Ultrasound-Assisted Wound (UAW) Debridement in the Treatment of Diabetic Foot Ulcer: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Literature Search
2.2. Article Selection
2.3. Data Collection
2.4. Assessment of Risk of Bias and Quality of Evidence
2.5. Statistical Analysis
3. Results
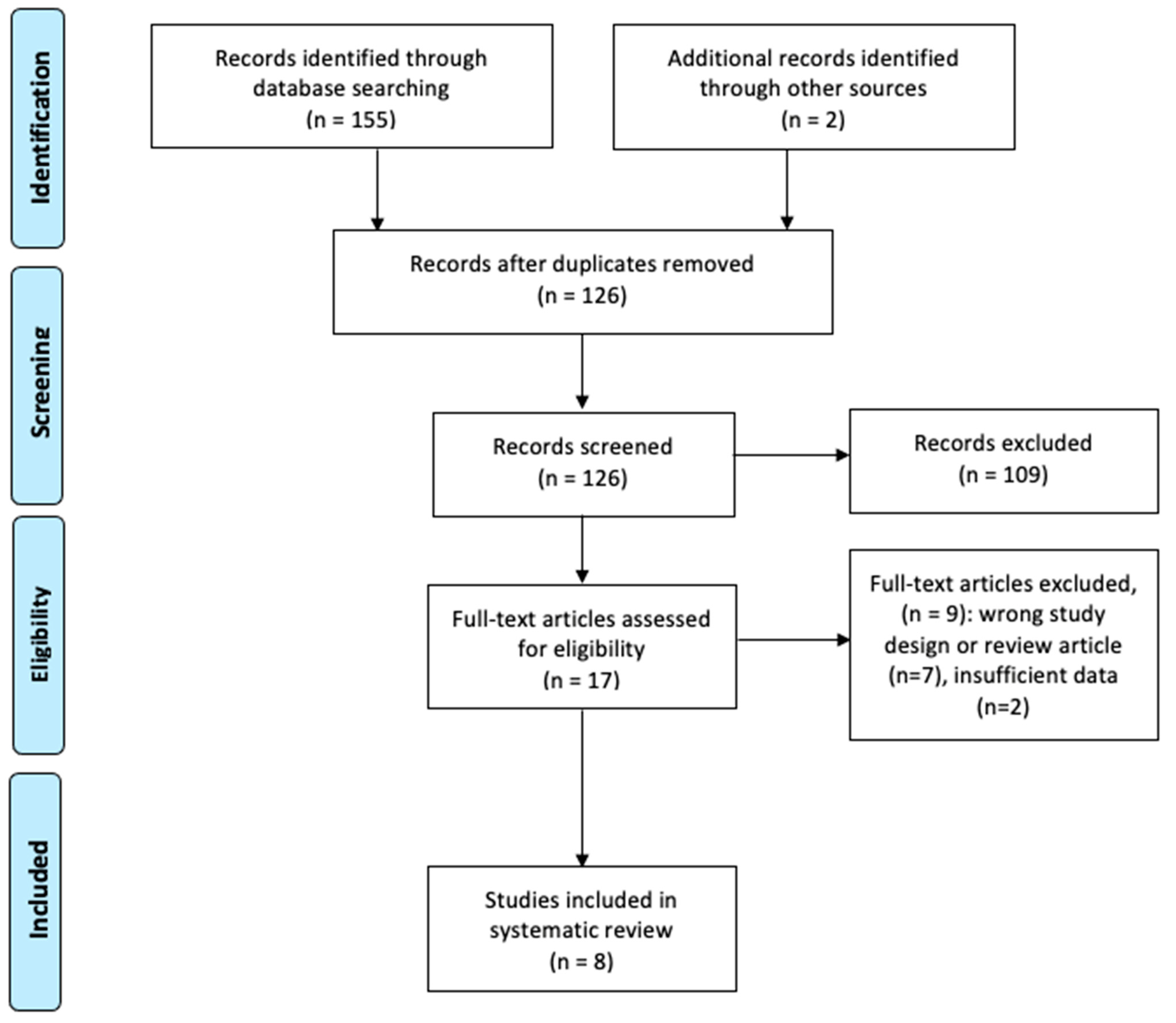
3.1. Literature Search
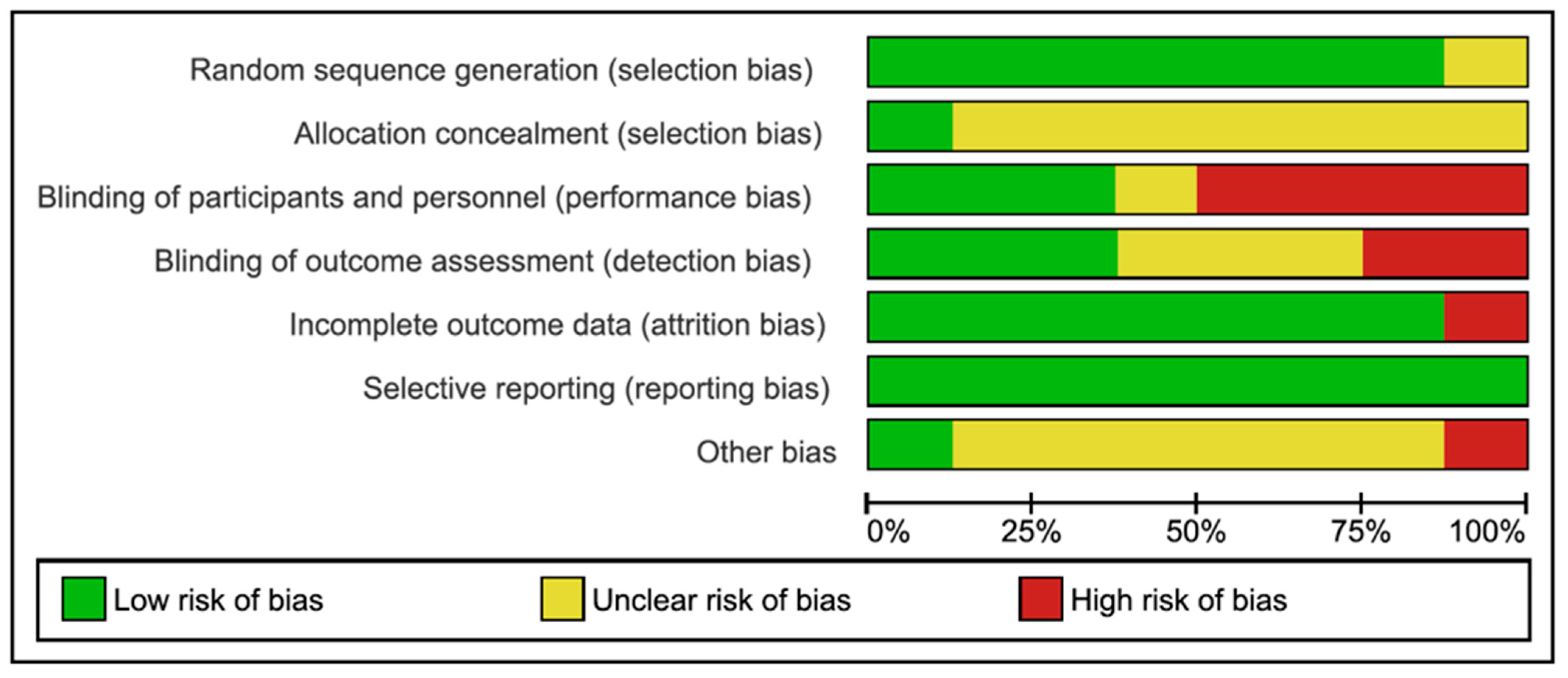
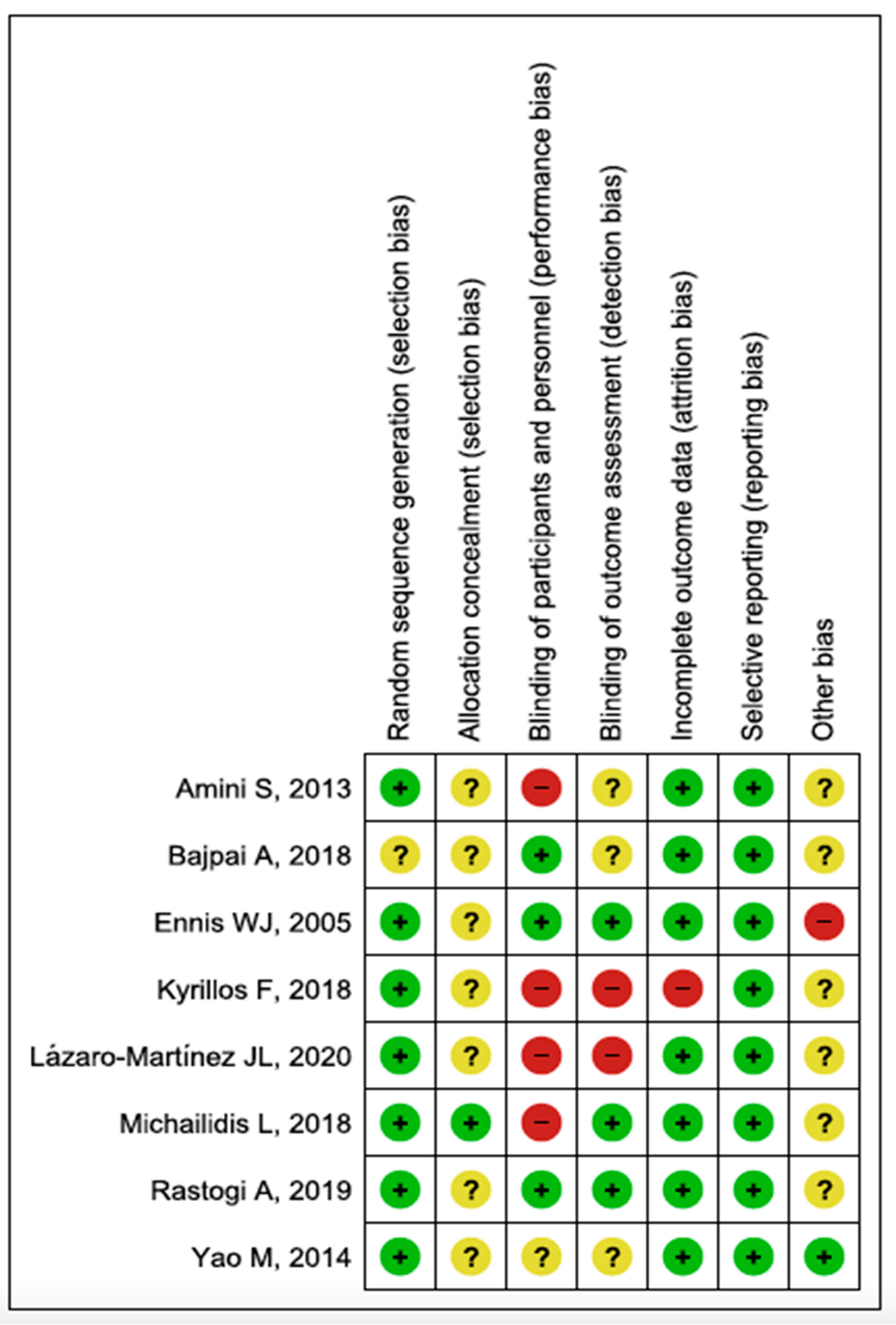
3.2. Assessment of Risk of Bias and Quality of Evidence
3.3. Outcome Measures
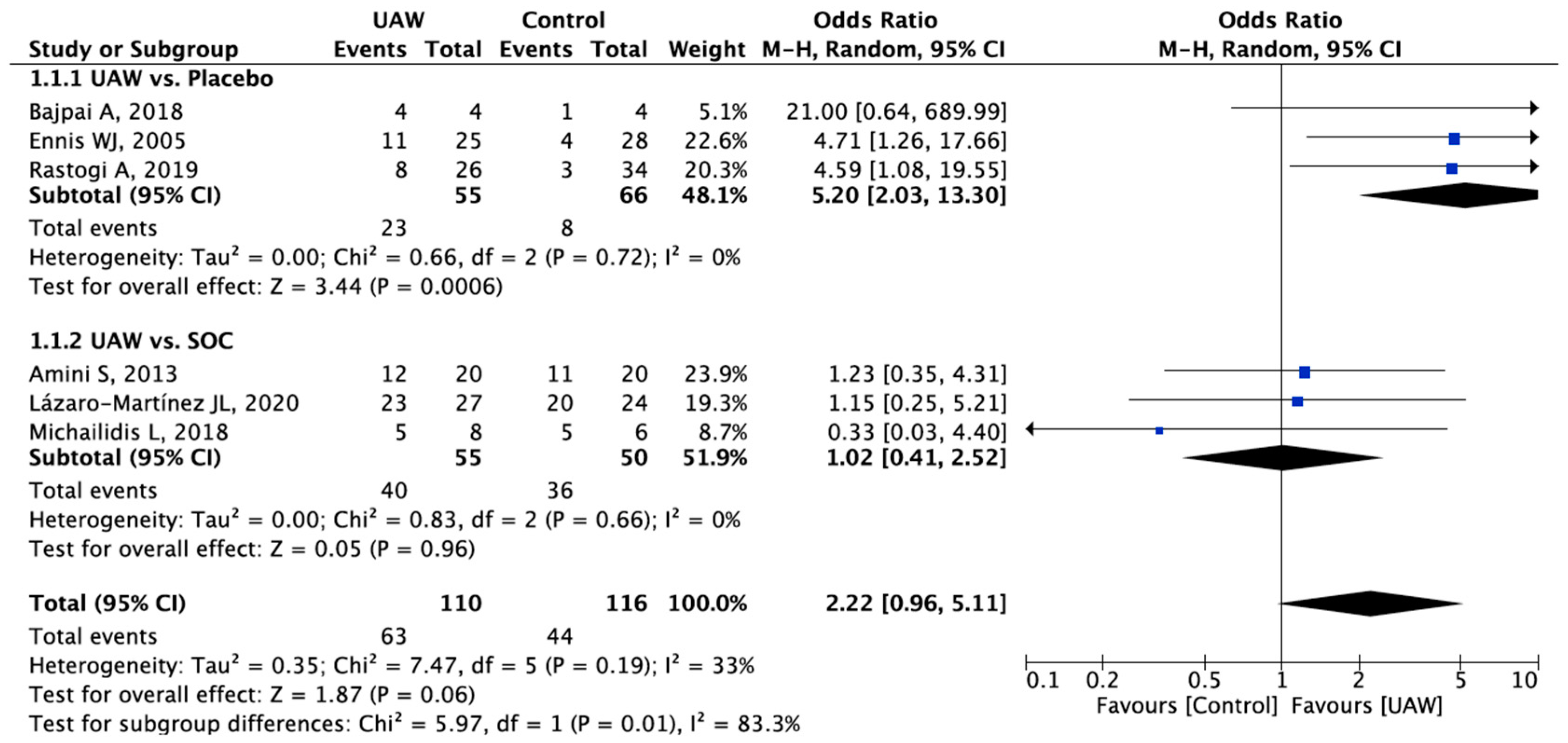
3.3.1. Healing Rate
3.3.2. Time to Healing
3.3.3. Wound Area Reduction
