Investigation of Clofazimine Resistance and Genetic Mutations in Drug-Resistant Mycobacterium tuberculosis Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Isolates of Mtb
2.2. Determination of MIC
2.3. Statistical Analysis
2.4. Clinical Data Analysis of CFZ-Resistant Isolates
2.5. PCR and DNA Sequencing
3. Results
3.1. CFZ MIC to Mtb
3.2. Clinical Data Analysis of CFZ Resistant Isolates
3.3. Sequencing of Genes Related to Resistance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barry, V.C.; Belton, J.G.; Conalty, M.L.; Denneny, J.M.; Edward, D.W.; O’Sullivan, J.F.; Twomey, D.; Winder, F. A new series of phenazine (rimino-compounds) with high anti-tuberculosis activity. Nature 1957, 179, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Van Rensburg, C.E.; Jooné, G.K.; O’Sullivan, J.F.; Anderson, R. Antimicrobial activities of clofazimine and B669 are mediated by lysophospholipids. Antimicrob. Agents Chemother. 1992, 36, 2729–2735. [Google Scholar] [CrossRef] [PubMed]
- Lechartier, B.; Cole, S.T. Mode of action of Clofazimine and combination therapy with benzothiazinones against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2015, 59, 4457–4463. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Kassovska-Bratinova, S.; Teh, J.S.; Winkler, J.; Sullivan, K.; Isaacs, A.; Schechter, N.M.; Rubin, H. Reduction of clofazimine by mycobacterial type 2 NADH: Quinone oxidoreductase: A pathway for the generation of bactericidal levels of reactive oxygen species. J. Biol. Chem. 2011, 286, 10276–10287. [Google Scholar] [CrossRef]
- Reddy, V.M.; O’Sullivan, J.F.; Gangadharam, P.R.J. Antimycobacterial activities of riminophenazines. J. Antimicrob. Chemother. 1999, 43, 615–623. [Google Scholar] [CrossRef]
- Van Rensburg, C.E.; Jooné, G.K.; Sirgel, F.A.; Matlola, N.M.; O’Sullivan, J.F. In vitro investigation of the antimicrobial activities of novel tetramethylpiperidine-substituted phenazines against Mycobacterium tuberculosis. Chemotherapy 2000, 46, 43–48. [Google Scholar] [CrossRef]
- Barry, V.C.; Buggle, K.; Byrne, J.; Conalty, M.L.; Winder, F. Absorption, distribution and retention of the riminocompounds in the experimental animal. Ir. J. Med. Sci. 1960, 416, 345–352. [Google Scholar] [CrossRef]
- Barry, V.C.; Conalty, M.L. The antimycobacterial activity of B663. Lepr. Rev. 1965, 36, 3–7. [Google Scholar]
- O’Connor, R.; O’Sullivan, J.F.; O’Kennedy, R. The pharmacology, metabolism and chemistry of clofazimine. Drug Metab. Rev. 1995, 27, 591–614. [Google Scholar] [CrossRef]
- Van Rensburg, C.E.; Anderson, R.; O’Sullivan, J.F. Riminophenazine compounds: Pharmacology and anti-neoplastic potential. Crit. Rev. Oncol. Hematol. 1997, 25, 55–67. [Google Scholar] [CrossRef]
- Van Deun, A.; Maug, A.K.J.; Salim, M.A.H.; Das, P.K.; Sarker, M.R.; Daru, P.; Rieder, H.L. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am. J. Respir. Crit. Care Med. 2010, 182, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Gopal, M.; Padayatchi, N.; Metcalfe, J.Z.; O’Donnell, M.R. Systematic review of clofazimine for the treatment of drug-resistant tuberculosis. Int. J. Tuberc. Lung. Dis. 2013, 17, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Treatment Guidelines for Drug-Resistant Tuberculosis, 2016 Update; WHO: Geneva, Switzerland, 2016; ISBN 9789241549639. [Google Scholar]
- Ahmad, N.; Ahuja, S.D.; Akkerman, O.W.; Alffenaar, J.-W.C.; Anderson, L.F.; Baghaei, P.; Bang, D.; Barry, P.M.; Bastos, M.L.; Behera, D.; et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: An individual patient data meta- analysis. Lancet 2018, 392, 821–834. [Google Scholar] [CrossRef]
- World Health Organization. Technical Report on Critical Concentrations for Drug Susceptibility Testing of Medicines Used in the Treatment of Drug-Resistant Tuberculosis; WHO: Geneva, Switzerland, 2018; Available online: http://www.who.int/iris/handle/10665/260470 (accessed on 5 January 2022).
