of Cajanus cajan, Callistemon viminalis, Melaleuca bracteata var. Revolut. Gold Syzygium Guineense. Ph.D. Thesis, 2010. Available online: https//researchspace.ukzn.ac.za/xmlui/handle/10413/8113 (accessed on 9 April 2022).
- Ouattara, B.; Jansen, O.; Angenot, L.; Guissou, I.; Frederich, M.; Fondu, P.; Tits, M. Antisickling properties of divanilloylquinic acids isolated from Fagara zanthoxyloides Lam. (Rutaceae). Phytomedicine 2009, 16, 125–129. [Google Scholar] [CrossRef]
- Tshilanda, D.D.; Mpiana, P.T.; Onyamboko, D.N.V.; Mbala, B.M.; Ngbolua, K.-T.; Tshibangu, D.S.T.; Bokolo, M.K.; Taba, K.M.; Kasonga, T.K. Antisickling activity of butyl stearate isolated from Ocimum basilicum (Lamiaceae). Asian Pac. J. Trop. Biomed. 2014, 4, 393–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tshilanda, D.D.; Onyamboko, D.N.; Babady-Bila, P.; Ngbolua, K.-T.; Tshibangu, D.S.; Dibwe, E.D.F.; Mpiana, P.T. Anti-sickling Activity of Ursolic Acid Isolated from the Leaves of Ocimum gratissimum L. (Lamiaceae). Nat. Prod. Bioprospect. 2015, 5, 215–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogundipe, O.O.; Moody, J.; Houghton, P.; Odelola, H. Bioactive chemical constituents from Alchornea laxiflora (benth) pax and hoffman. J. Ethnopharmacol. 2001, 74, 275–280. [Google Scholar] [CrossRef]
- Oloyede, G.; Onocha, P.A.; Soyinka, J.; Oguntokun, O.; Thonda, E. Phytochemical screening, antimicrobial and antioxidant activities of four Nigerian medicinal plants. Ann. Biol. Res. 2010, 1, 114–120. [Google Scholar]
- Borokini, T.I.; Omotayo, F.O. Phytochemical and ethnobotanical study of some selected medicinal plants from Nigeria. J. Med. Plants Res. 2012, 6, 1106–1118. [Google Scholar]
- Ogundipe, O.; Moody, J.; Houghton, P. Occurrence of Flavonol Sulphates in Alchornea laxiflora. Pharm. Biol. 2008, 39, 421–423. [Google Scholar] [CrossRef]
- Mohammed, R.K.; Ibrahim, S.; Atawodi, S.E.; Eze, E.D.; Suleiman, J.B.; Malgwi, I.S. Anti-diabetic and haematological effects of n-butanol fraction of Alchornea cordifolia leaf extract in streptozotocin-induced diabetic wistar rats. J. Biol. Sci. 2013, 2, 45–53. [Google Scholar]
- Rani, P.; Peta, D. Efficiency of Different Plant Foliar Extracts on Grain Protection and Seed Germination in Maize. Res. J. Seed Sci. 2011, 4, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zhong, X.-N.; Otsuka, H.; Ide, T.; Hirata, E.; Takushi, A.; Takeda, Y. Three flavonol glycosides from leaves of Myrsine seguinii. Phytochemistry 1997, 46, 943–946. [Google Scholar] [CrossRef]
- Jang, S.-Y.; Jang, S.Y.; Bae, J.S.; Lee, Y.H.; Oh, K.Y.; Park, K.H.; Bae, Y.S. Caffeic acid and quercitrin purified from Houttuynia cordata inhibit DNA topoisomerase I activity. Nat. Prod. Res. 2011, 25, 222–231. [Google Scholar] [CrossRef]
- Iwu, M.M.; Igboko, A.; Onwubiko, H.; Ndu, U. Effect of cajaminose from Cajanus cajan on gelation and oxygen affinity of sickle cell haemoglobin. J. Ethnopharmacol. 1988, 23, 99–104. [Google Scholar] [CrossRef]
