High Heterogeneity of Temporal Bone CT Aspects in Osteogenesis Imperfecta Is Not Linked to Hearing Loss
Abstract
:1. Introduction
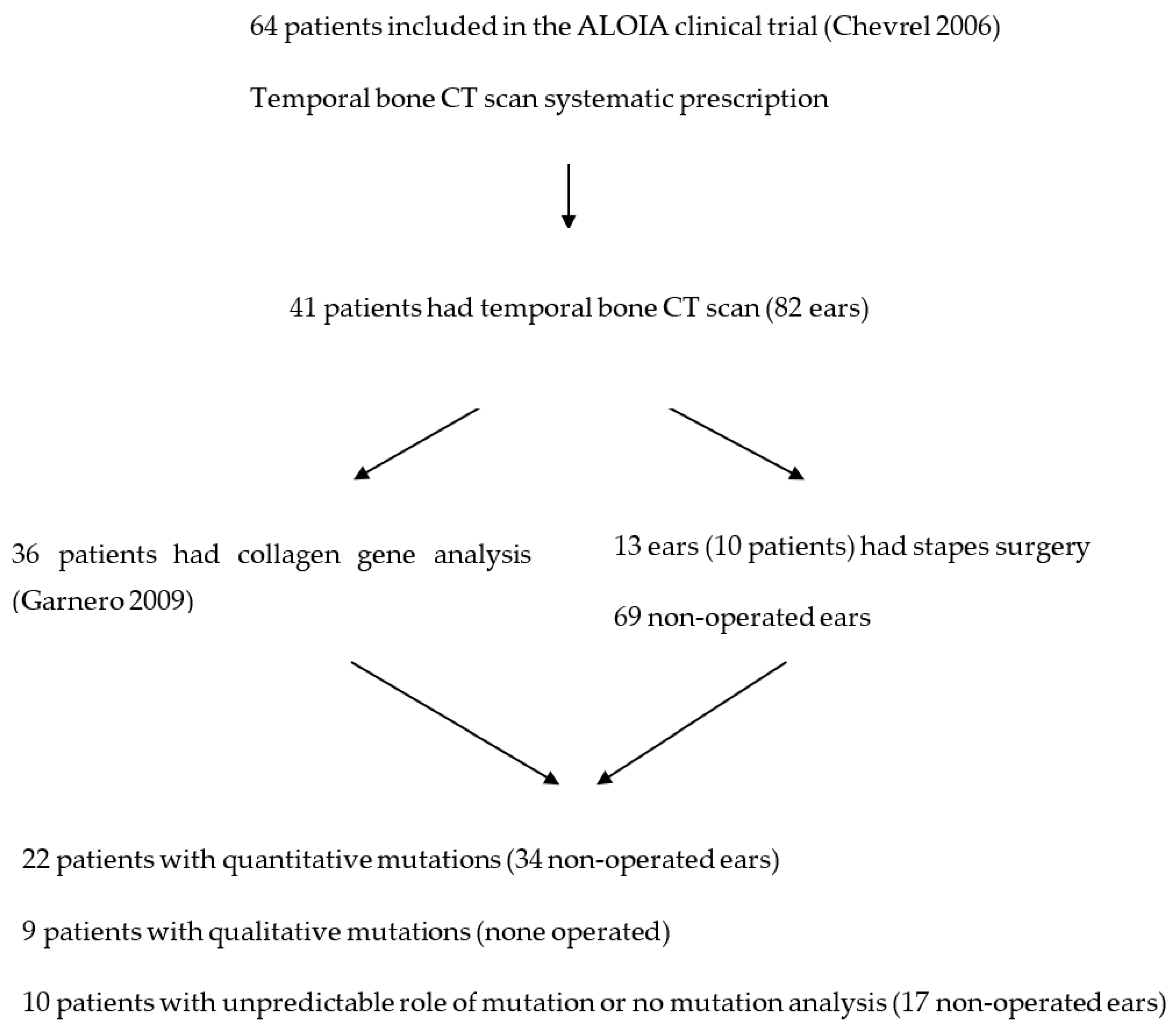
2. Material and Methods
2.1. Type of Study
2.2. Subjects
- A typical personal history of bone fragility fractures (fractures in the absence of a car crash or major trauma) before 20 years of age, with at least three fragility fractures.