- Diacon, A.H.; Dawson, R.; von Groote-Bidlingmaier, F.; Symons, G.; Venter, A.; Donald, P.R.; van Niekerk, C.; Everitt, D.; Hutchings, J.; Burger, D.A.; et al. Bactericidal activity of pyrazinamide and clofazimine alone and in combinations with pretomanid and bedaquiline. Am. J. Respir. Crit. Care. Med. 2015, 191, 943–953. [Google Scholar] [CrossRef]
- Schön, T.; Jureen, P.; Chryssanthou, E.; Giske, C.G.; Sturegård, E.; Kahlmeter, G.; Hoffner, S.; Angeby, K.A. Wild-type distributions of seven oral second-line drugs against Mycobacterium tuberculosis. Int. J. Tuberc. Lung. Dis. 2011, 15, 502–509. [Google Scholar] [CrossRef]
- Cavanaugh, J.S.; Jou, R.; Wu, M.H.; Dalton, T.; Kurbatova, E.; Ershova, J.; Cegielski, J.P.; Global PETTS Investigators. Susceptibilities of MDR Mycobacterium tuberculosis isolates to unconventional drugs compared with their reported pharmacokinetic/pharmacodynamic parameters. J. Antimicrob. Chemother. 2017, 72, 1678–1687. [Google Scholar] [CrossRef]
- Jagannath, C.; Reddy, M.V.; Kailasam, S.; O’Sullivan, J.F.; Gangadharam, P.R. Chemotherapeutic activity of clofazimine and its analogues against Mycobacterium tuberculosis. In vitro, intracellular, and in vivo studies. Am. J. Respir. Crit. Care. Med. 1995, 151, 1083–1086. [Google Scholar] [CrossRef]
- Pang, Y.; Zong, Z.; Huo, F.; Jing, W.; Ma, Y.; Dong, L.; Li, Y.; Zhao, L.; Fu, Y.; Huang, H. In vitro drug susceptibility of bedaquiline, delamanid, linezolid, clofazimine, moxifloxacin, and gatifloxacin against extensively drug-resistant tuberculosis in Beijing, China. Antimicrob. Agents Chemother. 2017, 61, e00900–e00917. [Google Scholar] [CrossRef]
- Aung, K.J.; van Deun, A.; Declercq, E.; Sarker, M.R.; Das, P.K.; Hossain, M.A.; Rieder, H.L. Successful 9-month Bangladesh regimen’ for multidrug-resistant tuberculosis among over 500 consecutive patients. Int. J. Tuberc. Lung Dis. 2014, 18, 1180–1187. [Google Scholar] [CrossRef]
