Subthreshold Exudative Choroidal Neovascularization (CNV): Presentation of This Uncommon Subtype and Other CNVs in Age-Related Macular Degeneration (AMD)
Abstract
1. Introduction
2. Exudative AMD
3. Type 1 MNV
4. Type 2 MNV
5. Type 3 MNV
6. Atypical Types of CNV (Identified by OCT-A)
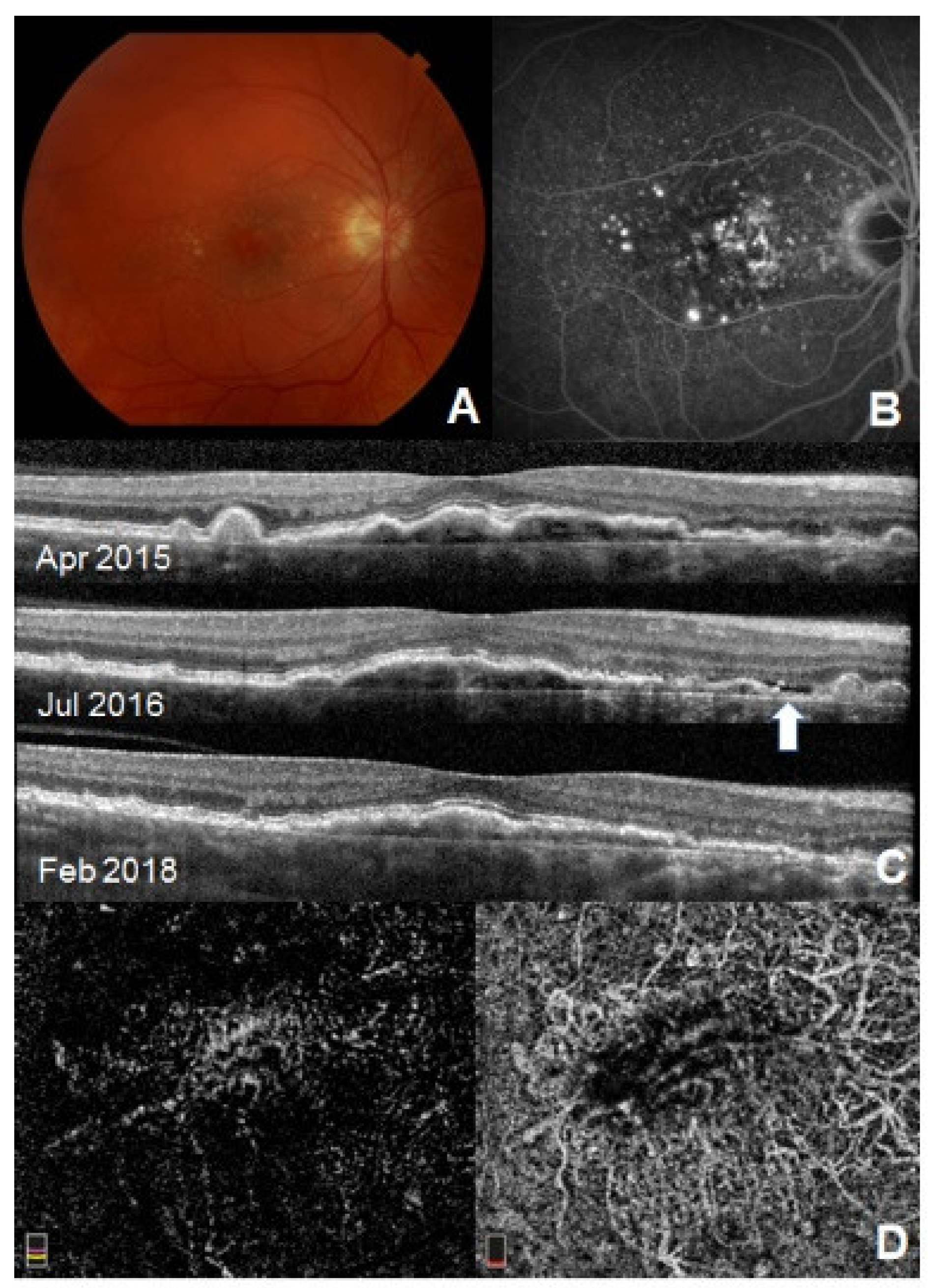
Subthreshold Exudative CNV
7. Nonexudative CNV (CNV in Dry AMD, Subclinical CNV, Quiescent CNV)
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-Related Macular Degeneration. N. Engl. J. Med. 2008, 358, 2606. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Friedman, D.S.; O’Colmain, B.J.; Muñoz, B.; Tomany, S.C.; McCarty, C.; DeJong, P.T.V.M.; Nemesure, B.; Mitchell, P.; Kempen, J.; Congdon, N. Prevalence of Age-Related Macular Degeneration in the United States. Arch. Ophthalmol. 2004, 137, 486–495. [Google Scholar] [CrossRef]
- Davis, M.D.; Gangnon, R.E.; Lee, L.Y.; Hubbard, L.D.; Klein, B.E.K.; Klein, R.; Ferris, F.L.; Bressler, S.B.; Milton, R.C. The Age-Related Eye Disease Study Severity Scale for Age-Related Macular Degeneration: AREDS Report No. 17. Arch. Ophthalmol. (Chicago Ill. 1960) 2005, 123, 1484–1498. [Google Scholar] [CrossRef]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef]
- Casalino, G.; Scialdone, A.; Bandello, F.; Chakravarthy, U. Hyperreflective Material as a Biomarker in Neovascular Age-Related Macular Degeneration. Expert Rev. Ophthalmol. 2020, 15, 83–91. [Google Scholar] [CrossRef]
- Green, W.R.; Enger, C. Age-Related Macular Degeneration Histopathologic Studies: The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology 1993, 114, 187–193. [Google Scholar] [CrossRef]
- Grossniklaus, H.E.; Green, W.R. Choroidal Neovascularization. Am. J. Ophthalmol. 2004, 137, 496–503. [Google Scholar] [CrossRef]
- Bacci, T.; Essilfie, J.O.; Leong, B.C.S.; Freund, K.B. Exudative Non-Neovascular Age-Related Macular Degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 259, 1123–1134. [Google Scholar] [CrossRef]
- Novotny, H.R.; Alvis, D.L. A Method of Photographing Fluorescence in Circulating Blood in the Human Retina. Circulation 1961, 24, 82–86. [Google Scholar] [CrossRef]
- Yannuzzi, L.A.; Slakter, J.S.; Sorenson, J.A.; Guyer, D.R.; Orlock, D.A. Digital Indocyanine Green Videoangiography and Choroidal Neovascularization. 1992. Retina 2012, 32, 191. [Google Scholar] [CrossRef] [PubMed]
- De Carlo, T.E.; Romano, A.; Waheed, N.K.; Duker, J.S. A Review of Optical Coherence Tomography Angiography (OCTA). Int. J. Retin. Vitr. 2015, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Francis, P.J.; George, S.; Schultz, D.W.; Rosner, B.; Klein, M.L. Association of CFH Y402H and LOC387715 A69S with Progression of Age-Related Macular Degeneration. J. Am. Med. Assoc. 2007, 297, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.W.M.; Adamis, A.P. Targeting Angiogenesis, the Underlying Disorder in Neovascular Age-Related Macular Degeneration. Can. J. Ophthalmol. 2005, 40, 352–368. [Google Scholar] [CrossRef]