- At least one criterion in the following list: blue sclerae, scoliosis (the Cobb method with an angle ≥10° but ≤40°), hearing loss (defined by elevated pure-tone thresholds above 20 dB on at least two octave frequencies or a mean air-bone gap above 10 dB), abnormal laxity of ligaments defined by at least three criteria of Carter and Wilkinson, dentinogenesis imperfecta defined by at least a yellow to brown tooth, and at least one family member with OI. [12]
- A low BMD of the lumbar spine or hip (total femur) measured by dual X-ray densitometry (DXA) with a T score of −2.5 or below at one site.
2.3. Methods
2.3.1. Audiometric Evaluation
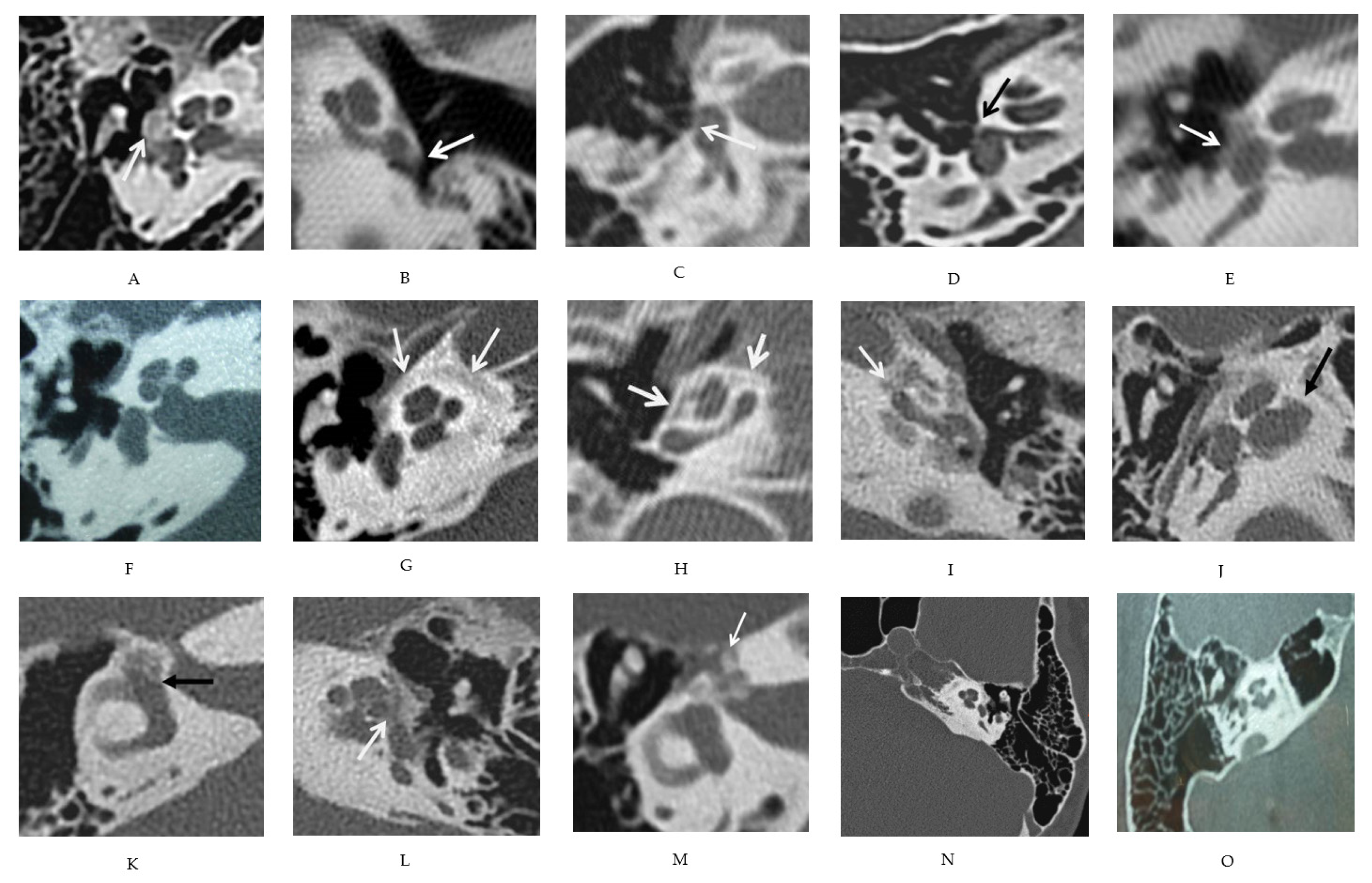
2.3.2. Radiologic Evaluation
2.3.3. Mutations
2.3.4. Bone Mineral Density
2.3.5. Statistical Analysis
3. Results
3.1. Description of Various CT Features
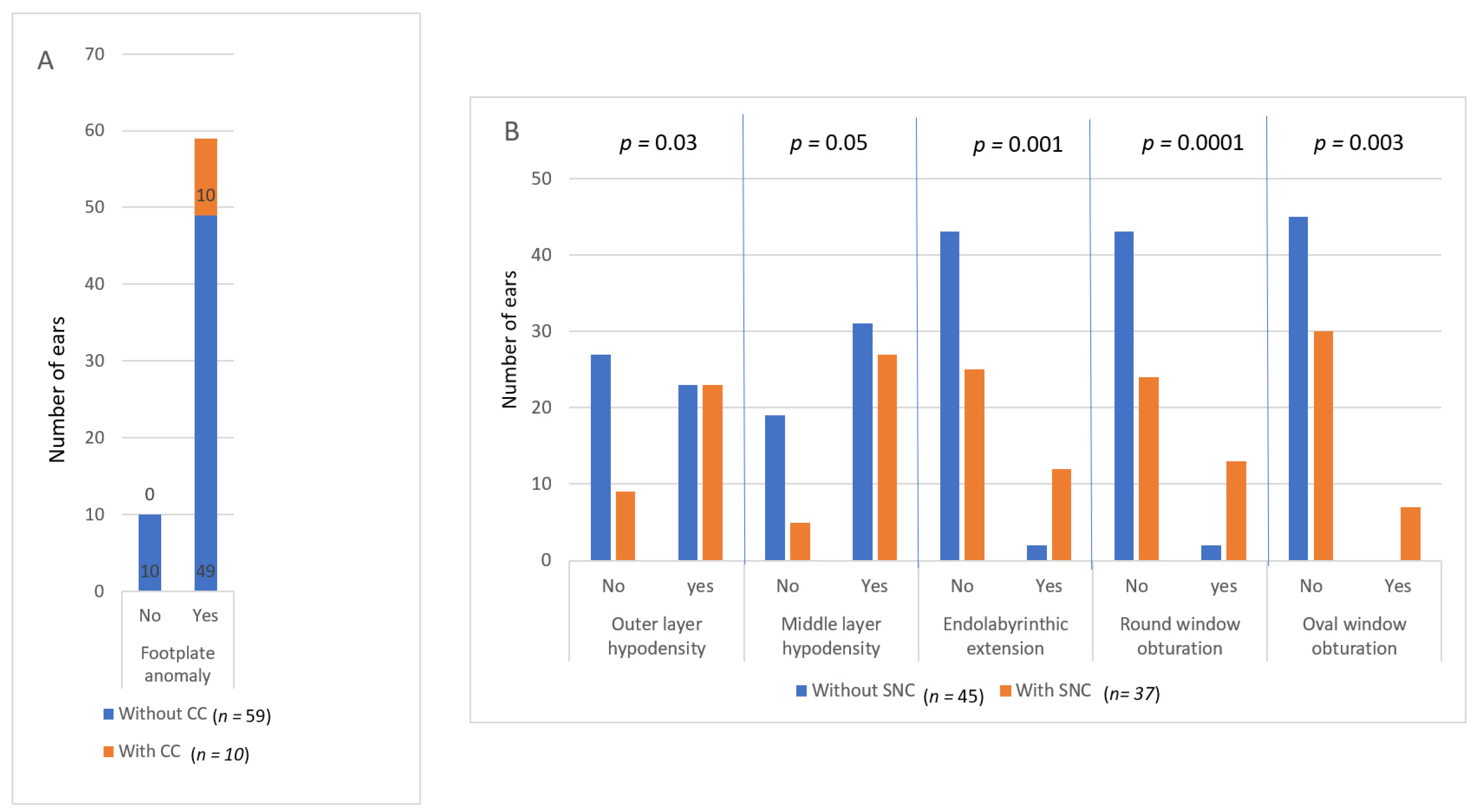
3.2. Comparison between CT and Hearing
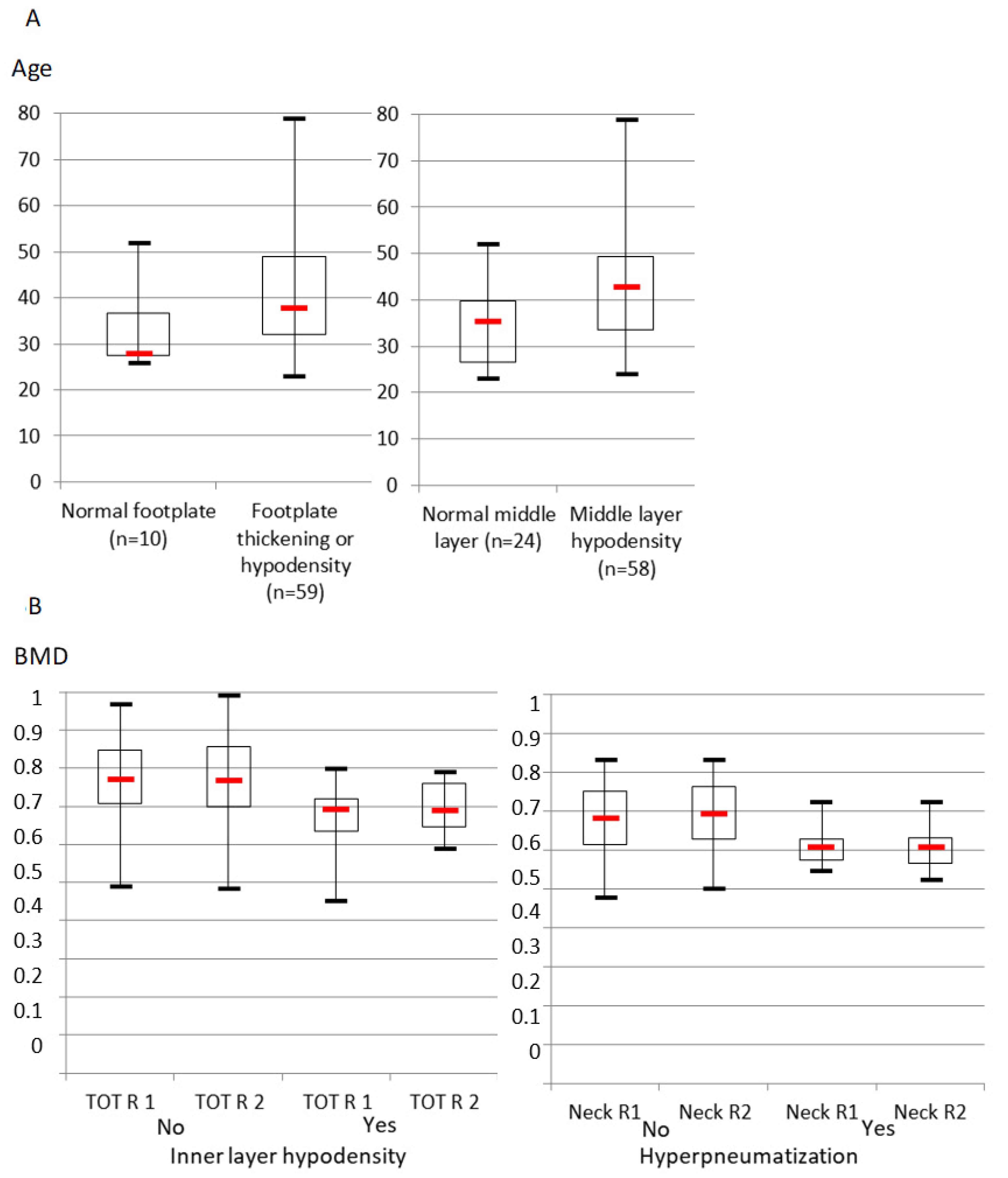
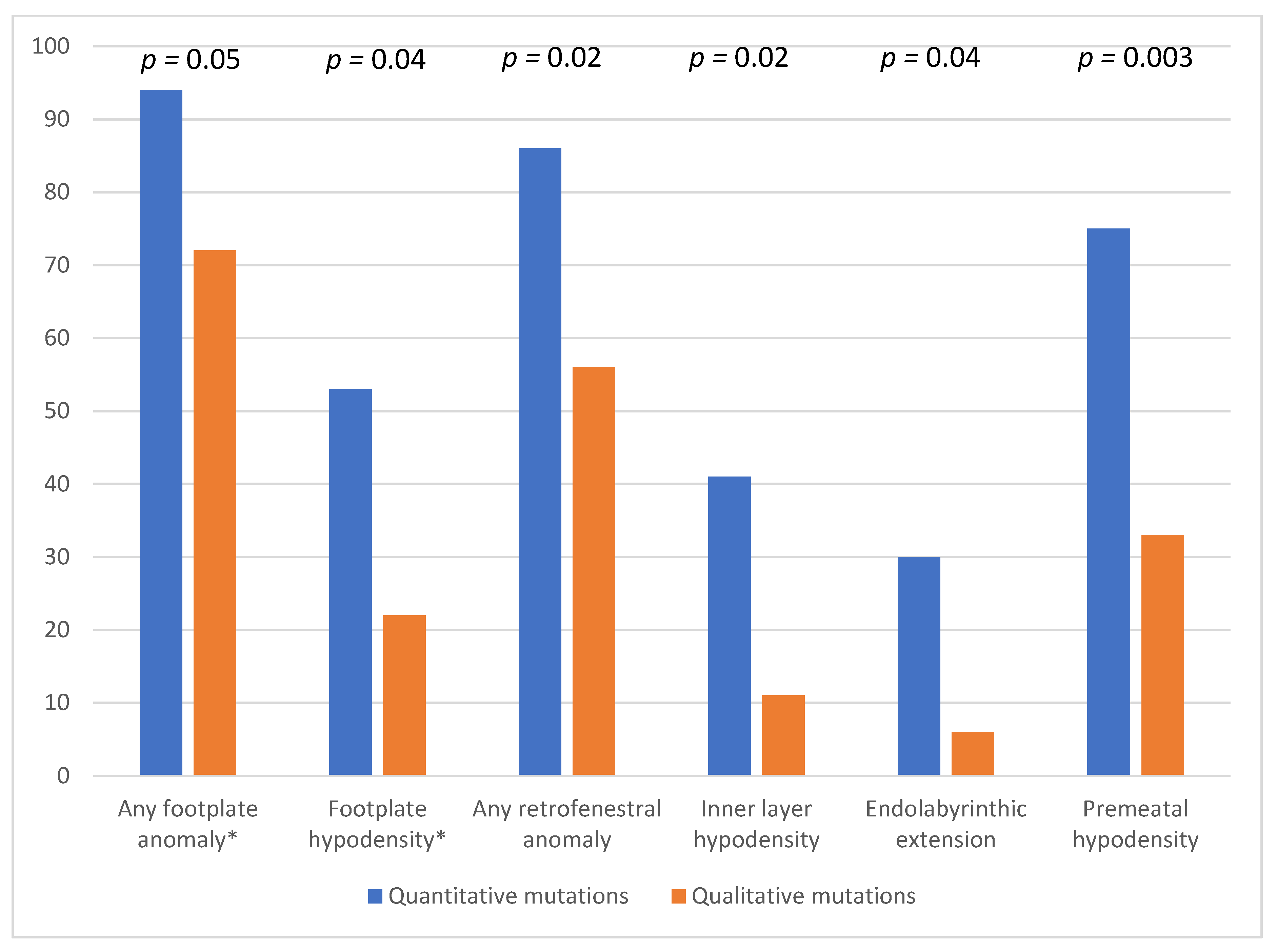
3.3. Comparison between CT and Age, Bone Mineral Density and Gene Mutations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMD | bone mineral density |
| CC | conductive component of hearing impairment |
| CT | computed tomography |
| HI | hearing impairment |
| ENT | ear, nose, throat |
| FP | stapes footplate |
| MHI | mixed hearing impairment |
| NH | normally hearing |
| OI | osteogenesis imperfecta |
| OS | otosclerosis |