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Apelqvist, J.; Bakker, K.; van Houtum, W.H.; Schaper, N.C. Practical guidelines on the management and prevention of the diabetic foot. Diabetes Metab. Res. Rev. 2008, 24 (Suppl. 1), S181–S187. [Google Scholar] [CrossRef] [PubMed]
- van Netten, J.J.; Bus, S.A.; Apelqvist, J.; Lipsky, B.A.; Hinchliffe, R.J.; Game, F.; Rayman, G.; Lazzarini, P.A.; Forsythe, R.O.; Peters, E.J.G.; et al. Definitions and criteria for diabetic foot disease. Diabetes Metab. Res. Rev. 2020, 36 (Suppl. 1), e3268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.C.; Andros, G.; Caporusso, J.; Harkless, L.B.; Mills, J.L.; Armstrong, D.G. Toe and flow: Essential components and structure of the amputation prevention team. J. Vasc. Surg. 2010, 52 (Suppl. 3), 23S–27S. [Google Scholar] [CrossRef] [Green Version]
- Noor, S.; Zubair, M.; Ahmad, J. Diabetic foot ulcer—A review on pathophysiology, classification and microbial etiology. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 192–199. [Google Scholar] [CrossRef]
- Schaper, N.C.; van Netten, J.J.; Apelqvist, J.; Bus, S.A.; Hinchliffe, R.J.; Lipsky, B.A.; Board, I.E. Practical guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. 1), e3266. [Google Scholar] [CrossRef] [Green Version]
- Rayman, G.; Vas, P.; Dhatariya, K.; Driver, V.; Hartemann, A.; Londahl, M.; Piaggesi, A.; Apelqvist, J.; Attinger, C.; Game, F. Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. 1), e3283. [Google Scholar] [CrossRef] [Green Version]
- Hingorani, A.; Lamuraglia, G.M.; Henke, P.; Meissner, M.H.; Loretz, L.; Zinszer, K.M.; Driver, V.R.; Frykberg, R.; Carman, T.L.; Marston, W.; et al. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J. Vasc. Surg. 2016, 63, 3S–21S. [Google Scholar] [CrossRef] [Green Version]
- Lázaro-Martínez, J.L.; Álvaro-Afonso, F.J.; Ahluwaila, R.; Baker, N.; Ríos-Ruh, J.; Rivera-San Martin, G.; Van Acker, K. Debridement and the Diabetic Foot. 2019. Available online: https://www.d-foot.org/images/Debridement.pdf (accessed on 26 March 2022).