- Srivastava, A.; Evans, K.J.; Sexton, A.E.; Schofield, L.; Creek, D.J. Metabolomics-Based Elucidation of Active Metabolic Pathways in Erythrocytes and HSC-Derived Reticulocytes. J. Proteome Res. 2017, 16, 1492–1505. [Google Scholar] [CrossRef] [PubMed]
- Sana, T.R.; Waddell, K.; Fischer, S.M. A sample extraction and chromatographic strategy for increasing LC/MS detection coverage of the erythrocyte metabolome. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 871, 314–321. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.C.; Chen, Y.-C.; Hsu, F.-L.; Lee, W.-R. Differential apoptosis-inducing effect of quercetin and its glycosides in human promyeloleukemic HL-60 cells by alternative activation of the caspase 3 cascade. J. Cell Biochem. 2003, 89, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Atabo, S.; Umar, I.A.; James, D.B.; Mamman, A.I. Sickled Erythrocytes Reversal and Membrane Stabilizing Compounds in Telfairia occidentalis. Scientifica 2016, 2016, 1568061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Park, Y.; Duraisingham, S.; Strobel, F.H.; Khan, N.; Soltow, Q.A.; Jones, D.P.; Pulendran, B. Predicting network activity from high throughput metabolomics. PLoS Comput. Biol. 2013, 9, e1003123. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Mpiana, P.T.; Tshibangu, D.; Shetonde, O.; Ngbolua, K. In vitro antidrepanocytary actvity (anti-sickle cell anemia) of some congolese plants. Phytomedicine 2007, 14, 192–195. [Google Scholar] [CrossRef]
- Mpiana, P.T.; Mudogo, V.; Tshibangu, D.; Ngbolua, K.; Shetonde, O.; Mangwala, K.; Mavakala, B. In vitro Antisickling Activity of Anthocyanins Extract of a Congolese Plant: Alchornea cordifolia M. Arg. J. Med. Sci. 2007, 7, 1182–1186. [Google Scholar] [CrossRef]
- Muzitano, M.F.; Cruz, E.A.; de Almeida, A.P.; Da Silva, S.A.G.; Kaiser, C.R.; Guette, C.; Rossi-Bergmann, B.; Costa, S.S. Quercitrin: An antileishmanial flavonoid glycoside from Kalanchoe pinnata. Planta Med. 2006, 72, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Maji, S.; Chakraborty, P. Quercitrin from Ixora coccinea Leaves and its Anti-oxidant Activity. J. PharmaSciTech 2013, 2, 72–74. [Google Scholar]
- Yamazaki, E.; Inagaki, M.; Kurita, O.; Inoue, T. Antioxidant activity of Japanese pepper (Zanthoxylum piperitum DC.) fruit. Food Chem. 2007, 100, 171–177. [Google Scholar] [CrossRef]
- Yin, Y.; Li, W.; Son, Y.-O.; Sun, L.; Lu, J.; Kim, D.; Wang, X.; Yao, H.; Wang, L.; Pratheeshkumar, P.; et al. Quercitrin protects skin from UVB-induced oxidative damage. Toxicol. Appl. Pharm. 2013, 269, 89–99. [Google Scholar] [CrossRef] [Green Version]
- de Medina, F.S.; Gálvez, J.; Romero, J.A.; Zarzuelo, A. Effect of quercitrin on acute and chronic experimental colitis in the rat. J. Pharmacol. Exp. Ther. 1996, 278, 771–779. [Google Scholar]