| SNC | sensorineural component of hearing impairment |
| TB | temporal bone |
References
- Van Dijk, F.S.; Sillence, D.O. Osteogenesis Imperfecta: Clinical Diagnosis, Nomenclature and Severity Assessment. Am. J. Med. Genet. A 2014, 164A, 1470–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forlino, A.; Marini, J.C. Osteogenesis Imperfecta. Lancet 2016, 387, 1657–1671. [Google Scholar] [CrossRef]
- Pedersen, U. Hearing Loss in Patients with Osteogenesis Imperfecta. A Clinical and Audiological Study of 201 Patients. Scand. Audiol. 1984, 13, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.; Dhooge, I.J.M.; Swinnen, F.K.R. Longitudinal Analysis of the Audiological Phenotype in Osteogenesis Imperfecta: A Follow-up Study. J. Laryngol. Otol. 2018, 132, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Carré, F.; Achard, S.; Rouillon, I.; Parodi, M.; Loundon, N. Hearing Impairment and Osteogenesis Imperfecta: Literature Review. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2019, 136, 379–383. [Google Scholar] [CrossRef]
- Sando, I.; Myers, D.; Harada, T.; Hinojosa, R.; Myers, E.N. Osteogenesis Imperfecta Tarda and Otosclerosis. A Temporal Bone Histopathology Report. Ann. Otol. Rhinol. Laryngol. 1981, 90, 199–203. [Google Scholar] [CrossRef]
- Nager, G.T. Osteogenesis Imperfecta of the Temporal Bone and Its Relation to Otosclerosis. Ann. Otol. Rhinol. Laryngol. 1988, 97, 585–593. [Google Scholar] [CrossRef]