- Hinchliffe, R.J.; Forsythe, R.O.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Hong, J.P.; Katsanos, K.; Mills, J.L.; Nikol, S.; Reekers, J.; et al. Guidelines on diagnosis, prognosis and management of peripheral artery disease in patients with a foot ulcer and diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. 1), e3276. [Google Scholar] [CrossRef]
- Elraiyah, T.; Domecq, J.P.; Prutsky, G.; Tsapas, A.; Nabhan, M.; Frykberg, R.G.; Hasan, R.; Firwana, B.; Prokop, L.J.; Murad, M.H. A systematic review and meta-analysis of débridement methods for chronic diabetic foot ulcers. J. Vasc. Surg. 2016, 63, 37S–45S. [Google Scholar] [CrossRef] [Green Version]
- Driver, V.R.; Yao, M.; Miller, C.J. Noncontact low-frequency ultrasound therapy in the treatment of chronic wounds: A meta-analysis. Wound Repair Regen. 2011, 19, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Kotronis, G.; Vas, P.R.J. Ultrasound Devices to Treat Chronic Wounds: The Current Level of Evidence. Int. J. Low. Extrem. Wounds 2020, 19, 341–349. [Google Scholar] [CrossRef]
- Kavros, S.J.; Miller, J.L.; Hanna, S.W. Treatment of ischemic wounds with noncontact, low-frequency ultrasound: The Mayo clinic experience, 2004–2006. Adv. Skin Wound Care 2007, 20, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Herberger, K.; Franzke, N.; Blome, C.; Kirsten, N.; Augustin, M. Efficacy, Tolerability and Patient Benefit of Ultrasound-Assisted Wound Treatment versus Surgical Debridement: A Randomized Clinical Study. Dermatology 2011, 222, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-J.R.; Perry, J.; Cross, K. Low-Frequency Ultrasound Debridement in Chronic Wound Healing: A Systematic Review of Current Evidence. Plast. Surg. 2017, 25, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Swanson, T.; Lázaro-Martínez, J.L.; Braumann, C.; Kirchhoff, J.-B.; Gaechter, B.; van Acker, K. Ultrasonic-assisted wound debridement: Report from a closed panel meeting. J. Wound Care 2020, 29, 128–135. [Google Scholar] [CrossRef]
- Stanisic, M.M.; Provo, B.J.; Larson, D.L.; Kloth, L.C. Wound debridement with 25 kHz ultrasound. Adv. Skin Wound Care 2005, 18, 484–490. [Google Scholar] [CrossRef]
- Voigt, J.; Wendelken, M.; Driver, V.; Alvarez, O.M. Low-frequency ultrasound (20–40 kHz) as an Adjunctive Therapy for Chronic Wound Healing: A Systematic Review of the Literature and Meta-Analysis of Eight Randomized Controlled Trials. Int. J. Low. Extrem. Wounds 2011, 10, 190–199. [Google Scholar] [CrossRef]
- Amini, S.; ShojaeeFard, A.; Annabestani, Z.; Hammami, M.; Shaiganmhr, Z.; Larijani, B.; Mohseni, S.; Afshani, H.R.; Rad, M.A.; Reza, M.-T.M. Low-Frequency Ultrasound Debridement in Patients with Diabetic Foot Ulcers and Osteomyelitis. Wounds 2013, 25, 193–198. [Google Scholar]
- Lázaro-Martínez, J.L.; Álvaro-Afonso, F.J.; García-Álvarez, Y.; Molines-Barroso, R.J.; García-Morales, E.; Sevillano-Fernández, D. Ultrasound-assisted debridement of neuroischaemic diabetic foot ulcers, clinical and microbiological effects: A case series. J. Wound Care 2018, 27, 278–286. [Google Scholar] [CrossRef]