- Study, P. Laser Photocoagulation of Subfoveal Recurrent Neovascular Lesions in Age-Related Macular Degeneration. Results of a Randomized Clinical Trial. Macular Photocoagulation Study Group. Arch. Ophthalmol. 1991, 109, 1220–1231. [Google Scholar]
- Gass, J.D.M. Biomicroscopic and Histopathologic Considerations Regarding the Feasibility of Surgical Excision of Subfoveal Neovascular Membranes. Am. J. Ophthalmol. 1994, 118, 285–298. [Google Scholar] [CrossRef]
- Lafaut, B.A.; Bartz-Schmidt, K.U.; Broecke, V.; Aisenbrey, S.; de Laey, J.J.; Heimann, K. Clinicopathological Correlation in Exudative Age Related Macular Degeneration: Histological DiVerentiation between Classic and Occult Choroidal Neovascularisation. Br. J. Ophthalmol. 2000, 84, 239–243. [Google Scholar] [CrossRef]
- Freund, K.B.; Yannuzzi, L.A.; Sorenson, J.A. Age-Related Macular Degeneration and Choroidal Neovascularization. Am. J. Ophthalmol. 1993, 115, 786–791. [Google Scholar] [CrossRef]
- Haddad, W.M.; Coscas, G.; Soubrane, G. Eligibility for Treatment and Angiographic Features at the Early Stage of Exudative Age Related Macular Degeneration. Br. J. Ophthalmol. 2002, 86, 663–669. [Google Scholar] [CrossRef]
- Jung, J.J.; Chen, C.Y.; Mrejen, S.; Gallego-Pinazo, R.; Xu, L.; Marsiglia, M.; Boddu, S.; Freund, K.B. The Incidence of Neovascular Subtypes in Newly Diagnosed Neovascular Age-Related Macular Degeneration. Am. J. Ophthalmol. 2014, 158, 769–779. [Google Scholar] [CrossRef]
- Invernizzi, A.; Nguyen, V.; Teo, K.; Barthelmes, D.; Fung, A.; Vincent, A.; Gillies, M. Five-Year Real-World Outcomes of Occult and Classic Choroidal Neovascularization: Data From the Fight Retinal Blindness! Project. Am. J. Ophthalmol. 2019, 204, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Freund, K.B.; Zweifel, S.A.; Engelbert, M. Edirorial: Do We Need a New Classification for Choroidal Neovascularization in Age-Related Macular Degeneration? Retina 2010, 30, 1333–1349. [Google Scholar] [CrossRef] [PubMed]
- Pece, A.; Bolognesi, G.; Introini, U.; Pacelli, G.; Calori, G.; Brancato, R. Indocyanine Green Angiography of Well-Defined Plaque Choroidal Neovascularization in Age-Related Macular Degeneration. Arch. Ophthalmol. 2000, 118, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Kriechbaum, K.; Oldag, A. Three-Dimensional Angiography of Classic and Occult Lesion Types in Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1751–1760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuehlewein, L.; Bansal, M.; Lenis, T.L.; Iafe, N.A.; Sadda, S.R.; Bonini Filho, M.A.; de Carlo, T.E.; Waheed, N.K.; Duker, J.S.; Sarraf, D. Optical Coherence Tomography Angiography of Type 1 Neovascularization in Age-Related Macular Degeneration. Am. J. Ophthalmol. 2015, 160, 739–748.e2. [Google Scholar] [CrossRef] [PubMed]