- Veillon, F. Otospongiose Juvénile et Ostéogenèse Imparfaite. In Imagerie de L’oreille et de l’os Temporal-Pédiatrie; Imagerie de L’oreille et de l’os Temporal; Éditions Lavoisier: Paris, France, 2014; Volume 5, pp. 1342–1348. [Google Scholar]
- Vincent, R.; Wegner, I.; Stegeman, I.; Grolman, W. Stapedotomy in Osteogenesis Imperfecta: A Prospective Study of 32 Consecutive Cases. Otol. Neurotol. 2014, 35, 1785–1789. [Google Scholar] [CrossRef]
- Swinnen, F.K.R.; Casselman, J.W.; De Leenheer, E.M.R.; Cremers, C.W.R.J.; Dhooge, I.J.M. Temporal Bone Imaging in Osteogenesis Imperfecta Patients with Hearing Loss. Laryngoscope 2013, 123, 1988–1995. [Google Scholar] [CrossRef] [Green Version]
- Chevrel, G.; Schott, A.-M.; Fontanges, E.; Charrin, J.E.; Lina-Granade, G.; Duboeuf, F.; Garnero, P.; Arlot, M.; Raynal, C.; Meunier, P.J. Effects of Oral Alendronate on BMD in Adult Patients with Osteogenesis Imperfecta: A 3-Year Randomized Placebo-Controlled Trial. J. Bone Miner. Res. 2006, 21, 300–306. [Google Scholar] [CrossRef]
- Carter, C.O.; Wilkinson, J.A. Persistent Joint Laxity and Congenital Dislocation of the Hip. J. Bone Joint Surg. Br. 1964, 46, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Schott, A.