- Lázaro-Martínez, J.L.; Álvaro-Afonso, F.J.; Sevillano-Fernández, D.; García-Álvarez, Y.; Sanz-Corbalan, I.; García-Morales, E. Cellular Proliferation, Dermal Repair, and Microbiological Effectiveness of Ultrasound-Assisted Wound Debridement (UAW) Versus Standard Wound Treatment in Complicated Diabetic Foot Ulcers (DFU): An Open-Label Randomized Controlled Trial. J. Clin. Med. 2020, 9, 4032. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. TPG Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. Chin. J. Evid.-Based Med. 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Ennis, W.J.; Formann, P.; Mozen, N.; Massey, J.; Conner-Kerr, T.; Meneses, P.; Group, M.U.D.F.S. Ultrasound therapy for recalcitrant diabetic foot ulcers: Results of a randomized, double-blind, controlled, multicenter study. Ostomy Wound Manag. 2005, 51, 12. [Google Scholar]
- Yao, M.; Hasturk, H.; Kantarci, A.; Gu, G.; Garcia-Lavin, S.; Fabbi, M.; Park, N.; Hayashi, H.; Attala, K.; French, M.A.; et al. A pilot study evaluating non-contact low-frequency ultrasound and underlying molecular mechanism on diabetic foot ulcers. Int. Wound J. 2014, 11, 586–593. [Google Scholar] [CrossRef]
- Bajpai, A.; Nadkarni, S.; Neidrauer, M.; Weingarten, M.S.; Lewin, P.A.; Spiller, K.L. Effects of Non-thermal, Non-cavitational Ultrasound Exposure on Human Diabetic Ulcer Healing and Inflammatory Gene Expression in a Pilot Study. Ultrasound Med. Biol. 2018, 44, 2043–2049. [Google Scholar] [CrossRef]
- Kyrillos, F.; Albehairy, A.; Roshdi, M.; Elkashef, W.; Tarshoby, M. Ultrasound versus sharp wound debridement in healing of recalcitrant neuropathic diabetic foot ulcers: Clinical and pathological study. Diabetologia 2018, 61, S6. [Google Scholar]
- Michailidis, L.; Bergin, S.M.; Haines, T.P.; Williams, C.M. Healing rates in diabetes-related foot ulcers using low frequency ultrasonic debridement versus non-surgical sharps debridement: A randomised controlled trial. BMC Res. Notes 2018, 11, 732. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, A.; Bhansali, A.; Ramachandran, S. Efficacy and Safety of Low-Frequency, Noncontact Airborne Ultrasound Therapy (Glybetac) For Neuropathic Diabetic Foot Ulcers: A Randomized, Double-Blind, Sham-Control Study. Int. J. Low. Extrem. Wounds 2019, 18, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F. The Dysvascular Foot: A System for Diagnosis and Treatment. Foot Ankle Int. 1981, 2, 64–122. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Lavery, L.A.; Harkless, L.B. Validation of a Diabetic Wound Classification System. The contribution of depth, infection and ischemia to risk of amputation. Diabetes Care 1998, 21, 855–859. [Google Scholar] [CrossRef] [PubMed]
| Outcome | Number of Studies | Study Design | Certainty Assessment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Effect | Certainty | |||
| Healing Rate | 6 | RCT | Serious a | Serious b | Not serious c | Serious d | Publication bias strongly suspected | OR (95% CI) 2.22 (0.96, 5.11) | ⨁◯◯◯◯◯ Very low |