- da Silva, E.R.; Cdo, C.M.; Magalhães, P.P. The leishmanicidal flavonols quercetin and quercitrin target Leishmania (Leishmania) amazonensis arginase. Exp. Parasitol. 2012, 130, 183–188. [Google Scholar] [CrossRef]
- Gálvez, J.; Crespo, M.E.; Jiménez, J.; Suárez, A.; Zarzuelo, A. Antidiarrhoeic activity of quercitrin in mice and rats. J. Pharm. Pharmacol. 1993, 45, 157–159. [Google Scholar] [CrossRef]
- Gadotti, V.M.; Santos, A.; Meyre-Silva, C.; Schmeling, L.O.; Machado, C.; Liz, F.H.; Filho, V.C. Antinociceptive action of the extract and the flavonoid quercitrin isolated from Bauhinia microstachya leaves. J. Pharm. Pharmacol. 2005, 57, 1345–1351. [Google Scholar] [CrossRef]
- Camuesco, D.; Comalada, M.; Rodriguez-Cabezas, M.E.; Nieto, A.; Lorente, M.D.; Concha, A.; Zarzuelo, A.; Gálvez, J. The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br. J. Pharmacol. 2004, 143, 908–918. [Google Scholar] [CrossRef] [Green Version]
- Pule, G.D.; Mowla, S.; Novitzky, N.; Wiysonge, C.; Wonkam, A. A systematic review of known mechanisms of hydroxyurea-induced fetal hemoglobin for treatment of sickle cell disease. Expert Rev. Hematol. 2015, 8, 669–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehanna, A.S. Sickle cell anemia and antisickling agents then and now. Curr. Med. Chem. 2001, 8, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Ilesanmi, O.O. Pathological basis of symptoms and crises in sickle cell disorder: Implications for counseling and psychotherapy. Hematol. Rep. 2010, 2, e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, M.; Doshi, P.J.; Dhavale, D.D.; Doshi, J.B.; Kate, S.L.; Kulkarni, G.; Ssharma, N.; Uppuladinne, M.; Sonavane, U.; Joshi, R.; et al. Potential of Isoquercitrin as Antisickling Agent: A Multi -Spectroscopic, Thermophoresis and Molecular Modeling Approach. J. Biomol. Struct. Dyn. 2019, 38, 1–27. [Google Scholar] [CrossRef]
- Oder, E.; Safo, M.K.; Abdulmalik, O.; Kato, G.J. New developments in anti-sickling agents: Can drugs directly prevent the polymerization of sickle haemoglobin in vivo? Br. J. Haematol. 2016, 175, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Křen, V.; Řezanka, T. Sweet antibiotics—The role of glycosidic residues in antibiotic and antitumor activity and their randomization. FEMS Microbiol. Rev. 2008, 32, 858–889. [Google Scholar] [CrossRef] [Green Version]
- Janisch, K.M.; Chen, Y.-C.; Hsu, F.-L.; Lee, W.-R. Properties of quercetin conjugates: Modulation of LDL oxidation and binding to human serum albumin. Free Radic. Res. 2004, 38, 877–884. [Google Scholar] [CrossRef]
- Tranchimand, S.; Brouant, P.; Iacazio, G. The rutin catabolic pathway with special emphasis on quercetinase. Biodegradation 2010, 21, 833–859. [Google Scholar] [CrossRef]
- Bentz, A.B. A Review of Quercetin: Chemistry, Antioxident Properties, and Bioavailability. J. Young Investig. 2017. Available online: https://www.semanticscholar.org/paper/A-Review-of-Quercetin%3A-Chemistry%2C-Antioxident-and-Bentz/f2c1a4d4bf6498bd098942b6171a9283966f1a6c (accessed on 9 April 2022).