-M.; Prockop, D.; Chevrel, G. Bone Turnover and Type I Collagen C-Telopeptide Isomerization in Adult Osteogenesis Imperfecta: Associations with Collagen Gene Mutations. Bone 2009, 44, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Curhan, S.G.; Stankovic, K.; Halpin, C.; Wang, M.; Eavey, R.D.; Paik, J.M.; Curhan, G.C. Osteoporosis, Bisphosphonate Use, and Risk of Moderate or Worse Hearing Loss in Women. J. Am. Geriatr. Soc. 2021, 69, 3103–3113. [Google Scholar] [CrossRef] [PubMed]
- Veillon, F.; Stierle, J.L.; Dussaix, J.; Ramos-Taboada, L.; Riehm, S. Otosclerosis imaging: Matching clinical and imaging data. J. Radiol. 2006, 87, 1756–1764. [Google Scholar] [CrossRef]
- Alkadhi, H.; Rissmann, D.; Kollias, S.S. Osteogenesis Imperfecta of the Temporal Bone: CT and MR Imaging in Van Der Hoeve-de Kleyn Syndrome. AJNR Am. J. Neuroradiol. 2004, 25, 1106–1109. [Google Scholar]
- Swinnen, F.K.R.; De Leenheer, E.M.R.; Coucke, P.J.; Cremers, C.W.R.J.; Dhooge, I.J.M. Audiometric, Surgical, and Genetic Findings in 15 Ears of Patients with Osteogenesis Imperfecta. Laryngoscope 2009, 119, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Berger, G.; Hawke, M.; Johnson, A.; Proops, D. Histopathology of the Temporal Bone in Osteogenesis Imperfecta Congenita: A Report of 5 Cases. Laryngoscope 1985, 95, 193–199. [Google Scholar] [CrossRef]
- Santos, F.; McCall, A.A.; Chien, W.; Merchant, S. Otopathology in Osteogenesis Imperfecta. Otol. Neurotol. 2012, 33, 1562–1566. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, J.R.; Pikus, A.; Weiss, G.; Rowe, D.W. Hearing and Middle Ear Function in Osteogenesis Imperfecta. JAMA 1982, 247, 2120–2126. [Google Scholar] [CrossRef]