- Wang, Q.; Pang, Y.; Jing, W.; Liu, Y.; Wang, N.; Yin, H.; Zhang, Q.; Ye, Z.; Zhu, M.; Li, F.; et al. Clofazimine for treatment of extensively drug-resistant pulmonary tuberculosis in China. Antimicrob. Agents Chemother. 2018, 62, e02149. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, J.; Cui, P.; Shi, W.; Zhang, W.; Zhang, Y. Identification of novel mutations associated with clofazimine resistance in Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2015, 70, 2507–2510. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, B.; Hu, M.; Huo, F.; Guo, S.; Jing, W.; Nuermberger, E.; Lu, Y. Primary clofazimine and bedaquiline resistance among isolates from patients with multidrug-resistant tuberculosis. Antimicrob. Agents Chemother. 2017, 61, e00239-17. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.; Ioerger, T.; Tyagi, S.; Li, S.Y.; Mdluli, K.; Andries, K.; Grosset, J.; Sacchettini, J.; Nuermberger, E. Mutations in pepQ Confer Low-Level Resistance to Bedaquiline and Clofazimine in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2016, 60, 4590–4599. [Google Scholar] [CrossRef] [PubMed]
- Andries, K.; Villellas, C.; Coeck, N.; Thys, K.; Gevers, T.; Vranckx, L.; Lounis, N.; de Jong, B.C.; Koul, A. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS ONE 2014, 9, e102135. [Google Scholar] [CrossRef]
- Bloemberg, G.V.; Keller, P.M.; Stucki, D.; Trauner, A.; Borrell, S.; Latshang, T.; Coscolla, M.; Rothe, T.; Hömke, R.; Ritter, C.; et al. Acquired resistance to bedaquiline and delamanid in therapy for tuberculosis. N. Engl. J. Med. 2015, 373, 1986–1988. [Google Scholar] [CrossRef]
- Hoffmann, H.; Kohl, T.A.; Hofmann-Thiel, S.; Merker, M.; Beckert, P.; Jaton, K.; Nedialkova, L.; Sahalchyk, E.; Rothe, T.; Keller, P.M.; et al. Delamanid and bedaquiline resistance in Mycobacterium tuberculosis ancestral Beijing genotype causing extensively drug-resistant tuberculosis in a Tibetan refugee. Am. J. Respir. Crit. Care Med. 2016, 193, 337–340. [Google Scholar] [CrossRef]