- Veritti, D.; Sarao, V.; Gorni, G.; Lanzetta, P. Anti-VEGF Drugs Dynamics: Relevance for Clinical Practice. Pharmaceutics 2022, 14, 265. [Google Scholar] [CrossRef] [PubMed]
- Horner, F.; Lip, P.L.; Mohammed, B.R.; Fusi-Rubiano, W.; Gokhale, E.; Mushtaq, B.; Chavan, R. Comparing Effectiveness of Three Different Anti-VEGF Treatment Regimens for Neovascular Age-Related Macular Degeneration: Two Years&rsquo Real-World Clinical Outcomes. Clin. Ophthalmol. 2021, 15, 1703–1713. [Google Scholar] [CrossRef]
- Naysan, J.; Jung, J.J.; Dansingani, K.K.; Balaratnasingam, C.; Freund, K.B. Type 2 (Subretinal) Neovascularization in Age related Macular Degeneration Associated with Pure Reticular Pseudodrusen Phenotype. Retina 2016, 36, 449–457. [Google Scholar] [CrossRef]
- Le, H.M.; Mimoun, G.; Cohen, S.Y.; Jung, C.; Semoun, O.; Souied, E.H. Progression from Type 2 Macular Neovascularization to Fibrovascular Pigment Epithelial Detachment. Vision 2021, 5, 16. [Google Scholar] [CrossRef]
- Yannuzzi, L.A.; Negrão, S.; Iida, T.; Carvalho, C.; Rodriguez-Coleman, H.; Slakter, J.; Freund, K.B.; Sorenson, J.; Orlock, D.; Borodoker, N. Retinal Angiomatous Proliferation in Age-Related Macular Degeneration. Retina 2001, 21, 416–434. [Google Scholar] [CrossRef]
- Yannuzzi, L.A.; Freund, K.B.; Takahashi, B.S. Review of Retinal Angiomatous Proliferation or Type 3 Neovascularization. Retina 2008, 28, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Lafaut, B.A.; Aisenbrey, S.; vanden Broecke, C.; Bartz-Schmidt, K.U. Clinicopathological Correlation of Deep Retinal Vascular Anomalous Complex in Age Related Macular Degeneration. Br. J. Ophthalmol. 2000, 84, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Kawamura, A.; Mori, R.; Yuzawa, M. Clinicopathological Findings of Retinal Angiomatous Proliferation. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2007, 245, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Cheung, C.M.G.; Arias-Barquet, L.; Ozdek, S.; Parachuri, N.; Kumar, N.; Hilely, A.; Zur, D.; Loewenstein, A.; Vella, G.; et al. Fluid-based visual prognostication in type 3 macular neovascularization-flip-3 study. Retina 2022, 42, 107–113. [Google Scholar] [CrossRef]
- Roh, M.; Miller, J.W.; Jeng-Miller, K.W.; Wang, J.C.; Laíns, I.; Silverman, R.F.; Loewenstein, J.I.; Husain, D.; Vavvas, D.G.; Miller, J.B. Subthreshold Exudative Choroidal Neovascularization Associated With Age-Related Macular Degeneration Identified by Optical Coherence Tomography Angiography. J. Vitr. Dis. 2020, 4, 377–385. [Google Scholar] [CrossRef]