- Paterson, C.R.; Monk, E.A.; McAllion, S.J. How Common Is Hearing Impairment in Osteogenesis Imperfecta? J. Laryngol. Otol. 2001, 115, 280–282. [Google Scholar] [CrossRef]
- Hartikka, H.; Kuurila, K.; Körkkö, J.; Kaitila, I.; Grénman, R.; Pynnönen, S.; Hyland, J.C.; Ala-Kokko, L. Lack of Correlation between the Type of COL1A1 or COL1A2 Mutation and Hearing Loss in Osteogenesis Imperfecta Patients. Hum. Mutat. 2004, 24, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, F.K.R.; Coucke, P.J.; De Paepe, A.M.; Symoens, S.; Malfait, F.; Gentile, F.V.; Sangiorgi, L.; D’Eufemia, P.; Celli, M.; Garretsen, T.J.T.M.; et al. Osteogenesis Imperfecta: The Audiological Phenotype Lacks Correlation with the Genotype. Orphanet J. Rare Dis. 2011, 6, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swinnen, F.K.R.; De Leenheer, E.M.R.; Goemaere, S.; Cremers, C.W.R.J.; Coucke, P.J.; Dhooge, I.J.M. Association between Bone Mineral Density and Hearing Loss in Osteogenesis Imperfecta. Laryngoscope 2012, 122, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Rolvien, T.; Schmidt, F.N.; Milovanovic, P.; Jähn, K.; Riedel, C.; Butscheidt, S.; Püschel, K.; Jeschke, A.; Amling, M.; Busse, B. Early Bone Tissue Aging in Human Auditory Ossicles Is Accompanied by Excessive Hypermineralization, Osteocyte Death and Micropetrosis. Sci. Rep. 2018, 8, 