| Time to Healing | 3 | RCT | Serious a | Serious b | Not serious c | Serious d | Publication bias strongly suspected | SMD (95% CI) −1.41 (−3.43, 0.61) | ⨁◯◯◯◯◯◯ Very low |
| Wound Area Reduction | 3 | RCT | Serious a | Not serious | Not serious c | Serious d | Publication bias strongly suspected | SMD (95% CI) 0.23 (−0.09, 0.55) | ⨁◯◯◯◯◯◯ Very low |
| Author/Year | Number of Participants | Intervention | Healing Rate (%) | Time to Healing (Weeks) | Wound Area Reduction (%) |
|---|---|---|---|---|---|
| Ennis [27]/2005 | Arm 1: 25 Arm 2: 28 Total: 55 | Arm 1: UAW Arm 2: Placebo | Arm 1: 11 (40.7%) Arm 2: 4 (14.3%) | Arm 1: 9.12 ± 0.58 w Arm 2: 11.74 ± 0.22 w | – |
| Amini [20]/2013 | Arm 1: 20 Arm 2: 20 Total: 40 | Arm 1: UAW Arm 2: SOC | Arm 1: 12 (60%) Arm 2: 11 (55%) | Arm 1: 8.8 ± 12 w Arm 2: 11.6 ± 11.2 w | Arm 1: 87.9 ± 33.8% Arm 2: 82.4 ± 33% |
| Yao [28]/2014 | Arm 1: 4 Arm 2: 4 Arm 3: 4 Total: 12 | Arm 1: UAW 3/w Arm 2: UAW 1/w Arm 3: SOC | – | – | Arm 1: 86% Arm 2: 25% Arm 3: 39% |
| Bajpai [29]/2018 | Arm 1: 4 Arm 2: 4 Total: 8 | Arm 1: UAW Arm 2: Placebo | Arm 1: 4 (100%) Arm 2: 1 (25%) | – | – |
| Kyrillos [30]/2018 | Arm 1: 12 Arm 2: 11 Total: 23 | Arm 1: UAW Arm 2: SOC | – | – | Arm 1: 43% Arm 2: 24.4% |
| Michailidis [31]/2018 | Arm 1: 8 Arm 2: 6 Total: 14 | Arm 1: UAW Arm 2: SOC | Arm 1: 5 (62.5%) Arm 2: 5 (83.3%) | Arm 1: 29.4 ± 10.07 w Arm 2: 15.4 ± 6.1 w | – |
| Rastogi [32]/2019 | Arm 1: 26 Arm 2: 34 Total: 60 | Arm 1: UAW Arm 2: Placebo | Arm 1: 8 (23.5%) Arm 2: 3 (11.5%) | – | Arm 1: 69.4 ± 23.2% Arm 2: 59.6 ± 24.9% |
| Lázaro-Martínez [22]/2020 | Arm 1: 27 Arm 2: 24 Total: 51 | Arm 1: UAW Arm 2: SOC | Arm 1: 23 (85.1%) Arm 2: 20 (83.3%) | Arm 1: 9.7 ± 3.8 w Arm 2: 14.8 ± 12 w | Arm 1: 86.6 ± 83.8% Arm 2: 78.94 ± 68.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Escobar, S.; Álvaro-Afonso, F.J.; García-Álvarez, Y.; López-Moral, M.; Lázaro-Martínez, J.L.; García-Morales, E. Ultrasound-Assisted Wound (UAW) Debridement in the Treatment of Diabetic Foot Ulcer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1911. https://doi.org/10.3390/jcm11071911
Flores-Escobar S, Álvaro-Afonso FJ, García-Álvarez Y, López-Moral M, Lázaro-Martínez JL, García-Morales E. Ultrasound-Assisted Wound (UAW) Debridement in the Treatment of Diabetic Foot Ulcer: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(7):1911. https://doi.org/10.3390/jcm11071911
Chicago/Turabian StyleFlores-Escobar, Sebastián, Francisco Javier Álvaro-Afonso, Yolanda García-Álvarez, Mateo López-Moral, José Luis Lázaro-Martínez, and Esther García-Morales. 2022. "Ultrasound-Assisted Wound (UAW) Debridement in the Treatment of Diabetic Foot Ulcer: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 7: 1911. https://doi.org/10.3390/jcm11071911
APA StyleFlores-Escobar, S., Álvaro-Afonso, F. J., García-Álvarez, Y., López-Moral, M., Lázaro-Martínez, J. L., & García-Morales, E. (2022). Ultrasound-Assisted Wound (UAW) Debridement in the Treatment of Diabetic Foot Ulcer: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(7), 1911. https://doi.org/10.3390/jcm11071911