- Uchida, A.; Srivastava, S.K.; Manjunath, D.; Singh, R.P.; Rachitskaya, A.V.; Kaiser, P.K.; Reese, J.L.; Ehlers, J.P. Impact of Drusen Burden on Incidence of Subclinical CNV with OCTA. Ophthalmic Surg. Lasers Imaging Retin. 2020, 51, 22–30. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Motulsky, E.H.; Thulliez, M.; Shi, Y.; Lyu, C.; de Sisternes, L.; Durbin, M.K.; Feuer, W.; Wang, R.K.; et al. Two-Year Risk of Exudation in Eyes with Nonexudative Age-Related Macular Degeneration and Subclinical Neovascularization Detected with Swept Source Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2019, 208, 1. [Google Scholar] [CrossRef]
- Querques, G.; Srour, M.; Massamba, N.; Georges, A.; Ben Moussa, N.; Rafaeli, O.; Souied, E.H. Functional Characterization and Multimodal Imaging of Treatment-Naive “Quiescent” Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6886–6892. [Google Scholar] [CrossRef]
- Carnevali, A.; Cicinelli, M.V.; Capuano, V.; Corvi, F.; Mazzaferro, A.; Querques, L.; Scorcia, V.; Souied, E.H.; Bandello, F.; Querques, G. Optical Coherence Tomography Angiography: A Useful Tool for Diagnosis of Treatment-Naïve Quiescent Choroidal Neovascularization. Am. J. Ophthalmol. 2016, 169, 189–198. [Google Scholar] [CrossRef]
- Sheth, J.; Anantharaman, G.; Chandra, S.; Sivaprasad, S. “Double-Layer Sign” on Spectral Domain Optical Coherence Tomography in Pachychoroid Spectrum Disease. Indian J. Ophthalmol. 2018, 66, 1796. [Google Scholar] [CrossRef]
- Shi, Y.; Motulsky, E.H.; Goldhardt, R.; Zohar, Y.; Thulliez, M.; Feuer, W.; Gregori, G.; Rosenfeld, P.J. Predictive Value of the OCT Double-Layer Sign for Identifying Subclinical Neovascularization in Age-Related Macular Degeneration. Ophthalmol. Retin. 2019, 3, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Narita, C.; Wu, Z.; Rosenfeld, P.J.; Yang, J.; Lyu, C.; Caruso, E.; McGuinness, M.; Guymer, R.H. Structural OCT Signs Suggestive of Subclinical Nonexudative Macular Neovascularization in Eyes with Large Drusen. Ophthalmology 2020, 127, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.G.; Christakis, P.G.; Chew, E.Y. 10-year follow-up of a subclinical choroidal neovascular membrane in a patient with age-related macular degeneration. Retin. Cases Brief Rep. 2018, 15, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Palejwala, N.V.; Jia, Y.; Gao, S.S.; Liu, L.; Flaxel, C.J.; Hwang, T.S.; Lauer, A.K.; Wilson, D.J.; Huang, D.; Bailey, S.T. Detection of Nonexudative Choroidal Neovascularization in Age-Related Macular Degeneration with Optical Coherence Tomography Angiography. Retina 2015, 35, 2204. [Google Scholar] [CrossRef]