1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duboeuf, F.; Burt-Pichat, B.; Farlay, D.; Suy, P.; Truy, E.; Boivin, G. Bone Quality and Biomechanical Function: A Lesson from Human Ossicles. Bone 2015, 73, 105–110. [Google Scholar] [CrossRef]
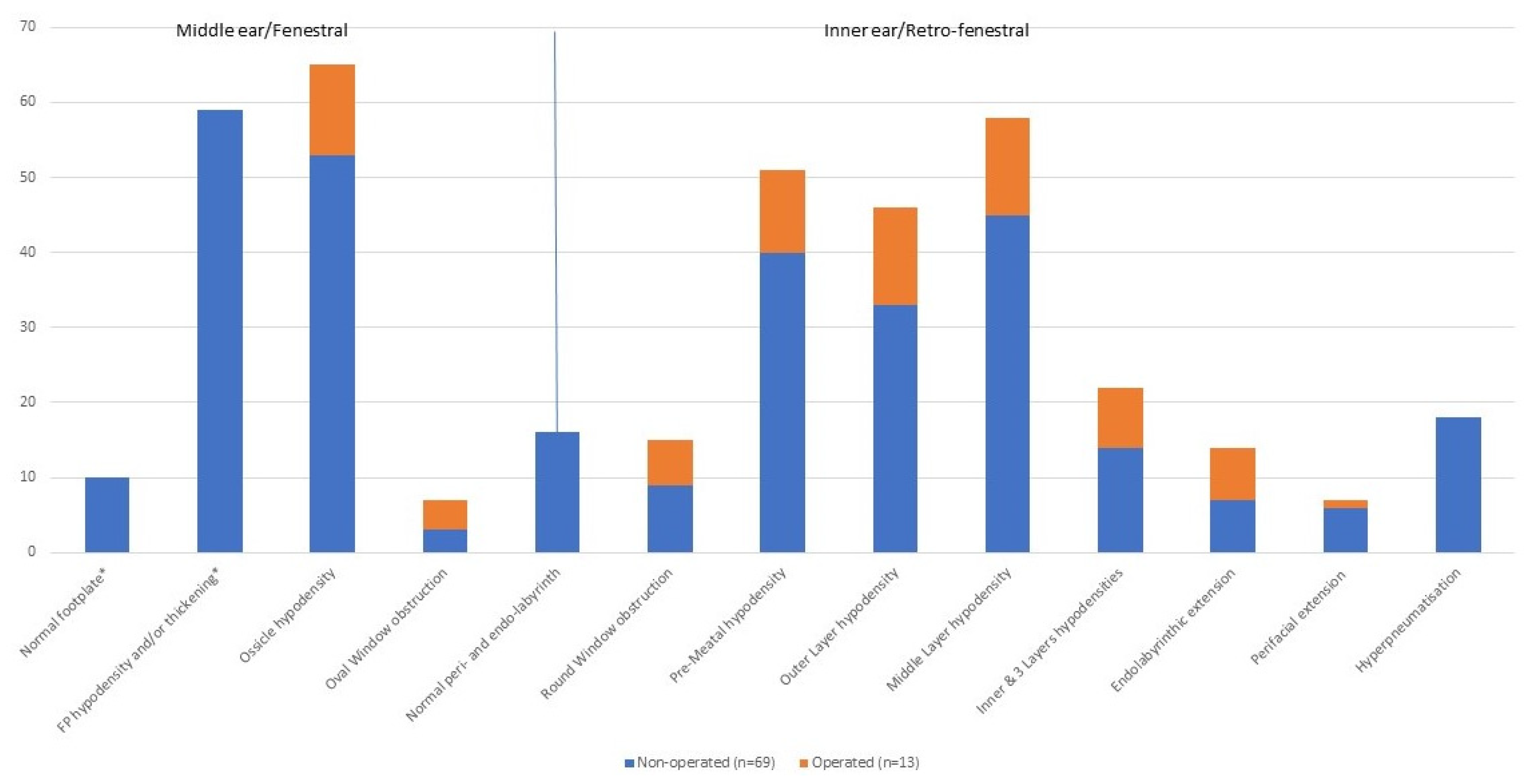
| Normal Footplate | Footplate Hypodensity or Thickening | Ossicle Hypodensity | Oval Window Obstruction | Round Window Obstruction | Pre-Meatal Hypodensity | Outer Layer Hypodensity | Middle Layer Hypodensity | Inner & 3 Layers Hypodensities | Endolabyrinthic Extension | Perifacial Extension | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ossicle hypodensity | 9 | 44 | ||||||||||
| Oval Window obstruction | 3 | 0 | ||||||||||
| Normal otic capsule | 4 | 12 | 14 | 0 | ||||||||
| Round Window obstruction | 0 | 9 | 13 | 5 | ||||||||
| Pre-Meatal hypodensity | 0 | 36 | 38 | 7 | 15 | |||||||
| Outer Layer hypodensity | 2 | 31 | 34 | 7 | 15 | 35 | ||||||
| Middle Layer hypodensity | 4 | 41 | 43 | 7 | 15 | 43 | 46 | |||||
| Inner & 3 Layers hypodensities | 1 | 13 | 18 | 6 | 9 | 17 | 22 | 22 | 22 | |||
| Endolabyrinthic extension | 0 | 7 | 11 | 5 | 9 | 12 | 14 | 14 | 14 | 14 | ||
| Perifacial extension | 0 | 6 | 5 | 2 | 4 | 7 | 6 | 7 | 4 | 2 | ||
| Hyperpneumatisation | 3 | 15 | 14 | 0 | 4 | 14 | 12 | 14 | 0 | 0 | 0 | 18 |
| Total non operated | 10 | 59 | 53 | 3 | 69 | |||||||
| Total | 65 | 7 | 15 | 51 | 46 | 58 | 22 | 14 | 7 | 82 |