- Muhammad, A.; Waziri, A.D.; Forcados, G.E.; Sanusi, B.; Sani, H.; Malami, I.; Abubakar, I.B.; Oluwatoyin, H.Y.; Adinoyi, O.A.; Mohammed, H.A. Sickling-preventive effects of rutin is associated with modulation of deoxygenated haemoglobin, 2,3-bisphosphoglycerate mutase, redox status and alteration of functional chemistry in sickle erythrocytes. Heliyon 2019, 5, e01905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, F.B.; Davis, P.J.; Blas, S.D.; Gombas, D.Z. Inositol phosphates modulate human red blood cell Ca(2+)-adenosine triphosphatase activity in vitro by a guanine nucleotide regulatory protein. Metabolism 1995, 44, 865–868. [Google Scholar] [CrossRef]
- Berrocal, M.; Corbacho, I.; Sepulveda, M.R.; Gutierrez-Merino, C.; Mata, A.M. Phospholipids and calmodulin modulate the inhibition of PMCA activity by tau. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Reue, K. Lipin proteins and glycerolipid metabolism: Roles at the ER membrane and beyond. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhou, Y. Crucial role of the pentose phosphate pathway in malignant tumors. Oncol. Lett. 2019, 17, 4213–4221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christodoulou, D.; Kuehne, A.; Estermann, A.; Fuhrer, T.; Lang, P.F.; Sauer, U. Reserve Flux Capacity in the Pentose Phosphate Pathway by NADPH Binding Is Conserved across Kingdoms. iScience 2019, 19, 1133–1144. [Google Scholar] [CrossRef] [Green Version]
- Chirico, E.N.; Pialoux, V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life 2012, 64, 72–80. [Google Scholar] [CrossRef]
- Hundekar, P.; Suryakar, A.; Karnik, A.; Ghone, R.; Vasaikar, M. Antioxidant Status and Lipid Peroxidation in Sickle Cell Anaemia. Biomed. Res. 2010, 21, 461–464. [Google Scholar]
- Rusanova, I.; Escames, G.; Cossio, G.; De Borace, R.G.; Moreno, B.; Chahboune, M.; López, L.C.; Díez, T.; Acuña-Castroviejo, A. Oxidative stress status, clinical outcome, and β-globin gene cluster haplotypes in pediatric patients with sickle cell disease. Eur. J. Haematol. 2010, 85, 529–537. [Google Scholar] [CrossRef]
- Dasgupta, T.; Fabry, M.E.; Kaul, D.K. Antisickling property of fetal hemoglobin enhances nitric oxide bioavailability and ameliorates organ oxidative stress in transgenic-knockout sickle mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R394–R402. [Google Scholar] [CrossRef] [Green Version]
- Frewin, R. Biochemical Aspects of Anaemia. Clinical Biochemistry: Metabolic and Clinical Aspects; Marshall, W.J., Ed.; Churchill Livingstone: London, UK, 2014. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeniyi, O.; Baptista, R.; Bhowmick, S.; Cookson, A.; Nash, R.J.; Winters, A.; Shen, J.; Mur, L.A.J. Isolation and Characterisation of Quercitrin as a Potent Anti-Sickle Cell Anaemia Agent from Alchornea cordifolia. J. Clin. Med. 2022, 11, 2177. https://doi.org/10.3390/jcm11082177
Adeniyi O, Baptista R, Bhowmick S, Cookson A, Nash RJ, Winters A, Shen J, Mur LAJ. Isolation and Characterisation of Quercitrin as a Potent Anti-Sickle Cell Anaemia Agent from Alchornea cordifolia. Journal of Clinical Medicine. 2022; 11(8):2177. https://doi.org/10.3390/jcm11082177
Chicago/Turabian StyleAdeniyi, Olayemi, Rafael Baptista, Sumana Bhowmick, Alan Cookson, Robert J. Nash, Ana Winters, Jianying Shen, and Luis A. J. Mur. 2022. "Isolation and Characterisation of Quercitrin as a Potent Anti-Sickle Cell Anaemia Agent from Alchornea cordifolia" Journal of Clinical Medicine 11, no. 8: 2177. https://doi.org/10.3390/jcm11082177
APA StyleAdeniyi, O., Baptista, R., Bhowmick, S., Cookson, A., Nash, R. J., Winters, A., Shen, J., & Mur, L. A. J. (2022). Isolation and Characterisation of Quercitrin as a Potent Anti-Sickle Cell Anaemia Agent from Alchornea cordifolia. Journal of Clinical Medicine, 11(8), 2177. https://doi.org/10.3390/jcm11082177