- Kloos, P.; Niederberger, H.; Valmaggia, C. Photodynamic Therapy in Secondary Sick RPE Syndrome after Repeated Intravitreal Injections of VEGF Inhibitors in Patients with Wet Age-Related Macular Degeneration. Klin. Mon. Augenheilkd. 2011, 228, 340–344. [Google Scholar] [CrossRef]

| Types of MNV/CNV | Description | Fundus Photography | FA/ICGA | OCT | OCT-A |
|---|---|---|---|---|---|
| Type 1 (old term: occult CNV) | Neovascular complexes arise from the choriocapillaris into and within the sub-RPE space | Nonspecific signs: drusen, pigment mottling, RPE elevation, hemorrhages, hard exudates | FA: Lack of early hyperfluorescence or poorly defined: stippled hyperfluorescence over an area of elevated RPE within the first 2 min. Staining and/or leakage corresponding to RPE abnormalities in later phases/ICGA: presence of hyperfluorescent area (plaque) | Elevation of RPE by material with heterogeneous reflectivity often with overlying SRF (less common IRF). | New vessels below the level of the RPE |
| Type 2 (old term: classic CNV) | Neovascular complexes arise from the choroid, traverse BM and the RPE monolayer to proliferate in the subretinal space | Grayish subretinal lesion, retinal edema, hard exudates, subretinal and intraretinal hemorrhages | FA: Early hyperfluorescence; late leakage pooling in the subretinal space/ICGA: intense choroidal hyperfluorescence can impede CNV detection. | Disruption of inner/outer segment photoreceptor junction and intraretinal cysts | New vessels above the level of the RPE penetrating the retina |
| Type 3 (RAP) | Neovascular complexes arise either from the retinal circulation or from both circulations (retinal-choroidal anastomosis) and grow toward the outer retina | Focal intraretinal hemorrhages, dilated retinal vessels | FA: Focal and poorly defined hyperfluorescence associated with intraretinal staining in the early and late phase. Cystoid macular edema might be present | Sub-RPE CNV with intraretinal angiomatous change along with subretinal neovascularization and cystic change. Outer retinal disruption. | RAP lesions at the level of the avascular zone. |
| Mixed CNV-Type 1 and Type 2 variant | Varied presentation with findings of both Type 1 and Type 2 CNV | Nonspecific signs: drusen, pigment mottling, RPE elevation, hemorrhages, hard exudates | Findings of both Type 1 and Type 2 CNV | Findings of both Type 1 and Type 2 CNV | Findings of both Type 1 and Type 2 CNV |
| Polypoidal choroidal vasculopathy (Type 1 variant) | Branching vascular network and nodular vascular agglomerations (“polyps”) | RPE elevation, exudation, | FA: Stippled hyperfluorescence over an area of elevated RPE on late phase FA/ICGA: branching vascular network and aneurysmal dilations located at the outer edge of the lesion | “Polyps” located below RPE. Findings similar to type 1 CNV | Well-delineated branching vascular network. Aneurysmal dilations may not be seen (flow detection threshold not reached). |