| Number of Ears | Normal Hearing | Conductive Component | Sensorineural Component | Both Conductive & Sensorineural Components | |
|---|---|---|---|---|---|
| Total sample | 82 | 41 | 11 | 37 | 7 |
| Operated | 13 | 1 | 1 | 12 | 1 |
| Non-operated | 69 | 40 | 10 | 25 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ltaief-Boudrigua, A.; Lina-Granade, G.; Truy, E.; Hermann, R.; Chevrel, G. High Heterogeneity of Temporal Bone CT Aspects in Osteogenesis Imperfecta Is Not Linked to Hearing Loss. J. Clin. Med. 2022, 11, 2171. https://doi.org/10.3390/jcm11082171
Ltaief-Boudrigua A, Lina-Granade G, Truy E, Hermann R, Chevrel G. High Heterogeneity of Temporal Bone CT Aspects in Osteogenesis Imperfecta Is Not Linked to Hearing Loss. Journal of Clinical Medicine. 2022; 11(8):2171. https://doi.org/10.3390/jcm11082171
Chicago/Turabian StyleLtaief-Boudrigua, Aïcha, Genevieve Lina-Granade, Eric Truy, Ruben Hermann, and Guillaume Chevrel. 2022. "High Heterogeneity of Temporal Bone CT Aspects in Osteogenesis Imperfecta Is Not Linked to Hearing Loss" Journal of Clinical Medicine 11, no. 8: 2171. https://doi.org/10.3390/jcm11082171
APA StyleLtaief-Boudrigua, A., Lina-Granade, G., Truy, E., Hermann, R., & Chevrel, G. (2022). High Heterogeneity of Temporal Bone CT Aspects in Osteogenesis Imperfecta Is Not Linked to Hearing Loss. Journal of Clinical Medicine, 11(8), 2171. https://doi.org/10.3390/jcm11082171