- Somoskovi, A.; Bruderer, V.; Homke, R.; Bloemberg, G.V.; Bottger, E.C. A mutation associated with clofazimine and bedaquiline cross-resistance in MDR-TB following bedaquiline treatment. Eur. Respir. J. 2015, 45, 554–557. [Google Scholar] [CrossRef]
- Martin, A.; Camacho, M.; Portaels, F.; Palomino, J.C. Resazurin microtiter assay plate testing of Mycobacterium tuberculosis susceptibilities to second-line drugs: Rapid, simple, and inexpensive method. Antimicrob. Agents Chemother. 2003, 7, 3616–3619. [Google Scholar] [CrossRef]
- Higgins, D.; Thompson, J.; Gibson, T.; Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- World Health Organization. Meeting Report of the WHO Expert Consultation on the Definition of Extensively Drug-Resistant Tuberculosis; WHO: Geneva, Switzerland, 2021; ISBN 978-92-4-0001866-2. [Google Scholar]
- Zhang, Z.; Li, T.; Qu, G.; Pang, Y.; Zhao, Y. In vitro synergistic activity of clofazimine and other antituberculous drugs against multidrug-resistant Mycobacterium tuberculosis isolates. Int. J. Antimicrob. Agents. 2015, 45, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lu, Y.; Fu, L.; Zhu, H.; Wang, B.; Mdluli, K.; Upton, A.M.; Jin, H.; Zheng, M.; Zhao, W.; et al. In vitro and in vivo activity of clofazimine against Mycobacterium tuberculosis persisters. Int. J. Tuberc. Lung Dis. 2012, 16, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Steel, H.C.; Matlola, N.M.; Anderson, R. Inhibition of potassium transport and growth of mycobacteria exposed to clofazimine and B669 is associated with a calcium-independent increase in microbial phospholipase A2 activity. J. Antimicrob. Chemother. 1999, 44, 209–216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.; Gao, J.; Du, J.; Shu, W.; Wang, L.; Wang, Y.; Xue, Z.; Li, L.; Xu, S.; Pang, Y. Acquisition of clofazimine resistance following bedaquiline treatment for multidrug-resistant tuberculosis. Int. J. Infect. Dis. 2021, 102, 392–963. [Google Scholar] [CrossRef] [PubMed]