| Quiescent CNV | Typical Type 1 CNV lacking exudation on repeated OCT for at least 6 months | Similar to type 1 CNV | FA: Late speckled hyperfluorescent lesions lacking well-demarcated borders. No late-phase leakage of undetermined source or pooling of dye in the subretinal space/In mid-late ICGA frames: visualization of hyperfluorescent “quiescent” CNV and plaques | Irregularly slightly elevated RPE without hyporeflective fluid accumulation in the intraretinal/subretinal space. Major axis in the horizontal plane, which is characterized by collections of moderately reflective material in the sub-RPE space and clear visualization of the hyperreflective Bruch’s membrane. Absence of CNV activity signs | During a period of stability or regression: capillaries and vessel loops no longer present. Remaining vessels are stiffer, thicker and less tortuous. |
| Subthreshold exudative CNV | Type 1 variant characterized by stable persistent or intermittent SRF | Drusen ± pigmentary changes, RPE elevation | FA: Stippled hyperfluorescence, late staining, no leakage/ICGA: absence of definite neovascular complexes and “hot spot,” rarely presence of plaque | Irregular RPE elevation with or without minimal SRF (wax and wane or persistent on FU) | Irregular vascular networks under the fovea or perifoveal areas |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douglas, V.P.; Garg, I.; Douglas, K.A.A.; Miller, J.B. Subthreshold Exudative Choroidal Neovascularization (CNV): Presentation of This Uncommon Subtype and Other CNVs in Age-Related Macular Degeneration (AMD). J. Clin. Med. 2022, 11, 2083. https://doi.org/10.3390/jcm11082083
Douglas VP, Garg I, Douglas KAA, Miller JB. Subthreshold Exudative Choroidal Neovascularization (CNV): Presentation of This Uncommon Subtype and Other CNVs in Age-Related Macular Degeneration (AMD). Journal of Clinical Medicine. 2022; 11(8):2083. https://doi.org/10.3390/jcm11082083
Chicago/Turabian StyleDouglas, Vivian Paraskevi, Itika Garg, Konstantinos A. A. Douglas, and John B. Miller. 2022. "Subthreshold Exudative Choroidal Neovascularization (CNV): Presentation of This Uncommon Subtype and Other CNVs in Age-Related Macular Degeneration (AMD)" Journal of Clinical Medicine 11, no. 8: 2083. https://doi.org/10.3390/jcm11082083
APA StyleDouglas, V. P., Garg, I., Douglas, K. A. A., & Miller, J. B. (2022). Subthreshold Exudative Choroidal Neovascularization (CNV): Presentation of This Uncommon Subtype and Other CNVs in Age-Related Macular Degeneration (AMD). Journal of Clinical Medicine, 11(8), 2083. https://doi.org/10.3390/jcm11082083