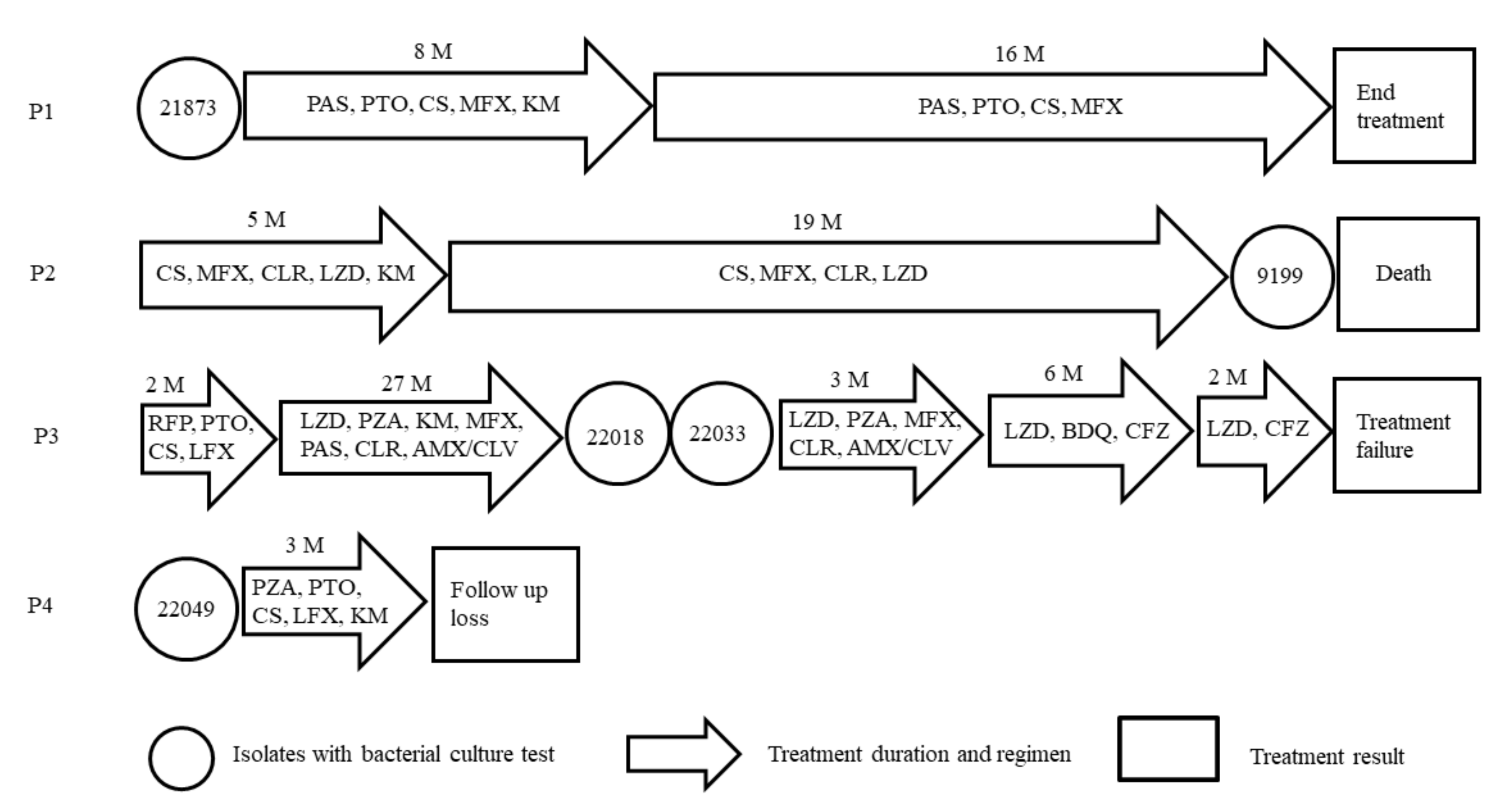
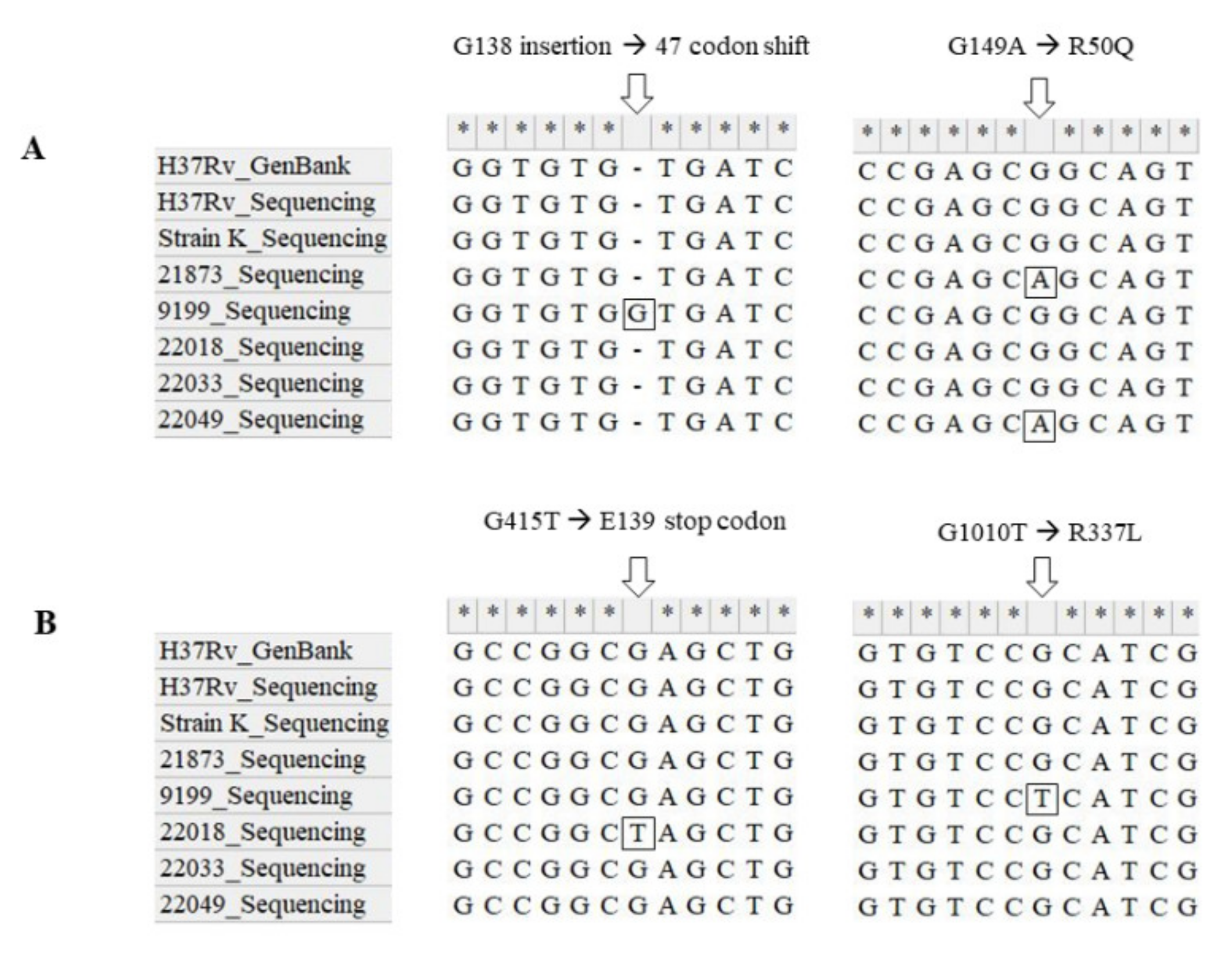
| Patients | Isolates | Type | Drug Resistance Profile | Year of Isolation |
|---|---|---|---|---|
| P1 | 21873 | MDR | INH, RFP, EMB, RBU, PTO | 2014 |
| P2 | 9199 | XDR | INH, RFP, EMB, RBU, SM, KM, AMK, OFX, MFX, PAS, PTO, CS | 2010 |
| P3 | 22018 | XDR | INH, RFP, EMB, RBU, SM, KM, AMK, OFX, LEV, PTO, CS | 2014 |
| 22033 | XDR | INH, RFP, EMB, RBU, SM, KM, AMK, OFX, LEV, PTO, CS | 2014 | |
| P4 | 22049 | XDR | INH, RFP, EMB, RBU, SM, KM, AMK, OFX, LEV, PTO, CS | 2014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Jung, J.; Kim, J.; Han, S.B.; Ryoo, S. Investigation of Clofazimine Resistance and Genetic Mutations in Drug-Resistant Mycobacterium tuberculosis Isolates. J. Clin. Med. 2022, 11, 1927. https://doi.org/10.3390/jcm11071927
Park S, Jung J, Kim J, Han SB, Ryoo S. Investigation of Clofazimine Resistance and Genetic Mutations in Drug-Resistant Mycobacterium tuberculosis Isolates. Journal of Clinical Medicine. 2022; 11(7):1927. https://doi.org/10.3390/jcm11071927
Chicago/Turabian StylePark, Sanghee, Jihee Jung, Jiyeon Kim, Sang Bong Han, and Sungweon Ryoo. 2022. "Investigation of Clofazimine Resistance and Genetic Mutations in Drug-Resistant Mycobacterium tuberculosis Isolates" Journal of Clinical Medicine 11, no. 7: 1927. https://doi.org/10.3390/jcm11071927
APA StylePark, S., Jung, J., Kim, J., Han, S. B., & Ryoo, S. (2022). Investigation of Clofazimine Resistance and Genetic Mutations in Drug-Resistant Mycobacterium tuberculosis Isolates. Journal of Clinical Medicine, 11(7), 1927. https://doi.org/10.3390/jcm11071927






